In this section, you will learn:
- Cancer is a collection of diseases characterized by uncontrolled cell multiplication.
- Alterations both within a cell and its surrounding environment contribute to cancer initiation and progression.
- An important part of the cancer care decision-making process and the foundation of precision medicine is the identification of alterations in DNA, RNA, and proteins that drive cancer.
- There are ancestry-related differences in cancer-driving cellular and molecular alterations.
- The majority of currently available information on cancer development is based on studies of individuals that are of European ancestry. Researchers are addressing this challenge through ongoing efforts to increase racial, ethnic, and ancestral diversity in cancer biology research studies.
- The biological differences among cancers in patients of different ancestries could provide novel targets for therapies and improve precision medicine
Decades of medical research have provided great insights into the underpinnings of cancer development. Knowledge gleaned from this research shows that cancer is not a single disease but a collection of diseases that arise when the processes that control normal cell growth, division, and life span go awry. As a result, cells start multiplying uncontrollably, fail to die when they should, and mobilize other cells and tissues, such as blood vessels and immune cells, all of which gives abnormal cells a growth advantage. In organs and tissues, the accumulating cancer cells form masses called tumors, whereas in the blood or bone marrow they crowd out normal cells.
During cancer development, abnormal or damaged cells acquire so-called “hallmarks” or characteristics that distinguish cancer cells from non-cancerous cells. Some of the hallmarks of cancer cells include their ability to multiply limitlessly by ignoring signals that tell normal cells to stop dividing or to die; sustain rapid growth by relying on nutrients that are different from those used by normal cells; accumulate multiple changes in their genetic material; evade the immune system responsible for eliminating abnormal or damaged cells; recruit blood vessels, thus increasing nutrients and oxygen supply to tumors; and leave the tissue of origin and spread to other tissues (182)Hanahan D (2022) Cancer Discov, 12: 31. . Cancer that has spread to other parts of the body, which is called metastatic disease, is the main cause of most cancer-related deaths.
It is important to note that there are many factors, from biological to environmental to behavioral, that influence cancer development. In the United States, centuries of systemic inequities and injustices have led to racial and ethnic minority groups and medically underserved populations being exposed to adverse social and built environmental factors, collectively referred to as structural and social drivers of health (SDOH), that contribute to the observed disparities in cancer burden among these population groups (see Figure 3). Adverse differences in SDOH can contribute to a higher cancer burden both indirectly, for example, by impeding health care access and promoting poorer health habits such as smoking and alcohol consumption, and directly through complex biological interplay that is still not fully understood but includes epigenetic modifications (see Epigenetic Changes), chronic inflammation, and altered metabolism (183)Gong J, et al. (2023) Nat Rev Urol, DOI: 10.1038/s41585-023-00828-w. [LINK NOT AVAILABLE].
Cancer Development: Generating Knowledge
Medical research is the backbone of progress against cancer because it is the driving force behind every breakthrough that enhances survival and quality of life, and every new policy or program designed to improve public health. Discoveries across the major areas of cancer research, including basic, clinical, translational, and population sciences, provide the foundation for advances in cancer prevention, detection, diagnosis, treatment, and survivorship.
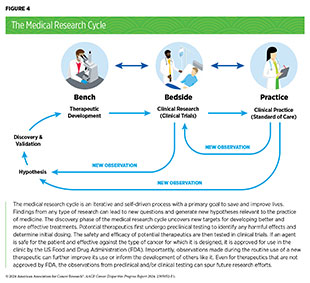
Much of the current knowledge of how cancer develops comes from basic research. Discoveries stemming from decades of basic research and population sciences have provided the foundational knowledge to drive preventive interventions and clinical breakthroughs, which have contributed to a 33 percent reduction in the overall US cancer mortality rate over the past three decades (38)Siegel RL, et al. (2024) CA Cancer J Clin, 74: 12. [LINK NOT AVAILABLE]. In the discovery phase of medical research (see Figure 4), hypotheses generated through basic research from observations with medical relevance are tested in experiments performed using cell- and animal-based models that attempt to mimic healthy and disease conditions, such as cancer. Cancer research uses models that mimic specific characteristics of cancer (e.g., increased cell growth) or types of cancer (e.g., breast cancer). A major challenge in cancer research, and one of the main barriers to studying cancer disparities, has been the historical lack of representation of biospecimens from medically underserved populations among basic research models (see Sidebar 11).
Cancer disparities among racial and ethnic minority groups are driven by complex interactions between adverse influences of SDOH (see Figure 3) and genetic and epigenetic differences that may be attributable to ancestral differences between populations. Unfortunately, due to the convenience of researchers, most established cancer cell lines—models that have provided much of the fundamental knowledge of the underpinnings of cancer initiation and progression (see Sidebar 11)—have been derived from patients with European ancestry (184)Halmai NB, et al. (2022) Trends Cancer, 8: 291. [LINK NOT AVAILABLE](185)Raghavan S (2022) Dis Model Mech, 15. [LINK NOT AVAILABLE]. Lack of diversity in genetic ancestry and/or lack of racial and ethnic representation while building research models and biorepositories leads to the generation of data that do not apply to all populations, thereby minimizing the applicability of research results. Diversity and inclusion in cancer research models is especially vital when investigating diseases that disproportionately impact patients from certain racial and ethnic minority groups and medically underserved populations. It is imperative that the medical research community work together to promote the development of more diverse cancer research models that represent the populations affected by the diseases.
To enhance our knowledge of the biological and genetic contributors to cancer disparities, additional resources are needed, including cancer models and biospecimens derived from patients representing a diverse array of racial and ethnic groups. In this regard, it should be noted that NCI has established the PDX (see Sidebar 11) Development and Trial Centers Research Network to accelerate translational research using patient-derived xenograft (PDX) datasets, and two of the six PDX Development and Trial Centers focus exclusively on developing minority PDXs.
