Understanding the Path to Cancer Development
In this section, you will learn:
- Cancer is a collection of diseases that are characterized by unchecked cell multiplication.
- Basic research is an essential driver of our understanding of the biology of cancer.
- Both acquired and inherited genetic mutations can contribute to cancer initiation and progression.
- Cancer development is influenced by modifications inside the cell as well as changes in the environment that surrounds a tumor.
- Technological advances are revolutionizing the identification of alterations in DNA, RNA, protein and cells that drive cancer.
- The knowledge gleaned from an integrated approach to understanding cancer development is fueling progress in the field of precision medicine.
Cancer is not a single disease but a collection of related diseases, in which some of the body’s cells divide uncontrollably and spread to other parts of the body.
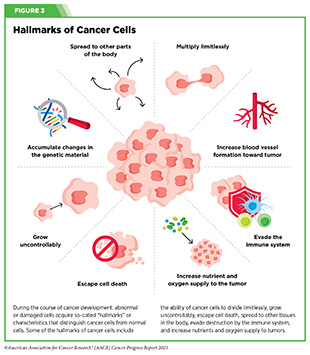
During the course of cancer development, abnormal or damaged cells acquire so-called “hallmarks” or characteristics that distinguish cancer cells from normal cells. Some of the hallmarks of cancer cells include their ability to: multiply limitlessly by ignoring signals that tell cells to stop dividing or to die; sustain rapid growth by relying on unique nutrients that are different from those used by normal cells; accumulate multiple changes in their genetic material; leave the tissue of origin and spread to other tissues; evade the immune system responsible for eliminating abnormal or damaged cells; and recruit blood vessels, thus increasing nutrients and oxygen supply to tumors (see Figure 3) (62)Hanahan D (2022) Cancer Discov, 12: 31. [LINK NOT AVAILABLE].
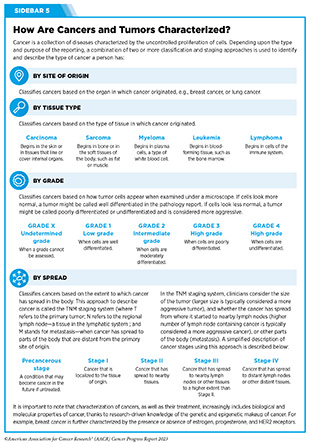
There are several ways to characterize cancers, and the methodology used depends on the type and purpose of the research and/or reporting (see Sidebar 5). In most research and clinical settings, multiple classification methods are used simultaneously to identify and describe the type of cancer that a person has.
Cancer Development: Generating Knowledge
Much of the current knowledge of how cancer develops comes from basic research. Sometimes called “pure” or “fundamental” science, basic research helps researchers understand living systems and life processes. This knowledge is essential to develop better ways to predict, prevent, detect, diagnose, and treat disease.
Knowledge gained from basic research provides the foundation for new advances in clinical care and refines existing practices. Recognizing the critical role of basic research in improving the overall health for all individuals, NIH has spent more than 50 percent of its budget on basic research every year over the past two decades (64)National Institutes of Health. FY 2003 – FY 2022 Distribution of Budget Authority Percentages for Basic and Applied Research. Accessed: July 5, 2023. .
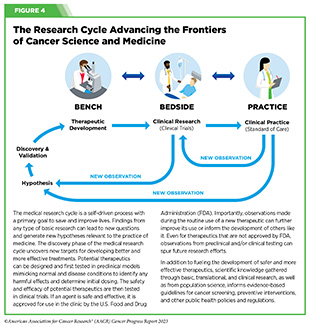
Basic research is a fundamental component of medical research (see Figure 4). Scientists working across the spectrum of medical research seek to address biological and/or clinical significance of a new discovery through experiments in a wide range of models that mimic healthy and diseased conditions.
Findings from these experiments can identify new targets for drug development; develop the technologies needed to generate drugs that are selective for cancer cells or cancer targets; inform approaches for preventive intervention; determine new strategies for early detection; or uncover predictive or prognostic biomarkers, each of which has the potential to improve public health.
Once researchers identify a potential therapeutic target, potential therapeutic agents can be generated and pursued in translational and clinical studies designed to carefully test candidate therapeutics against the target and determine the appropriate dosage, dosing schedules, and toxicities. These preclinical studies help determine the most promising candidates, which are then tested in clinical trials (see Sidebar 27).
Progress made against cancer in recent decades is a direct result of years-long cross disciplinary collaborations among stakeholders working throughout the medical research cycle (see Sidebar 1 and Figure 4).
