Understanding Cancer Development in the Context of Cancer Health Disparities
In this section you will learn:
- Cancer is a highly diverse group of diseases characterized by uncontrolled cellular growth.
- Biological determinants of cancer development include changes within and outside the cell.
- Pathogenic germline mutations predispose individuals to diseases such as cancer. These mutations can be more common in groups that share genetic ancestry.
- Identification of genetic, epigenetic, transcriptomic, and protein alterations that drive cancer are an important part of the cancer care decision-making process and form the foundation of precision medicine. The majority of currently available cancer treatments are based on studies of individuals that are of European ancestry and have consequently benefited these populations more compared to groups of non-European ancestry.
- To equitably treat cancer, continued research is needed to deepen our understanding of cancer biology in racial and ethnic minorities and other underserved populations.
Cancer is not a single disease but rather a collection of disorders broadly characterized by the inability of a cell to respond to normal biological cues related to proliferation, growth, and death. As a result, uncontrolled division leads to a mass of cells called a tumor. The development of cancer is extremely complex, and the field of cancer research is rapidly evolving. Our understanding of the hallmarks that define cancer development has increased tremendously in the past two decades, thanks to major advances in medical research resulting from generous federal investments.
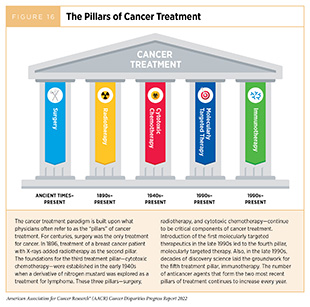
If detected early, small tumors are treatable with surgery, radiation, or systemic therapies. However, if left undetected, tumors continue to grow and crowd out the surrounding healthy cells within organs, disrupting normal function and leading to organ failure. Cancer cells can continue to divide, acquiring changes that allow them to grow faster, and eventually use the blood and lymphatic systems to move to distant organs. Growth of cancer cells in another organ distant from its original site is called metastasis and is the primary cause of death from most cancers. Many cancers have already metastasized through the bloodstream to other organs even when the primary tumor is detected early (158)Klein CA. Parallel Progression of Primary Tumours and Metastases. Nature Reviews Cancer 2009;9:302-12. [LINK NOT AVAILABLE]. The aggressiveness of a cancer often refers to the speed at which a single cancer cell progresses to form a tumor and metastasizes throughout the body. This process is highly dependent on the site of cancer, the health of the patient, lifestyle, and environmental factors. Survival rates from cancers will therefore depend upon equitable access to the main pillars of cancer treatment (Figure 16) at the time of diagnosis.
Centuries of systemic racism and discrimination in the United States have led to many racial and ethnic minorities and underserved groups being disproportionately exposed to detrimental social and built environmental factors that directly or indirectly contribute to increased incidence, advanced-stage diagnoses, and higher mortality from cancer (33)Cackowski FC, Mahal B, Heath EI, Carthon B. Evolution of Disparities in Prostate Cancer Treatment: Is This a New Normal? Am Soc Clin Oncol Educ Book 2021;41:1-12. [LINK NOT AVAILABLE](159)Martini R, Newman L, Davis M. Breast Cancer Disparities in Outcomes; Unmasking Biological Determinants Associated with Racial and Genetic Diversity. Clinical & Experimental Metastasis 2021;39:7-14.. [LINK NOT AVAILABLE](160)Freedman JA, Abo MA, Allen TA, Piwarski SA, Wegermann K, Patierno SR. Biological Aspects of Cancer Health Disparities. Annual Review of Medicine 2021;72:229-41. [LINK NOT AVAILABLE](161)Nelson B. How Structural Racism Can Kill Cancer Patients. Cancer Cytopathology 2020;128:83-4. [LINK NOT AVAILABLE](162)Ashing KT, Jones V, Bedell F, Phillips T, Erhunmwunsee L. Calling Attention