Cancer Development: Interpreting Knowledge
Cancer is a genetic disease caused by changes in genes that control vital functions, such as cell multiplication and cell growth. However, transformation of noncancerous cells into cancer cells, accumulation of cancer cells to form tumors, and spread of tumors to distant sites in the body are all complex, multistep processes that are influenced by alterations inside the cell as well as changes outside the cell.
Changes That Contribute to Cancer Initiation
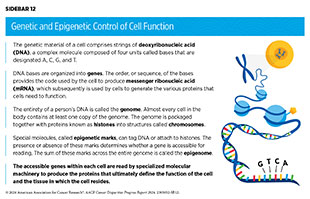
Cells of the human body rely on instructions from genetic material known as deoxyribonucleic acid (DNA) to function. DNA is made up of four types of building blocks called bases, that are designated A, T, C, and G (see Sidebar 12). Anywhere from 50 million to 250 million of these bases link together to form individual strands, with two strands of the same length paired together to form a double-stranded, helical structure; the paired strands are packaged together with proteins known as histones into structures called chromosomes. Each chromosome contains hundreds to thousands of genes, which are segments of DNA that contain the code for a protein, the functional unit of the cell.
To make a protein, a cell reads a gene from the DNA to make another type of molecule called messenger ribonucleic acid (mRNA) in a process called transcription. The cell can make many copies of mRNA from a single sequence of DNA, increasing the amount of message in the cell. The cells then “translate” the information in the mRNA into proteins; therefore, usually the more mRNA present, the more protein is made.
All humans share roughly 99.9 percent sequence similarity in their DNA, with only 0.1 percent being different from one human to another; yet this 0.1 percent encompasses millions of changes and is what makes each of us unique. Many of the genetic differences found in DNA across groups with different genetic ancestries are a result of human migration out of continental Africa roughly 100,000 years ago to neighboring continents (collectively termed the human diaspora). The subsequent adaptations to new climates, diseases, and environments shaped human genetics, which results in the human diversity we see today (190)Timmermann A, et al. (2016) Nature, 538: 92. [LINK NOT AVAILABLE].
Biological traits that arise from genetic differences can be positive, such as adaptation to unfavorable climates and altitudes, tolerance of particular food sources, or resistance to infections with parasites. However, genetic differences can also predispose certain population groups to genetic diseases like cancer. Recent migrations (forced or intentional) have led to genetic mixture of ancestral groups among most minority populations in the United States. The differences in genetic composition that result from this mixing are what make measurements of ancestry important in cancer studies in human populations.
In the following sections, we describe the cellular and molecular alterations that lead to cancer initiation and progression. We also highlight some of the known ancestry-related differences in such alterations. It should be emphasized that US racial categories are sociopolitical constructs and the racial or ethnic disparities in cancer burden are driven largely by decades of systemic and structural inequities that directly and/or indirectly impact human biology and population health. However, the greater prevalence of advanced-stage disease and more aggressive cancers in certain population groups may indicate factors beyond socioeconomic and structural differences. The interactions of inherent genetic ancestry with environmental influences, such as systemic racism and SDOH, and the relative contributions of each on driving cancer disparities is an area of extensive research.
Genetic Alterations
Alterations in the DNA sequence, referred to as mutations, can disrupt or modify normal protein function and are among the hallmarks of cancer cells. Genetic alterations can change the sequence or amount of mRNA and the resulting protein that is produced, which in turn can contribute to cancer development (see Sidebar 13). Genetic alterations can be inherited (called germline mutations) or acquired during a person’s lifetime (called somatic mutations). In about 10 percent of cancer cases, the mutations are inherited.
To identify genetic alterations in cancer and other diseases, a patient’s genome must be compared against the human reference genome. Unfortunately, the original reference genome that was used by researchers lacked the genetic diversity that naturally exists among different populations because it was derived from a very small pool of individuals, mostly of European ancestry. Therefore, a major advance in the field of genomic medicine has been the recent release of the updated human reference pangenome, which is built from a more diverse cohort of individuals (192)Wang T, et al. (2022) Nature, 604: 437. [LINK NOT AVAILABLE].
Germline mutations are passed on from parents to children and become incorporated into the DNA of every cell in the body of the offspring and increase their risk of developing cancer. Not all germline mutations contribute to cancer development. Inherited genetic alterations that play a role in cancer development are among the pathogenic germline mutations.
Much of the research on pathogenic germline mutations has been conducted in individuals of European ancestry, limiting our understanding of many identified pathogenic variants in patients of other ancestries. Because of limited information from racial and ethnic minority individuals, there is often insufficient evidence to determine with confidence whether a mutation is truly cancer causing, and these mutations are often categorized as variants of undetermined significance (VUS). Consequently, genetic counseling for racial and ethnic minority individuals becomes less precise and less informative than it is for those of European ancestry. There is an urgent need to increase research on examining differences in inherited genetic alterations in people from different ancestral backgrounds because these differences can inform early detection, surveillance, and treatment decisions.
Thanks to a sharper focus on the science of cancer disparities over the past decade, along with rapid advances in technology, such as sophisticated DNA and RNA sequencing methods, we are beginning to understand ancestry-related differences in pathogenic germline mutations. African ancestry is a significant risk factor for prostate cancer, with mortality rates for patients across sub-Saharan Africa being nearly three-fold higher than global averages (193). In the United States, Black patients have disproportionately higher prostate cancer incidence and mortality compared to other racial and ethnic groups. Emerging data suggest that germline mutations may contribute to the increased prostate cancer risk among Black men and that prostate cancers from Black men exhibit higher rates of pathogenic germline mutations in BRCA1 genes (183)Gong J, et al. (2023) Nat Rev Urol, DOI: 10.1038/s41585-023-00828-w. [LINK NOT AVAILABLE].