Basic Research: Vital for Making Progress Against Cancer
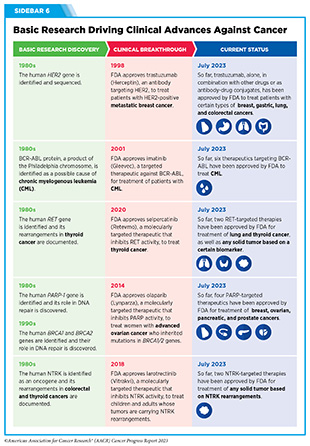
Basic research, together with translational and clinical studies and population sciences, has played a pivotal role in reducing the burden of cancer in the United States. Discoveries stemming from decades of basic research have provided the foundational knowledge, which has led to a 33 percent reduction in the overall cancer mortality rate over the last three decades (see Sidebar 6). One example of how basic research contributes to progress against cancer is the development of small molecule inhibitors of Kirsten rat sarcoma viral oncogene homologue (KRAS), a protein that is essential for the growth and survival of normal cells and is altered in many cancers.
Several teams of basic researchers simultaneously identified the human KRAS gene in 1982 (65)Fernandez-Medarde A, et al. (2021) Genes (Basel), 12. [LINK NOT AVAILABLE]. Subsequent discoveries linked KRAS activity with growth and survival of normal cells and identified alterations in the gene that were associated with human cancer (65)Fernandez-Medarde A, et al. (2021) Genes (Basel), 12. [LINK NOT AVAILABLE]. More recent studies have established that the KRAS gene is frequently mutated (see Sidebar 7) in several cancer types, including cancers of the pancreas, lungs, and colon and rectum (66)Huang L, et al. (2021) Signal Transduct Target Ther, 6: 386. [LINK NOT AVAILABLE]. Up until 2021, KRAS was considered an undruggable protein, and patients with cancer harboring KRAS mutations had limited treatment options.
Thanks to the research-driven technological innovations, advances in synthetic chemistry and structural biology, and a deeper understanding of the genetic alterations in lung cancer, researchers have developed several small molecular inhibitors that successfully inhibit the activity of the mutant KRAS in patients with NSCLC. One of the compounds, sotorasib (Lumakras), was approved by FDA in May 2021 (4)American Association for Cancer Research. AACR Cancer Progress Report 2021. Accessed: June 30, 2023. , and a second compound, adagrasib (Krazati), was approved in December 2022 (see Expanding Treatment Options for Patients with Lung Cancer).
Basic research provides the foundational knowledge about what triggers cancer development; how cancer evades the body’s defenses; and how cancer spreads within the body, among other aspects of cancer biology (see Cancer Development: Interpreting Knowledge). Basic research is also fueling the development of molecules that can help visualize cancer cells better, deliver drugs to tumors more precisely, and kill cancer cells more selectively and effectively. Collectively, basic research-driven advances are contributing to progress against cancer that is saving lives and improving health outcomes for countless patients.
Cancer Development: Interpreting Knowledge
At a fundamental level, cancer is a genetic disease that is caused by changes in genes that control vital functions, such as cell multiplication and cell growth (see Sidebar 7). However, transformation of normal cells into cancer cells, accumulation of cancer cells to form tumors, and spread of tumors to distant sites in the body are all complex, multistep processes that are influenced by alterations inside the cell as well as changes outside the cell.
Changes That Contribute to Cancer Initiation
Cellular functions are dictated by instructions that are encoded in deoxyribonucleic acid (DNA), a complex molecule that constitutes the genetic material of cells. Four unique molecules or bases, designated A, T, C, and G, make up a DNA strand, and two DNA strands are then paired together to form a double helix. The entirety of a person’s DNA is called the genome.
Inside the nucleus of human cells, the DNA double helix is wrapped around bead-like structures called nucleosomes, which are composed of proteins called histones. The packaged DNA is further compacted in multiple layers, making up structures we know as chromosomes. Nearly all human cells have 46 chromosomes.
Genes are pieces of DNA and there are hundreds to thousands of genes contained in each chromosome. Genes carry instructions or messages for making proteins, which are functional units of the cell. The cell uses a complex process, called transcription, to copy the message embedded in a gene to make another type of molecule called messenger ribonucleic acid (mRNA). The information in mRNA is subsequently “translated” into proteins. Cellular needs influence how much mRNA or protein will be produced.
The following section describes the types of changes within a cell that impact cancer development.