to the Role of Race-Driven Societal Determinants of Health on Aggressive Tumor Biology: A Focus on Black Americans. JCO Oncology Practice 2022;18:15-22. [LINK NOT AVAILABLE](163)Zeigler-Johnson CM, Tierney A, Rebbeck TR, Rundle A. Prostate Cancer Severity Associations with Neighborhood Deprivation. Prostate Cancer 2011;2011:846263. [LINK NOT AVAILABLE]. These factors, collectively referred to as SDOH, contribute to a greater burden of many types of cancers in racial and ethnic minorities and other medically underserved populations (see Factors That Drive Cancer Health Disparities) (43)Zavala VA, Bracci PM, Carethers JM, Carvajal-Carmona L, Coggins NB, Cruz-Correa MR, et al. Cancer Health Disparities in Racial/Ethnic Minorities in the United States. Br J Can 2021;124:315-32. [LINK NOT AVAILABLE](164)Mbemi A, Khanna S, Njiki S, Yedjou CG, Tchounwou PB. Impact of Gene–Environment Interactions on Cancer Development. International Journal of Environmental Research and Public Health 2020;17:8089. [LINK NOT AVAILABLE]. While SDOH individually and collectively play an undeniable role in driving cancer health disparities, it must be noted that research has uncovered ancestry-related biological differences in cancers among patients from different populations. These differences may help explain the higher incidence or aggressiveness of certain cancers and differential responses to therapy that persist even after accounting for SDOH.
Influences Inside the Cell
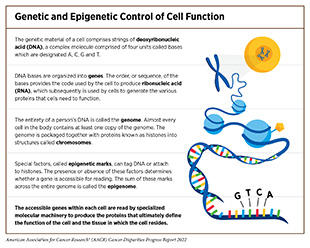
Cells of the human body rely on instructions from genetic material known as deoxyribonucleic acid (DNA) to function. DNA is made up of four types of building blocks called bases which are designated A, T, C, and G (see sidebar on Genetic and Epigenetic Control of Cell Function). Anywhere from 50–250 million of these bases are linked together to form individual strands, with two strands of the same length paired together to form a double-stranded, helical structure; these pairs of strands are packaged together with proteins known as histones into structures called chromosomes. Each chromosome contains hundreds to thousands of genes, which are segments of DNA that contain the code for a protein, the functional unit of the cell. To make a protein, a cell copies a gene from the DNA to make another type of molecule called ribonucleic acid (RNA) in a process called transcription. The cell can make many copies of RNA from a single sequence of DNA, increasing the amount of message in the cell. The cells then “translate” the information in the RNA into proteins; therefore, the more RNA present, the more protein that is made.
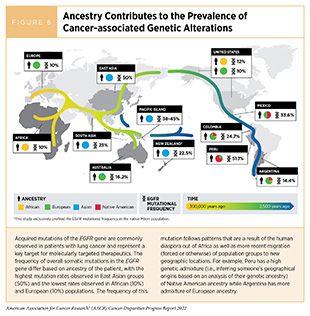
The human species shares roughly 99.9 percent sequence similarity in its DNA, with only 0.1 percent being different from one human to another; yet this 0.1 percent encompasses millions of changes and is what makes each of us unique. Many of the genetic differences found in DNA across groups with different genetic ancestries are a result of human migration out of continental Africa roughly 100,000 years ago to neighboring continents (collectively termed the human diaspora). The subsequent adaptations to new climates, diseases, and environments shaped human genetics, which results in the human diversity we see today (Figure 6) (166)Timmermann A, Friedrich T. Late Pleistocene Climate Drivers of Early Human Migration. Nature 2016;538:92-5. [LINK NOT AVAILABLE]. Biological traits that arise from genetic differences can be positive, such as adaptability to unfavorable climates and altitudes, tolerance of particular food sources, or being more resistant to infections by parasites. However, these genetic differences can also predispose certain groups to genetic diseases like cancer. Recent migrations (forced or otherwise) have led to further genetic mixture, which is the reality of most minority populations in the U.S. The differences in genetic composition that result from this mixing are what make measurements of ancestry important in comparative tumor studies.