Black women in the United States have a 40 percent higher mortality from breast cancer, attributable in part to advanced stage at diagnosis and more aggressive tumors such as the triple-negative subtype, a particularly intractable form of breast cancer. Studies show that breast cancer patients with West African ancestry have a higher prevalence of pathogenic mutations in BRCA1 or BRCA2 compared to women from Western Europe; the rate is even higher among patients from the Bahamas (194)George SHL, et al. (2021) JAMA Netw Open, 4: e210307. [LINK NOT AVAILABLE]. Among women with endometrial cancer and epithelial ovarian cancer, higher rates of germline pathogenic mutations are found in patients from Ashkenazi Jewish ancestry compared to those with European ancestry (138)Liu YL, et al. (2024) Cancer, 130: 576. [LINK NOT AVAILABLE](195)Sia TY, et al. (2023) JCO Precis Oncol, 7: e2300137. [LINK NOT AVAILABLE]. Hispanic children, adolescents, and young adults have a higher risk of acute lymphoblastic leukemia (ALL) compared to other US racial or ethnic groups. Recent studies have identified a genetic alteration that is associated with Native American ancestry and increases the risk of childhood ALL. The genetic variant was detected among self-reported Hispanic/Latino individuals but not NH White individuals (196)de Smith AJ, et al. (2024) Cell Genom: 4: 100526. [LINK NOT AVAILABLE].
Somatic mutations or acquired genomic alterations occur over an individual’s lifetime because of internal errors arising during cell multiplication or because of external influences such as environmental exposures and lifestyle factors (see Disparities in the Burden of Preventable Cancer Risk Factors), or because of underlying health conditions such as Crohn’s disease. Comprehensive analyses of cancer cell DNA have revealed numerous cancer-causing somatic mutations. The Cancer Genome Atlas (TCGA), an initiative supported by NCI and the National Human Genome Research Institute, looked at the genetic content of about 11,000 tumors across 33 different cancer types. The data have provided a comprehensive map of the somatic mutational landscape across many cancer types.
Ancestry analysis of the TCGA samples has reported that only 9.8 percent of tumors are of African ancestry and only 0.4 percent are of Native or Latin American ancestry (184)Halmai NB, et al. (2022) Trends Cancer, 8: 291. [LINK NOT AVAILABLE]. The disparity becomes even more striking in certain cancer types, such as gastric cancer, which has a disproportionately higher burden among AI/AN, Asian, Black, Hispanic, and Native Hawaiian populations but for which data are underrepresented or completely lacking in TCGA (3,184,197). Additionally, lower-quality analysis of African ancestral samples, attributable to lower sequencing coverage—a metric that ensures reliability of DNA sequencing data—has led to underdetection of mutations from these population groups, thereby deepening research gaps that perpetuate cancer disparities (198)Wickland DP, et al. (2022) J Natl Cancer Inst, 114: 1192. [LINK NOT AVAILABLE].
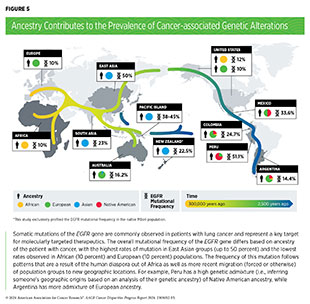
The evidence is mounting that there are considerable differences in somatic mutational profiles of most cancer types when comparing patients of different ancestries, with potential implications for therapeutic interventions (see Figure 5) (137)Arora K, et al. (2022) Cancer Discov, 12: 2552. (199)Casolino R, et al. (2024) CA Cancer J Clin, 1–22. [LINK NOT AVAILABLE](200)Jiagge E, et al. (2023) Cancer Cell, 41: 1963. [LINK NOT AVAILABLE](201)Shi J, et al. (2023) Nat Commun, 14: 3043. [LINK NOT AVAILABLE](202)Byun J, et al. (2022) Nat Genet, 54: 1167. [LINK NOT AVAILABLE]. As one example, a series of recent studies have interrogated the cancer genome in patients from diverse ancestries and compiled a comprehensive list of novel genetic mutations associated with prostate cancer risk, some with therapeutic implications, and the likelihood of aggressive disease among men of African ancestry (193)Jaratlerdsiri W, et al. (2022) Nature, 609: 552. [LINK NOT AVAILABLE](203)Wang A, et al. (2023) Nat Genet, 55: 2065. [LINK NOT AVAILABLE](204)Soh PXY, et al. (2023) Nat Commun, 14: 8037. [LINK NOT AVAILABLE](205)Gong T, et al. (2022) Genome Med, 14: 100. [LINK NOT AVAILABLE]. As another example, many recent reports indicate that there are unique somatic mutations among patients with early-onset colorectal cancers—colorectal cancer among individuals younger than 50 years—based on race, ethnicity, ancestry, and geography (206)Hein DM, et al. (2022) J Natl Cancer Inst, 114: 775. [LINK NOT AVAILABLE](207)Holowatyj AN, et al. (2023) Cancer Discov, 13: 570. (208)Perez-Mayoral J, et al. (2023) Genes (Basel), 14. [LINK NOT AVAILABLE]. Notably, early-onset cancer incidence has been rising globally (35)Centers for Disease Control and Prevention. Declines in Cancer Death Rates Among Youth: United States, 2001-2021. Accessed: Mar 13, 2024. (36)Miller KD, et al. (2021) CA Cancer J Clin, 71: 466. [LINK NOT AVAILABLE].
Encouragingly, these data highlight recent efforts among researchers to achieve equity in cancer genomics and ensure that benefits of precision medicine (see Figure 6) are accessible to populations from all ancestral backgrounds. Another area of urgent need is to address the underrepresentation of rural patients in cancer genomic databases (210)Shultz C, et al. (2023) Cancer Rep (Hoboken), 6: e1746. [LINK NOT AVAILABLE].