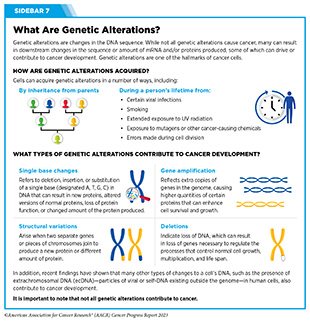
Genetic Alterations
Alterations in the DNA sequence, referred to as mutations, can disrupt or modify normal protein function and are among the hallmarks of cancer cells. Gene alterations can change the sequence or amount of mRNA and the resulting proteins that are produced, which in turn can contribute to cancer development (see Sidebar 7). Genetic alterations can be inherited (called germline mutations) or acquired during a person’s lifetime (called somatic mutations). In about 10 percent of cancer cases, the mutations are inherited.
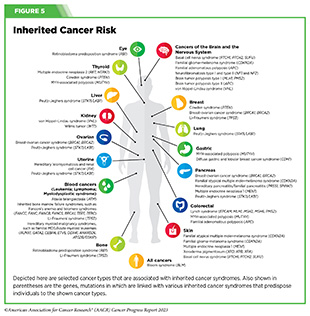
Germline mutations occur in a body’s reproductive cells (egg or sperm) that are passed on from parents to children and become incorporated into the DNA of every cell in the body of the offspring. These types of mutations can increase their risk of developing cancer, although not all germline mutations contribute to cancer development. Inherited genetic alterations that play a role in cancer development are among the pathogenic germline mutations (see Figure 5).
We are making great strides in understanding germline pathogenic mutations, thanks to advances in research and technology. As one example, a recent study utilizing cutting edge DNA sequencing found that 13 percent of patients with endometrial cancer had germline pathogenic mutations, and 63 percent of these mutations occurred in genes that are known to drive cancer development (69)Gordhandas S, et al. (2023) J Natl Cancer Inst, 115: 560. [LINK NOT AVAILABLE]. These findings can help researchers identify new ways to test individuals and their family members for risk of developing endometrial cancer, as well as devise new strategies for minimizing the possibility of developing cancer.
Somatic or acquired mutations occur over an individual’s lifetime due to errors arising during normal cell divisions or because of environmental exposures, lifestyle factors, and/ or health conditions. Research has revealed that tumors originating from different sites, as well as different tumor masses originating from a primary tumor within a person, contain different somatic mutations. For example, two recent studies evaluated metastatic tumors from different patients and from different sites within each patient to understand how metastatic lung cancer grows and spreads inside the body. Findings from these studies showed that genetic features of metastatic lung tumors from separate sites differed greatly within a patient and across patients and that these differences determined when and how the cancer would recur (70)Frankell AM, et al. (2023) Nature, 616: 525. [LINK NOT AVAILABLE](71)Al Bakir M, et al. (2023) Nature, 616: 534. [LINK NOT AVAILABLE].
Researchers are leveraging knowledge of genetic alterations in cancer to develop approaches that are driven by artificial intelligence for establishing comprehensive maps of somatic mutations across different cancer types. In a recent study, researchers developed a deep learning model that identified known and previously unknown somatic mutations as drivers of cancer development across 37 types of cancers (73)Sherman MA, et al. (2022) Nat Biotechnol, 40: 1634. [LINK NOT AVAILABLE]. Characterizing cancers at a molecular level based on the types of genetic alterations, as well as understanding how genetic mutations contribute to cancer, has led to the development and FDA approval of many molecularly targeted therapies (74)National Cancer Institute. Targeted Therapy Drug List by Cancer Type. Accessed: July 5, 2023. . Recent research efforts are focused on understanding how germline and somatic mutations work together to cause cancer, and whether this knowledge can be applied to find new ways to test for and treat cancer, monitor responses to therapy and/or determine an individual’s risk of developing cancer over their lifetime (75)Vali-Pour M, et al. (2022) Nat Commun, 13: 3724. [LINK NOT AVAILABLE](76)Gabriel AAG, et al. (2022) J Natl Cancer Inst, 114: 1159. [LINK NOT AVAILABLE](77)Srinivasan P, et al. (2021) Nat Genet, 53: 1577. [LINK NOT AVAILABLE].
RNA Variations
Most human genes contain information for making proteins in fragments of DNA, called exons. Exons are interspersed by DNA sequences, called introns, that do not contain information necessary to make a functional protein. When a gene is transcribed into mRNA, the initial mRNA molecule contains a copy of both exons and introns. An intricate “cut and paste” process, called splicing, removes introns and joins exons together to produce an mRNA molecule that is subsequently translated into a functional protein by the cellular machinery.