Genetic Changes in Cancer
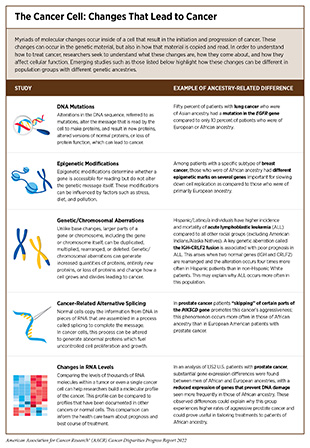
Genetic mutations are changes that occur to the sequence of bases in the DNA. These include single base changes such as substitution, losing, or gaining of a base. Mutations can also be changes to a larger number of bases such as deletions, amplifications, and exchange within and between chromosomes (168)Raca G, Abdel-Azim H, Yue F, Broach J, Payne JL, Reeves ME, et al. Increased Incidence of Ikzf1 Deletions and Igh-Crlf2 Translocations in B-All of Hispanic/Latino Children—a Novel Health Disparity. Leukemia 2021;35:2399-402. [LINK NOT AVAILABLE]. In fact, even within a single tumor, there can be subpopulations of cancer cells with different mutations, a phenomenon known as intratumor heterogeneity, which helps cancer cells evade anticancer therapies.
Most cancer-causing mutations are acquired over an individual’s lifetime due to errors arising during normal cell duplication or because of environmental exposures, lifestyle factors, or health conditions that fuel chronic inflammation. These acquired mutations are referred to as somatic mutations. About 10 percent of cancer-causing mutations are inherited. When multiple individuals in a family carry a mutation in a gene that is important in cancer-causing processes, and there is strong evidence that the mutation significantly increases risk of cancer, these types of inherited mutations are called “pathogenic” as experienced by Alejandro Mirazo who has lynch syndrome. Decades of research have led to the identification of numerous genes that are associated with cancers as well as specific inherited mutations that are pathogenic.
Unfortunately, much of the research studies to understand these genetic predispositions have been done primarily in groups of European ancestry, limiting our understanding of many identified pathogenic variants in other groups, such as those from African, Native American, Asian, and Hispanic ancestries. Research studies focused on examining differences in genetic predispositions in people from different ancestries are vital because they can inform early detection, surveillance, and treatment decisions.
Epigenetic Changes in Cancer
DNA inside the cell is tightly packaged around proteins called histones. This packaging serves many purposes, the most important of which is to control access to the genes that are encoded in the DNA. To regulate access to the genetic code, cells make small changes to the DNA and/or the histones. These changes, called epigenetic modifications, do not alter the DNA sequence and can be reversible, but they can still be passed on to children. The “epigenome” describes all of the epigenetic modifications to the DNA in a single cell. A key function of epigenetic modifications is to grant access to the genetic code when cells need to generate a specific protein and restrict access to it when cells do not need the protein. In cancer cells, the epigenetic processes that grant or restrict access to the genetic code can become aberrant, leading to cancer (171)Ahmad A, Azim S, Zubair H, Khan MA, Singh S, Carter JE, et al. Epigenetic Basis of Cancer Health Disparities: Looking Beyond Genetic Differences. Biochimica et Biophysica Acta (BBA) Reviews on Cancer 2017;1868:16-28. [LINK NOT AVAILABLE]. Emerging evidence shows that environmental influences such as diet, stress, and exposure to pollutants can result in epigenetic changes to the DNA. This is especially relevant to racial and ethnic minorities and other underserved populations, who continue to be disproportionately negatively affected by environmental influences, potentially experiencing epigenetic changes that can aid cancer development.