RNA Variations
RNA is the transcript of the original genetic code embedded in the DNA and is used to make proteins, which are the molecules that perform important functions that dictate a cell’s fate. Most human genes contain information for making proteins in fragments of DNA, called exons. Exons are interspersed by DNA sequences, called introns, that do not contain information necessary to make a functional protein. When a gene is transcribed into mRNA, the initial mRNA molecule contains a copy of both exons an introns. An intricate “cut and paste” process, called splicing, removes introns and joins exons together to produce an mRNA molecule that is subsequently translated into a functional protein by the cellular machinery. RNA splicing plays a pivotal role in maintaining normal cellular functions and aberration to normal splicing pathways can lead to cancer (211)Bradley RK, et al. (2023) Nat Rev Cancer, 23: 135. [LINK NOT AVAILABLE].
Ancestry-related differences in mRNA levels or processing in cancer have been demonstrated in many analyses (212)Freedman JA, et al. (2021) Annu Rev Med, 72: 229. [LINK NOT AVAILABLE](213)Asare A, et al. (2022) Cancer Res Commun, 2: 99. [LINK NOT AVAILABLE]. As one example, comparison of RNA data from patients with breast cancer versus healthy individuals of Asian and European ancestry led to the identification of new details of unique breast cancer risks across these population groups (214)Jia G, et al. (2022) Am J Hum Genet, 109: 2185. [LINK NOT AVAILABLE].
Of interest, research has shown that RNA may be spliced differently in people of different ancestry. One study found that the PIK3CD-S gene, which increases the aggressiveness of prostate cancer, was spliced differently in patients of African ancestry, compared to those of European ancestry. Researchers hypothesize that, because of this difference, response to common treatments targeted against the PIK3C protein may not be as effective in African American patients (215)Wang BD, et al. (2017) Nat Commun, 8: 15921. [LINK NOT AVAILABLE].
In contrast to mRNA, non-coding RNAs are a heterogeneous group of molecules that are not translated into proteins. Since their discovery, non-coding RNAs have emerged as important regulators of multiple biological functions across a range of cell types and tissues. Micro(mi)RNAs are one type of non-coding RNA that blocks the ability of mRNAs to be translated to proteins. Dysregulation of miRNAs has been implicated in cancer. Additionally, ancestry-related differences in miRNA levels and/or function that may contribute to cancer disparities have been identified (213)Asare A, et al. (2022) Cancer Res Commun, 2: 99. [LINK NOT AVAILABLE](216)Kulkarni A, et al. (2022) Cells, 11: 2448. [LINK NOT AVAILABLE](217)Ottman R, et al. (2023) Cancers (Basel), 15: 2331. [LINK NOT AVAILABLE]. For example, recent studies indicate that ancestry-related differences in the levels or function of miRNAs may mediate disparities in breast cancer survival and therapeutic outcomes among African American patients (218)Angajala A, et al. (2022) Sci Rep, 12: 22178. [LINK NOT AVAILABLE](219)Zhao D, et al. (2022) Nat Commun, 13: 7734. [LINK NOT AVAILABLE](220)Mani C, et al. (2023) Breast Cancer Res, 25: 44. [LINK NOT AVAILABLE]. Long non-coding RNAs are another type of non-coding RNA and their role in cancer as well as ancestry-associated expression in certain cancers are areas of active research (221)Abu-Shihab Y, et al. (2023) Blood, 142: 722. [LINK NOT AVAILABLE].
Protein Modifications
Proteins are vital for normal cellular functions. The human proteome—the complete set of proteins made by humans—contains about 20,000 unique proteins. After being produced from mRNA, proteins can undergo additional modifications, providing great versatility and variability in protein functions to meet cellular needs. Examining the proteome of cancer cells can unveil additional information about how cancer develops. For example, a recent study that evaluated proteomes of nearly 1,000 cancer cell lines identified common and unique cancer-related changes in levels of many proteins that were not detected at DNA or RNA levels (222)Goncalves E, et al. (2022) Cancer Cell, 40: 835. [LINK NOT AVAILABLE].
In the United States, NCI is playing a vital role in supporting research on proteomic alterations in cancer through the Clinical Proteomic Tumor Analysis Consortium (CPTAC) (224)Rodriguez H, et al. (2021) Cell, 184: 1661. [LINK NOT AVAILABLE]. CPTAC researchers are already generating data on how alterations in protein modifications play a role in cancer and discovering novel avenues for therapeutic intervention (225)Geffen Y, et al. (2023) Cell, 186: 3945. [LINK NOT AVAILABLE].
Epigenetic Changes
DNA inside the cell’s nucleus is tightly packaged around proteins called histones. Epigenetic alterations refer to modifications that do not involve a change in the DNA sequence. Epigenetic alterations occur when chemical marks are added to or removed from DNA or the histone proteins. Epigenetic modifications regulate how and when genes are turned on or off. Specialized proteins add or remove unique epigenetic modifications to and from DNA and histones (226)Lu Y, et al. (2020) Mol Cancer, 19: 79. [LINK NOT AVAILABLE]. The complete set of all the epigenetic changes in a cell is called the epigenome. In contrast to genetic mutations, most epigenetic changes are reversible.
Epigenetic alterations are frequently observed in cancer cells. Because most epigenetic changes are reversible, they are attractive targets for drug development. Cancer-associated epigenetic changes may be acquired with age and/or exposure to environmental factors (e.g., air pollution) or behavioral factors (e.g., cigarette smoking) or psychosocial stressors (e.g., systemic racism and discrimination) and may be passed from parent to child (227)Feil R, et al. (2012) Nat Rev Genet, 13: 97. [LINK NOT AVAILABLE](228)Rasmussen L, et al. (2021) Nutrients, 13: 2821. [LINK NOT AVAILABLE](229)Johnstone SE, et al. (2010) Nat Rev Genet, 11: 806. [LINK NOT AVAILABLE].
One area of research is ancestry-related epigenetic differences in tumors and how such differences may contribute to cancer disparities among different patient populations (230)Ensenyat-Mendez M, et al. (2023) JAMA Netw Open, 6: e2335821. [LINK NOT AVAILABLE](231)Jordan IK, et al. (2022) Oncoscience, 9: 23. [LINK NOT AVAILABLE](232)Craddock J, et al. (2023) Cancers (Basel), 15: 3465. [LINK NOT AVAILABLE]. There is increasing evidence suggesting that social and built environmental factors (such as redlining, segregation, or neighborhood deprivation) (see Understanding and Addressing Drivers of Cancer Disparities) may drive cancer disparities through modulation of the tumor epigenome (233)Gohar J, et al. (2022) Breast Cancer Res Treat, 191: 653. [LINK NOT AVAILABLE](234)Miller-Kleinhenz JM, et al. (2023) Front Oncol, 13: 1154554. [LINK NOT AVAILABLE]. For example, high neighborhood deprivation has been shown to be associated with epigenetic changes and differential gene expression in breast tumors among Black women (235)Jenkins BD, et al. (2023) JAMA Netw Open, 6: e2341651. [LINK NOT AVAILABLE]. These alterations may lead to more aggressive tumors in Black women, highlighting the vital need for investments in public health interventions and policy changes at the neighborhood level.
Systems That Enable Cancer Progression
A hallmark of cancer is the ability of tumor cells to break away from the primary tissue and travel to other parts of the body. Systems that enable cancer to spread from the primary tissue to other organs of the body include the circulatory system (blood and lymphatics) and the immune system. There is emerging evidence that cancer initiation or progression, as it becomes worse or spreads in the body, is also affected by the microbiome (microorganisms that live in our bodies).
The Circulatory System
The blood and lymphatic systems form the roads and bridges that connect organs and tissues and help in the delivery of nutrients and oxygen and removal of waste such as dead cells or carbon dioxide. These circulatory networks are also the primary conduits for the process of cancer metastasis, whereby cancer cells leave their primary sites and form secondary tumors in distant organs. The ability to promote blood vessel formation toward and within a tumor is a hallmark of cancer. Because of the high demand of fuel and oxygen required to sustain the rapid growth of cancer cells, blood vessels connecting to tumors also grow quickly, making tumors highly vascularized. The degree to which tumors become vascularized can be an indicator of tumor aggressiveness and patient outcomes.
Interestingly, studies have shown increased vascularization in breast tumors of patients of African ancestry compared to those of European ancestry (236)Martin DN, et al. (2009) PLoS ONE, 4: e4531. [LINK NOT AVAILABLE](237)Madu CO, et al (2020) J Cancer, 11: 4474. [LINK NOT AVAILABLE]. In fact, a notable difference between cancer patients of African and European ancestry is in the biology of tumor blood vessel formation (238)Kim G, et al. (2020) Front Oncol, 10: 1022. [LINK NOT AVAILABLE].
The Immune System
The immune system is composed of a variety of organs, tissues, cells, and molecules that work together to defend the body against external (virus, bacteria) and internal (cancer) threats. How the immune system responds to these threats depends on the types of exposures individuals encounter in their lifetime. Groups that share common ancestral history can also have comparable immune systems because of evolutionary shaping at both genetic and environmental levels. The immune cells found within a tumor can identify and eliminate cancer cells, although in many cases the immune system is suppressed, permitting the formation and progression of a tumor (239)Nédélec Y, et al. (2016) Cell, 167: 657. [LINK NOT AVAILABLE](240)Freedman JA, et al. (2021) Annual Review of Medicine, 72: 229. [LINK NOT AVAILABLE].
Recent studies have highlighted the role of circulating immune cells and proteins in cancer metastasis, potentially through protection of circulating tumor cells as well as the promotion of tumor cell implantation in distant sites (241)Kiely M, et al. (2022) Trends Cancer, 8: 316. [LINK NOT AVAILABLE]. Understanding how and why immune systems in individuals from different ancestries are different can give us a better understanding of the biology of cancer disparities. Notably, the levels of immune molecules in the blood correlate with ancestry, suggesting a potential role in cancer disparities. Emerging data point to distinct ancestry-related immune and inflammatory markers in the circulation among patients with prostate cancer and lung cancer, among other diseases (241)Kiely M, et al. (2022) Trends Cancer, 8: 316. [LINK NOT AVAILABLE]. Additionally, there is evidence that systemic inequities such as neighborhood deprivation can impact the immune system and may predispose African American men to aggressive prostate cancer (242)Pichardo MS, et al. (2023) JAMA Netw Open, 6: e2251745. [LINK NOT AVAILABLE].
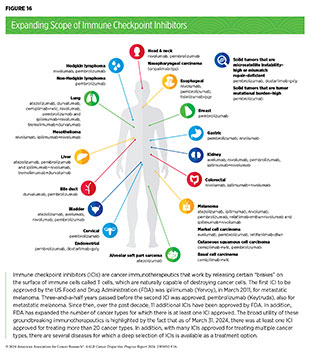
Insights into the interplay between the immune system and cancer form the basis for developing immunotherapies, one of the most effective cancer treatment pillars available today; so far, immunotherapies have been approved to treat more than 20 different types of cancer (see Figure 16). Despite this success, it must be noted that the development of immunotherapies has been primarily based on clinical samples from individuals of European ancestry, with limited data from minority populations. This is troubling, since immune function has been found to be different between different ancestral groups, indicating there cannot be a “one-size-fits-all” approach (see Treatment With Molecularly Targeted Therapy and Immunotherapy) (239)Nédélec Y, et al. (2016) Cell, 167: 657. [LINK NOT AVAILABLE](240)Freedman JA, et al. (2021) Annual Review of Medicine, 72: 229. [LINK NOT AVAILABLE]. Comprehensive analysis of the immune system of cancer patients from diverse racial and ethnic backgrounds is vital to develop precise interventions that are effective in these populations.