The study of how social experiences can lead to epigenetic changes in DNA is known as social epigenomics. Individuals and their communities are exposed to these societal risks to varying degrees. Understanding how SDOH affect biology therefore represents an important area for developing intervention strategies to combat cancer disparities (see Factors That Drive Cancer Health Disparities). While the exact mechanisms by which these factors influence biology are multifaceted, it has been shown that the epigenetic regulation of genes that cause breast and prostate cancers is different between African American and Caucasian patients (172)Mehrotra J, Ganpat MM, Kanaan Y, Fackler MJ, McVeigh M, Lahti-Domenici J, et al. Estrogen Receptor/Progesterone Receptor-Negative Breast Cancers of Young African-American Women Have a Higher Frequency of Methylation of Multiple Genes Than Those of Caucasian Women1. Clinical Cancer Research 2004;10:2052-7. [LINK NOT AVAILABLE](173)Kwabi-Addo B, Wang S, Chung W, Jelinek J, Patierno SR, Wang B-D, et al. Identification of Differentially Methylated Genes in Normal Prostate Tissues from African American and Caucasian Men. Clinical Cancer Research 2010;16:3539-47. [LINK NOT AVAILABLE]. One area of active investigation is understanding allostatic load, which refers to the cumulative lifetime effects of stressors such as racism on epigenomics and the body’s stress response system, and how these interactions influence cancer risk and development. Because epigenetic modifications are potentially reversible, intervention strategies that remove adverse environmental and social risks may provide effective approaches for improving outcomes.
The Transcriptome
As described earlier in this chapter, when a cell “reads” a gene, it copies the information from the DNA and makes it into RNA. By changing the number of times the DNA is copied into RNA, the cell can increase or decrease the level of the RNA present in the cell, which helps regulate the amount of protein a cell generates; how much protein is made can drastically affect cell function. By changing the levels of RNA, a cell can adapt to changes that occur in the body. Because a cell is usually making copies of RNA from hundreds to thousands of genes at once, determining the RNA levels of each of these genes can help create a molecular profile, which often matches with the function of the cell. Researchers compare these profiles from tumor and normal cells to identify specific characteristics that drive a cancer cell or a tumor and aid clinicians in making treatment decisions. To date, there are limited studies that have compared tumor signatures of people from diverse genetic ancestries. However, emerging evidence shows different RNA expression patterns in triple-negative breast tumors of African Americans compared to European Americans (174)Davis M, Martini R, Newman L, Elemento O, White J, Verma A, et al. Identification of Distinct Heterogenic Subtypes and Molecular Signatures Associated with African Ancestry in Triple Negative Breast Cancer Using Quantified Genetic Ancestry Models in Admixed Race Populations. Cancers (Basel) 2020;12. [LINK NOT AVAILABLE]. Studies such as these highlight the contributions of ancestry to the unique genetic and molecular profiles of tumors. Understanding these different profiles across groups will be key to understanding why certain cancers are more aggressive in certain ancestral groups and, more importantly, to determining the best type of diagnostic or treatment.
After RNA is copied from DNA a process called RNA splicing helps complete the message by removing those bases from the RNA that are not needed to make a protein. In cancer cells, this process can be altered to generate abnormal proteins, which can fuel uncontrolled cell proliferation and growth (see sidebar on The Cancer Cell: Changes that Lead to Cancer). Research has shown that RNA may be spliced differently in people of different genetic ancestry. One study found that the PIK3CD-S gene, which increases the aggressiveness of prostate cancer, was spliced differently in African American patients, compared to European American patients. Researchers theorized that, because of the function of the gene involved, response to common treatments targeted against PIK3C may not be as effective in African American patients (175)Wang B-D, Ceniccola K, Hwang S, Andrawis R, Horvath A, Freedman JA, et al. Alternative Splicing Promotes Tumour Aggressiveness and Drug Resistance in African American Prostate Cancer. Nature communications 2017;8:15921. [LINK NOT AVAILABLE].
Influences Outside the Cell
Genetic mutations, epigenetic modifications, and RNA splicing are factors that influence cancer development from inside the cell. However, the environment surrounding the cell is equally important in cancer initiation and progression. Cross talk between the cancer cell and its surroundings (also known as the tumor microenvironment) is a key contributor to tumor growth and metastasis (see sidebar on The Tumor Microenvironment: External Influences on Cancer Development and Progression). Cancer cells can release molecules that shape their surrounding environment to provide them with nutrients, oxygen, and a supportive structure. This reorganization also aids in the process of metastasis, where cancer cells leave the tumor through blood and lymphatic systems. In turn, the microenvironment can influence the tumor by promoting or suppressing its growth.