The Microbiome
The human microbiome is the collection of all microorganisms (e.g., bacteria and fungi) and viruses that live in the gut, skin, and mouth, among other sites in the body. Most microorganisms that make up the human microbiome are beneficial to our health, but some are potentially harmful. Accruing evidence suggests that the balance between helpful and potentially harmful microorganisms in the gut microbiome contributes to overall health, and an imbalance can contribute to a number of diseases, including cancer (243)Kho ZY, et al. (2018) Front Microbiol, 9: 1835. [LINK NOT AVAILABLE]. Research has also shown an association between a patient’s microbial composition and their response to therapeutics, with implications that modulating the microbiome can boost the effectiveness of certain anticancer treatments such as immunotherapies (244)Villemin C, et al. (2023) Trends Immunol, 44: 44. [LINK NOT AVAILABLE].
The gut microbiome can be affected by SDOH, such as food insecurity, access to spaces for physical activity, and chronic stress, implying potential differences across racial and ethnic groups (245)Byrd D, et al. (2024) Nat Rev Cancer, 24: 89. [LINK NOT AVAILABLE]. The diversity of the gut microbiome within an individual and between different individuals, as well as the abundance of specific microbes within a person, could differ by race and ethnicity, and these differences may be relevant to cancer disparities (245)Byrd D, et al. (2024) Nat Rev Cancer, 24: 89. [LINK NOT AVAILABLE]. In fact, unique, race-specific associations of harmful microbes with colorectal cancer have been reported (245)Byrd D, et al. (2024) Nat Rev Cancer, 24: 89. [LINK NOT AVAILABLE](246)Piawah S, et al. (2023) Cancers (Basel), 15: 4546. [LINK NOT AVAILABLE].
The microbiome in other sites in the body has also been associated with the risk of precancerous states or cancer. As one example, the interplay between vaginal microbiome and human papillomavirus (HPV) in cervical precancers and cancer development is an area of ongoing research (247)Kyrgiou M, et al. (2022) Semin Cancer Biol, 86: 189. [LINK NOT AVAILABLE](248)Sharifian K, et al. (2023) Virol J, 20: 73. [LINK NOT AVAILABLE]. Emerging data suggest that the risk of cervical cancer associated with vaginal microbiome may differ by race (249)Tossas KY, et al. (2023) J Womens Health (Larchmt), 32: 553. [LINK NOT AVAILABLE]. Future studies should examine whether early detection and manipulation of specific microbes may aid in cancer prevention or interception.
Research has shown that tumors themselves also harbor microorganisms and the type of microorganism present in tumors can predict health outcomes (250)Chen Y, et al. (2022) Front Immunol, 13: 935846. [LINK NOT AVAILABLE]. Race-specific microbial associations have been identified in breast tumors with potential associations with genes involved in tumor aggressiveness, blood vessel formation, and metastasis (251)Parida S, et al. (2023) NPJ Breast Cancer, 9: 4. [LINK NOT AVAILABLE].
Targeting the microbiome may hold promise for reducing racial and ethnic disparities in cancer. However, many outstanding questions remain before such interventions can become a part of routine clinical care (245)Byrd D, et al. (2024) Nat Rev Cancer, 24: 89. [LINK NOT AVAILABLE](252)Ahmad S, et al. (2022) World J Gastroenterol, 28: 2782. [LINK NOT AVAILABLE]. These include a better understanding of the influence of host genetics on the microbiome and vice versa, and how that interplay may lead to cancer development. Current and future preclinical and clinical studies evaluating the microbiome as a driver of cancer and cancer disparities must recruit diverse participants and collect detailed data on SDOH to accurately ascertain the microbial contribution to cancer risk across races and ethnicities.
Processes That Promote Cancer Progression
As cancer cells grow and divide, a mass of cells, or tumor, develops. The tumor is not just made up of cancer cells; rather, it is heterogeneous, and is made up of diverse cancerous and noncancerous cells. Research has shown that tumor progression and metastasis are largely dependent upon complex interactions between cancer cells and their surrounding tissue. Cancer metastasis refers to the spread of cancer cells from the tissue where they first originated to another part of the body. More than 90 percent of cancer-related deaths result from metastatic disease (254)Mittal V (2018) Annu Rev Pathol, 13: 395. [LINK NOT AVAILABLE].
Researchers are continually uncovering complex processes that facilitate cancer metastasis. One area of investigation is the ancestry-related differences in inflammatory and immune biomarkers in the circulation and their contribution to disparities in cancer progression and treatment response.
Tumor Heterogeneity
During the course of disease, as cancer cells divide, they continue to acquire new alterations in their genomes, epigenomes, transcriptomes, and proteomes in various combinations and become more heterogeneous. Researchers use the term “tumor heterogeneity” to describe the differences between cancer cells within a single tumor, the differences between tumors of the same type in different patients, or the differences between a primary (original) tumor and the metastatic tumor.
Tumor heterogeneity plays a crucial role in cancer development and influences how cancer spreads and how it responds to treatment. The heterogeneity of cancer cells in the primary tumor enables some tumor cells to acquire properties that facilitate their spread to other parts of the body. Tumor heterogeneity is also one of the major reasons for treatment resistance (255)Dagogo-Jack I, et al. (2018) Nat Rev Clin Oncol, 15: 81. [LINK NOT AVAILABLE].
Evaluating ancestry-related differences in the genetic drivers of cancer and the differential patterns of cancer cell evolution over time is an area of ongoing research (256)Lord BD, et al. (2022) Trends Cancer, 8: 276. [LINK NOT AVAILABLE]. For example, studies in gastrointestinal cancers such as stomach and liver cancer have aimed to characterize tumor heterogeneity and identify shared features of liver cancer across diverse populations to improve equitable utilization of precision cancer medicine (257)Ma L, et al. (2022) Trends Cancer, 8: 286. [LINK NOT AVAILABLE](258)Toal TW, et al. (2022) Cancer Res Commun, 2: 1487. [LINK NOT AVAILABLE]. A recent report demonstrated population-specific patterns of tumor heterogeneity with a unique tumor mutational landscape among stomach cancer patients of Latino ancestry (258)Toal TW, et al. (2022) Cancer Res Commun, 2: 1487. [LINK NOT AVAILABLE]. The burden of stomach cancer is disproportionately higher among individuals of Latino ancestry, and the study found a higher frequency of a poor prognosis–associated molecular subtype in Latino patients.
Tumor Microenvironment
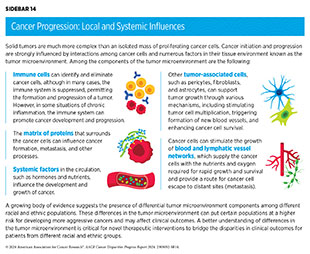
Research has shown that tumor initiation and progression are largely dependent upon complex interactions between cancer cells and the surrounding tissue, which is known as the tumor microenvironment (see Sidebar 14). Bidirectional communication between cancer cells and the tumor microenvironment has a profound influence on cancer progression. Moreover, the tumor microenvironment can shelter cancer cells from the effects of some cancer treatments, including radiation, chemotherapy, and immunotherapy, thus modifying a patient’s response to treatment (259)Jin MZ, et al. (2020) Signal Transduct Target Ther, 5: 166. [LINK NOT AVAILABLE](260)Anderson NM, et al. (2020) Curr Biol, 30: R921. [LINK NOT AVAILABLE].
Recent research suggests that several components of the tumor microenvironment could differ among different racial and ethnic patient populations (261)Maynard JP, et al. (2022) Prostate, 82: 1505. [LINK NOT AVAILABLE](262)Peres LC, et al. (2022) Cancer Epidemiol Biomarkers Prev, 31: 1006. (263)Zajac KK, et al. (2023) Cancer Rep (Hoboken), 6: e1779. [LINK NOT AVAILABLE]. These differences may result from factors associated with inherited genetic ancestry, increased levels of chronic inflammation, innate differences in immune response, or a combination of these (264)Tang W, et al. (2018) Clin Cancer Res, 24: 5471. (265)Jenkins BD, et al. (2019) Cancer Epidemiol Biomarkers Prev, 28: 690. (266)Wallace TA, et al. (2008) Cancer Res, 68: 927. . As an example, several recent studies that have examined breast tumors have shown that patients of African ancestry have unique architecture and cellular composition within the tumor microenvironment (267)Kumar B, et al. (2023) Nat Commun, 14: 5683. [LINK NOT AVAILABLE](268)Bassiouni R, et al. (2023) Cancer Res, 83: 34. . Additionally, a number of these studies reported a higher mobilization and greater abundance of immune cells within the breast tumor microenvironment in patients of African ancestry, albeit the cells exhibited an inactive or suppressed state indicative of a more aggressive disease (223)Singhal SK, et al. (2022) JCI Insight, 7: e157465. [LINK NOT AVAILABLE](269)Bauer M, et al. (2023) Cancer Immunol Res, 11: 720. (270)Martini R, et al. (2022) Cancer Discov, 12: 2530. .
A better understanding of how cancer cells and the tumor microenvironment—in particular, immune components—differ among diverse patient populations will help to better identify causes of cancer disparities, determine better treatment strategies such as immunotherapies, and provide information on how to eliminate cancer disparities.
Epithelial-to-mesenchymal Transition
Epithelial cells tightly connect with each other to form the covering of all body surfaces and line body cavities and hollow organs. Roughly 90 percent of cancers develop in epithelial cells (271)Holly JM, et al. (2013) Cancer Metastasis Rev, 32: 673. [LINK NOT AVAILABLE].
Cancers that develop in epithelial cells acquire properties of another type of cells, called mesenchymal cells, which form the connective tissue, blood vessels, and lymphatic tissue, and have the ability to migrate within the body. Cancer cells acquire the mesenchymal characteristic of moving within the body by hijacking pathways fundamental for epithelial-to-mesenchymal cell transition, or EMT, which is an essential process for the formation of organs during normal embryonic development (272)Castaneda M, et al. (2022) Semin Cancer Biol, 87: 17. [LINK NOT AVAILABLE]. Hijacking of EMT pathways by cancer cells is one of the hallmarks of cancer. EMT plays a critical role in the ability of cancer cells to evade the immune system (273)Wang G, et al. (2021) NPJ Precis Oncol, 5: 56. [LINK NOT AVAILABLE].
Recent studies have demonstrated ancestry-related differences in cellular and molecular pathways associated with EMT (274)Kinseth MA, et al. (2014) Int J Cancer, 134: 81. [LINK NOT AVAILABLE](275)Awasthi S, et al. (2021) Clin Cancer Res, 27: 320. (276)Liadi Y, et al. (2023) Prostate Cancer Prostatic Dis. DOI: 10.1038/ s41391-023-00667-1. [LINK NOT AVAILABLE]. Ongoing research is exploring whether therapeutically targeting EMT could improve clinical outcomes.