Supporting the Tumor Cells
Directly surrounding the tumor is the extracellular matrix, a platform of noncellular components on which cancer cells grow. During proliferation, cancer cells instruct the surrounding matrix to support their growth and in turn the matrix provides cues to the tumor that influence cancer progression and metastasis (186)Elgundi Z, Papanicolaou M, Major G, Cox TR, Melrose J, Whitelock JM, et al. Cancer Metastasis: The Role of the Extracellular Matrix and the Heparan Sulfate Proteoglycan Perlecan. Frontiers in Oncology 2020;9:1482. [LINK NOT AVAILABLE]. Because some cancer types are more aggressive in patients of certain genetic ancestry, there is an interest in understanding if the tumor matrix is different across populations, whether this difference drives tumor aggressiveness, and if the matrix could be targeted therapeutically (187)Angel PM, Saunders J, Jensen-Smith H, Bruner E, Ford ME, Berkhiser S, et al. Abstract P2-10-18: Deciphering Racial Disparities in Breast Cancer Collagen Reorganization by Targeted Extracellular Matrix Proteomics. Cancer Research 2020;80:P2-10-18. [LINK NOT AVAILABLE].
The blood and lymphatic networks form the roads and bridges that connect the body’s organs and tissues and help in the delivery of nutrients and oxygen and removal of waste such as dead cells or carbon dioxide. These networks also make an important component of the tumor microenvironment. Because a lot of fuel and oxygen is required to sustain the rapid growth of cancer cells, blood vessels connecting to tumors also grow quickly, making tumors highly vascularized. The degree to which tumors become vascularized can be an indicator of tumor aggressiveness and patient outcomes. Interestingly, some studies have shown increased vascularization in breast tumors of patients of African ancestry compared to those of European ancestry (183)Martin DN, Boersma BJ, Yi M, Reimers M, Howe TM, Yfantis HG, et al. Differences in the Tumor Microenvironment between African-American and European-American Breast Cancer Patients. PLOS ONE 2009;4:e4531. [LINK NOT AVAILABLE](188)Kim G, Pastoriza JM, Condeelis JS, Sparano JA, Filippou PS, Karagiannis GS, et al. The Contribution of Race to Breast Tumor Microenvironment Composition and Disease Progression. Frontiers in Oncology 2020;10. [LINK NOT AVAILABLE]. Although data are still emerging, the difference in tumor vascularization between the two populations may explain in part why, despite having lower incidence of breast cancer, African American patients continue to have a 40 percent higher risk of breast cancer-related death.
Hormones are molecules that travel in the bloodstream and are naturally produced by organs. The main function of hormones is to act as communication signals that relay information from one place in the body to another. Notably, hormones also play a key role in the development of several cancers including breast, prostate, uterine, ovarian, testicular, thyroid, and bone. Often, the primary treatment for these cancers is limiting the levels of hormones using hormone therapy. While hormone therapy is a useful cancer treatment, hormones can also be used in gender-affirming therapy by members of the SGM community. How, under these circumstances, hormones could contribute to cancer development is an area of active investigation. Notably, studies suggest that hormone therapies may promote cancer
development in individuals using these treatments for cancer- or non-cancer-related purposes (181)de Blok CJM, Wiepjes CM, Nota NM, van Engelen K, Adank MA, Dreijerink KMA, et al. Breast Cancer Risk in Transgender People Receiving Hormone Treatment: Nationwide Cohort Study in the Netherlands. BMJ 2019;365:l1652. [LINK NOT AVAILABLE](182)de Nie I, de Blok CJM, van der Sluis TM, Barbé E, Pigot GLS, Wiepjes CM, et al. Prostate Cancer Incidence under Androgen Deprivation: Nationwide Cohort Study in Trans Women Receiving Hormone Treatment. The Journal of Clinical Endocrinology & Metabolism 2020;105:e3293-e9. [LINK NOT AVAILABLE] (see sidebar on The Tumor Microenvironment: External Influences on Cancer Development and Progression).
The Immune System
The immune system is composed of a variety of organs, tissues, cells, and molecules that all work together to defend the body against external (virus, bacteria) and internal (cancer) threats by recognizing and eliminating them. How the immune system responds to these threats depends on the types of exposures individuals encounter in their lifetime. Groups that share common ancestral history can also have comparable immune systems because of evolutionary shaping at both genetic and environmental levels. The immune cells found within a tumor can identify and eliminate cancer cells, although in many cases the immune system is suppressed, permitting the formation and progression of a tumor (160)Freedman JA, Abo MA, Allen TA, Piwarski SA, Wegermann K, Patierno SR. Biological Aspects of Cancer Health Disparities. Annual Review of Medicine 2021;72:229-41. [LINK NOT AVAILABLE](189)Nédélec Y, Sanz J, Baharian G, Szpiech ZA, Pacis A, Dumaine A, et al. Genetic Ancestry and Natural Selection Drive Population Differences in Immune Responses to Pathogens. Cell 2016;167:657-69.e21. [LINK NOT AVAILABLE]. Understanding how and why immune systems in individuals from different ancestry are different can give us a better understanding of how cancers develop and the unique role the immune system plays.
Insights into the interplay between the immune system and cancer form the basis for developing immunotherapies, which are some of the most effective cancer treatments available today; so far, immunotherapies have been approved for the treatment of more than 26 different types of cancer.
Despite this success, it must be noted that the development of immunotherapies has been primarily based on clinical trials involving individuals of European ancestry, with limited data from minority populations. This is troubling, since immune function has been found to be different between different ancestral groups, indicating there cannot be a “one size fits all” approach (160)Freedman JA, Abo MA, Allen TA, Piwarski SA, Wegermann K, Patierno SR. Biological Aspects of Cancer Health Disparities. Annual Review of Medicine 2021;72:229-41. [LINK NOT AVAILABLE](189)Nédélec Y, Sanz J, Baharian G, Szpiech ZA, Pacis A, Dumaine A, et al. Genetic Ancestry and Natural Selection Drive Population Differences in Immune Responses to Pathogens. Cell 2016;167:657-69.e21. [LINK NOT AVAILABLE]. Comprehensive analysis of the immune system of cancer patients from diverse racial and ethnic backgrounds is vital to develop precise therapeutic interventions that are effective in these populations.
Integrating and Translating Our Knowledge
In this chapter we have highlighted how cancer is a complex, multifaceted collection of diseases. The most effective cancer control efforts must take a comprehensive look at the genetic, epigenetic, lifestyle, and environmental influences and apply approaches tailored to each individual patient. In fact, in recent years there has been a shift from a “one size fits all” approach to cancer prevention, screening, and treatment to a more personalized approach called precision medicine. The aim of precision medicine is to use information about an individual’s biology as well as other factors to prevent, diagnose, and treat disease. Precision medicine has the potential to further revolutionize cancer care. However, collection of relevant data from racial and ethnic minorities and other underserved populations including recruitment of diverse population groups in to clinical studies is vital if we are to improve patient outcomes in an equitable manner.