Cancer Development: Integrating and Translating Our Knowledge
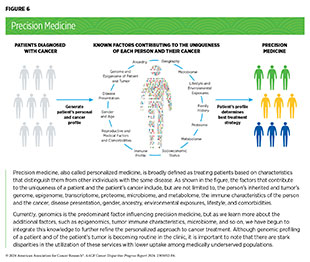
One of the most important insights gleaned from our current knowledge of the complexities underpinning cancer initiation and progression is that each patient’s cancer is unique because of their distinct biological, lifestyle, environmental, and ancestral influences. The most effective cancer control efforts, therefore, must take a comprehensive look at multiple factors such as the person’s inherited genome, the genome and epigenome of the cancer, family history, disease presentation, gender, exposures, lifestyle, microbiome, and other comorbidities and apply approaches tailored to each individual patient. In fact, in the past decade there has been a shift from a “one-size-fits-all” approach to cancer prevention, screening, and treatment to a more personalized approach called precision medicine (see Figure 6).
The aim of precision medicine is to use information about an individual’s biology as well as other factors to prevent, diagnose, and treat disease. Precision medicine has the potential to further revolutionize cancer care. While genomics is the predominant factor influencing current precision cancer medicine, researchers are rapidly accumulating data on additional cancer-associated measures, including immune, metabolic, microbial, and protein profiles, to better understand and tackle cancer. Furthermore, the role of additional biological factors, such as vitamins or lipids, that contribute to cancer development and may have ancestry-related differences is being identified (278)Siddappa M, et al. (2023) Cancer Res Commun, 3: 621. [LINK NOT AVAILABLE](279)Boyd AE, et al. (2023) Cancers (Basel), 15: 2238. [LINK NOT AVAILABLE].
Unfortunately, much of the current information fueling precision medicine is derived from patients who are White and/or of European ancestry. Lack of relevant data from racial and ethnic minority groups and medically underserved populations, as well as a lack of diversity in cancer clinical studies undermines the true success of precision medicine. Collecting biospecimens from patients with different sociodemographic backgrounds will create diverse datasets that researchers can use to better understand race-, ethnicity-, and ancestry-related differences in cancers. All constituents invested in public health must come together to ensure that institutions serving historically underserved populations and underresourced communities have the infrastructure, including access to advanced technologies such as the latest DNA and RNA sequencing techniques, that is needed for proper implementation of precision medicine.
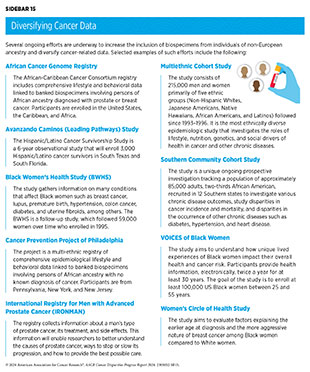
Several private and federal initiatives are being undertaken to diversify cancer-related data (see Sidebar 15). As one example, the AACR Project Genomics Evidence Neoplasia Information Exchange (GENIE) has sequenced tumors from over 172,000 patients across 19 leading cancer centers in the United States and Europe, nearly 12 percent of whom are from racial and ethnic minorities (281)American Association for Cancer Research. AACR Project GENIE. Accessed: March 17, 2024. . Researchers are already using these databases to address gaps in knowledge about cancer biology and the genetic changes that occur specifically in underrepresented and underserved populations (282)Goel N, et al. (2022) JAMA Network Open, 5: e220573. [LINK NOT AVAILABLE](283)Kamran SC, et al. (2021) JCO Precis Oncol, 5: 1654. [LINK NOT AVAILABLE](284)Schumacher FR, et al. (2021) JCO Precis Oncol, 5: 1650. [LINK NOT AVAILABLE].
To advance the science of cancer disparities, it is imperative that researchers integrate biological data with structural and social drivers of health that contribute to cancer development and are known contributors to cancer disparities. In this regard it should be noted that the Research on Prostate Cancer in Men of African Ancestry: Defining the Roles of Genetics, Tumor Markers and Social Stress (RESPOND) is one such study that is looking at the underlying factors and reasons that put African American men at higher risk for prostate cancer. By using surveys and tumor characterization, RESPOND is evaluating how exposure to stress over a lifetime, inherited susceptibility (i.e., genes), and tumor characteristics contribute to the development of prostate cancer (285)Public Health Institute. National RESPOND Study Takes Closer Look at Health Disparities in Prostate Cancer for African American Men. Accessed: April 22, 2024. (286)African American Prostate Cancer Study. What is RESPOND? Accessed: April 22, 2024. .
Precision medicine holds immense promise to deliver better outcomes with reduced toxicity for patients with cancer. However, many questions remain unanswered, such as the cost effectiveness of multidimensional profiling required for precision medicine, and the extent to which such profiling improves outcomes for individuals from diverse populations. Additionally, there are disparities in the access and utilization of these services, with lower uptake among racial and ethnic minority groups and underserved populations (see Treatment With Molecularly Targeted Therapy and Immunotherapy) (277)Halbert CH (2022) Annu Rev Genomics Hum Genet, 23: 613. [LINK NOT AVAILABLE](287)Dutta R, et al. (2023) Nature Cancer, 4: 787. [LINK NOT AVAILABLE](288)Khoury MJ, et al. (2022) Genet Med, 24: 1630. [LINK NOT AVAILABLE]. It is vital that researchers across cancer science, medicine, and public health work together with policymakers to ensure that all patients with cancer can equitably benefit from the unprecedented breakthroughs being made in cancer care and that precision medicine approaches reduce and not further exacerbate cancer disparities.
Next Section: Disparities in the Burden of Preventable Cancer Risk Factors Previous Section: Understanding and Addressing Drivers of Cancer Disparities