Collecting biospecimens from people with different backgrounds will create diverse datasets that researchers can use to better understand the ancestry-related differences in cancers. Currently, many of the large cancer datasets including The Cancer Genome Atlas, an NIH-supported collaborative effort to genetically profile cancers based on patient tumors, contain samples largely from White people (77 percent of all samples), reducing the likelihood of discovering cancer-causing alterations in underrepresented population groups. As one such example, in a recent study among Black patients with prostate cancer, tumor sequencing identified a genetic mutation in a tumor suppressor gene that was more common in Black men (five percent) compared to White men (only one percent) (192)Huang FW, Mosquera JM, Garofalo A, Oh C, Baco M, Amin-Mansour A, et al. Exome Sequencing of African-American Prostate Cancer Reveals Loss-of-Function Erf Mutations. Cancer Discovery 2017;7:973-83. [LINK NOT AVAILABLE]. Recent reports have also shown that tumors sequenced from African American patients were of lower quality and reduced coverage, leading to lower detection of possible variants (193)Wickland DP, Sherman ME, Radisky DC, Mansfield AS, Asmann YW. Lower Exome Sequencing Coverage of Ancestrally African Patients in the Cancer Genome Atlas. JNCI: Journal of the National Cancer Institute 2022. [LINK NOT AVAILABLE]. To accelerate progress in this area, data integration and sharing across institutions, companies, and countries worldwide is critical. A main focus of these research endeavors must be to build a highly diverse reference genome, which can increase our understanding of gene alterations found in underrepresented groups (194)Casaletto J, Parsons M, Markello C, Iwasaki Y, Momozawa Y, Spurdle AB, et al. Federated Analysis of Brca1 and Brca2 Variation in a Japanese Cohort. Cell Genomics 2022;2:100109. [LINK NOT AVAILABLE](195)Sirén J, Monlong J, Chang X, Novak AM, Eizenga JM, Markello C, et al. Pangenomics Enables Genotyping of Known Structural Variants in 5202 Diverse Genomes. Science 2021;374:abg8871. [LINK NOT AVAILABLE].
Several initiatives are being spearheaded by private and public organizations to facilitate the expansion of diverse biospecimens. As one example, in 2018, the All of Us research program was launched by the NIH to enroll 1 million people in the United States and “build a diverse database that can inform thousands of studies on a variety of health conditions.” In March 2022, this project had sequenced the entire genomes of 100,000 people, 50 percent of whom self-reported as being racially or ethnically diverse (196)NIIH’s All of Us Research Program Releases First Genomic Dataset of Nearly 100,000 Whole Genome Sequences. [updated cited. Additionally, the AACR Project Genomics Evidence Neoplasia Information Exchange (GENIE)® has sequenced tumors from over 121,000 patients across 19 leading cancer centers in the U.S. and Europe, nearly 13.4 percent of which are from racial and ethnic minorities. Researchers are already using these databases to address gaps in our knowledge about cancer biology and the genetic changes that occur specifically in minority groups (197)Goel N, Kim DY, Guo JA, Zhao D, Mahal BA, Alshalalfa M. Racial Differences in Genomic Profiles of Breast Cancer. JAMA Netw Open 2022;5:e220573. [LINK NOT AVAILABLE](198)Kamran SC, Xie J, Cheung ATM, Mavura MY, Song H, Palapattu EL, et al. Tumor Mutations across Racial Groups in a Real-World Data Registry. JCO Precision Oncology 2021:1654-8. [LINK NOT AVAILABLE](199)Schumacher FR, Basourakos SP, Lewicki PJ, Vince R, Spratt DE, Barbieri CE, et al. Race and Genetic Alterations in Prostate Cancer. JCO Precision Oncology 2021:1650-3. [LINK NOT AVAILABLE].
Researchers use a variety of model systems to understand how genetic alterations result in cancers. Establishment of research models that better represent genetic diversity for biomedical research is paramount to developing treatments that are safe and effective for all populations. Emerging studies are beginning to utilize “patient-derived xenografts,” tumor cells surgically resected from a patient and grown in immunosuppressed mice. These models are often used to evaluate personalized cancer treatments (200)Halmai NB, Carvajal-Carmona LG. Diversifying Preclinical Research Tools: Expanding Patient-Derived Models to Address Cancer Health Disparities. Trends in Cancer 2022;8:291-4. [LINK NOT AVAILABLE](201)Siddharth S, Parida S, Muniraj N, Hercules S, Lim D, Nagalingam A, et al. Concomitant Activation of Gli1 and Notch1 Contributes to Racial Disparity of Human Triple Negative Breast Cancer Progression. eLife 2021;10:e70729. [LINK NOT AVAILABLE]. Diversifying these preclinical cancer research tools will be key to the success of precision medicine in treating disease for diverse populations.
Discoveries in cancer genomics have led to the development of numerous therapeutics which target the cellular changes that arise due to mutations. Unfortunately, most of these therapies have been developed in patients with cancer who are of primarily European ancestry. To develop new therapies for diverse groups, studies that incorporate the tumor and patient genetic factors as well as SDOH will need to be utilized. One such study is the Research on Prostate Cancer in Men of African Ancestry: Defining the Roles of Genetics, Tumor Markers and Social Stress (RESPOND), which is “one of the largest studies ever to look at the underlying factors and reasons that put African American men at higher risk for prostate cancer.” By using surveys, current DNA sequencing, and tumor samples, RESPOND will “study how exposure to stress over a lifetime, inherited susceptibility (i.e. genes), and tumor characteristics contribute to the development of prostate cancer” (202)African American Prostate Cancer Study. Home [Updated April 22, 2022, cited 2022 April 22].(203)African American Prostate Cancer Study. What Is Respond? [updated April 22, 2022, cited 2022 April 22]..
The use of molecularly targeted therapeutics relies on genetic tests that detect mutations. Currently, only a fraction of patients with cancer have their tumors tested (204)Kehl KL, Lathan CS, Johnson BE, Schrag D. Race, Poverty, and Initial Implementation of Precision Medicine for Lung Cancer. JNCI: Journal of the National Cancer Institute 2018;111:431-4. [LINK NOT AVAILABLE](205)Palazzo LL, Sheehan DF, Tramontano AC, Kong CY. Disparities and Trends in Genetic Testing and Erlotinib Treatment among Metastatic Non–Small Cell Lung Cancer Patients. Cancer Epidemiology, Biomarkers & Prevention 2019;28:926-34. [LINK NOT AVAILABLE], with disparities in who gets tested (206)Childers KK, Maggard-Gibbons M, Macinko J, Childers CP. National Distribution of Cancer Genetic Testing in the United States: Evidence for a Gender Disparity in Hereditary Breast and Ovarian Cancer. JAMA Oncol 2018;4:876-9. [LINK NOT AVAILABLE]. For instance, following the expansion of Medicare to cover tumor sequencing, compared to NHW individuals, use of tumor sequencing was 14 percent lower in African Americans and 23 percent lower in Hispanic/Latinos (207)Sheinson DM, Wong WB, Meyer CS, Stergiopoulos S, Lofgren KT, Flores C, et al. Trends in Use of Next-Generation Sequencing in Patients with Solid Tumors by Race and Ethnicity after Implementation of the Medicare National Coverage Determination. JAMA Netw Open 2021;4:e2138219. [LINK NOT AVAILABLE]. Current disparities in the implementation of precision medicine (205)Palazzo LL, Sheehan DF, Tramontano AC, Kong CY. Disparities and Trends in Genetic Testing and Erlotinib Treatment among Metastatic Non–Small Cell Lung Cancer Patients. Cancer Epidemiology, Biomarkers & Prevention 2019;28:926-34. [LINK NOT AVAILABLE](208)Verma V, Haque W, Cushman TR, Lin C, Simone CBI, Chang JY, et al. Racial and Insurance-Related Disparities in Delivery of Immunotherapy-Type Compounds in the United States. Journal of Immunotherapy 2019;42:55-64. [LINK NOT AVAILABLE] can be attributed to lack of information given to patients, lower recruitment into clinical trials, implicit bias, insurance disparities, and financial cost (209)Stein JN, Charlot M, Cykert S. Building toward Antiracist Cancer Research and Practice: The Case of Precision Medicine. JCO Oncology Practice 2021;17:273-7. [LINK NOT AVAILABLE].
Overall, reducing disparities in treatment and understanding of cancer across different groups will require a multilevel approach. Identification of mutations that lead to cancer through the use of large and inclusive genomic databases as well as studying them in diverse research models will increase our knowledge surrounding the genetic and other changes that occur in different ancestral groups. Translating this knowledge into the clinic will require sequencing of tumors from individuals to understand the mutations that have occurred in that tumor; these mutations will then allow clinicians to select the most appropriate therapy for the patient.