RNA splicing plays a pivotal role in normal cellular functions. In cancer cells, changes in proteins necessary for splicing can produce aberrant mRNA molecules, which subsequently make abnormal proteins that can fuel cancer development, lead to treatment resistance, and alter immune cell function (78)Bradley RK, et al. (2023) Nat Rev Cancer, 23: 135. [LINK NOT AVAILABLE]. Ongoing research is focused on understanding how cancer-related changes in RNA splicing can be leveraged for therapeutic purposes (79)Stanley RF, et al. (2022) Nat Cancer, 3: 536. [LINK NOT AVAILABLE].
Researchers are also using transcriptomics—the study of all RNA molecules in a cell—to establish comprehensive patterns of the types and levels of RNA, or transcriptomes, present in healthy tissues versus tumors. Knowledge gleaned from such studies helps researchers understand how different types of RNA may contribute to cancer development. Technological breakthroughs in RNA sequencing with higher precision and accuracy have further allowed researchers to determine transcriptomes of single cells. Such analyses are unveiling previously undiscovered mechanisms that contribute to cancer development (81)Martinez-Ruiz C, et al. (2023) Nature, 616: 543. [LINK NOT AVAILABLE], tumor heterogeneity (see Tumor Heterogeneity), and treatment resistance (82)Wild SA, et al. (2022) Elife, 11. [LINK NOT AVAILABLE].
Protein Modifications
Proteins are vital for normal cellular functions. The human proteome—the complete set of proteins made by humans— contains about 20,000 unique proteins. After being produced from mRNA, proteins can undergo additional modifications, providing great versatility and variability in their functions to meet cellular needs. Examining the proteome of cancer cells can unveil additional information about how cancer develops. For example, a recent study evaluated proteomes of nearly 1,000 cancer cell lines—tumor-derived cells that keep dividing and growing under certain conditions in the laboratory and are commonly used in medical research to understand molecular underpinnings of cancer development. Researchers identified common and unique cancer-related changes in levels of many proteins that were not detected at DNA or RNA levels (83)Goncalves E, et al. (2022) Cancer Cell, 40: 835. [LINK NOT AVAILABLE].
Modifications of proteins, also called posttranslational modifications (PTMs), are often necessary for normal cellular functions, such as responding to signals from outside the cell (84)Uversky V. Posttranslational Modification. 2013. p 425. [LINK NOT AVAILABLE]. Changes in normal PTMs of proteins can contribute to cancer (85)Wang H, et al. (2023) Cancer Gene Ther, 30: 529. [LINK NOT AVAILABLE]. Identifying cancer-related PTM(s) has been central to developing effective anticancer therapeutics. Research has shown that many enzymes—specialized proteins that speed up chemical reactions in the body—that mediate PTMs exhibit altered function in cancer cells and are an attractive target for therapeutic development. This knowledge has led to the development and FDA approval of numerous molecularly targeted therapies that function by inhibiting the activity of such enzymes (74)National Cancer Institute. Targeted Therapy Drug List by Cancer Type. Accessed: July 5, 2023. , thus expanding treatment options available to patients with cancer.
Epigenetic Changes
Epigenetic changes alter the structure of DNA without changing the DNA sequence. Epigenetic alterations occur when chemical marks are added to or removed from DNA, or when histone proteins undergo PTMs. Epigenetic changes may be acquired with age and/or exposure to environmental factors (e.g., air pollution) or behavioral factors (e.g., smoking) and psychosocial stressors (e.g., systemic racism), and may affect a person’s risk of cancer as well as be passed from parent to child.
Epigenetic modifications regulate how and when genes are turned on or off. Specialized proteins add or remove unique epigenetic modifications to and from DNA and histones (86)Lu Y, et al. (2020) Mol Cancer, 19: 79. [LINK NOT AVAILABLE]. In contrast to genetic mutations, most epigenetic changes are reversible.
Cancer cells exhibit an altered epigenome—the complete set of all of the epigenetic changes in a cell. Because epigenetic changes are attractive targets for drug development, an area of active research is understanding how changes to the epigenome contribute to cancer development and how such changes can be targeted. Research over the years has led to development and approval of several anticancer therapeutics that function by modifying the cancer epigenome (87)Nepali K, et al. (2021) J Biomed Sci, 28: 27. [LINK NOT AVAILABLE].
Researchers are also leveraging the knowledge of the epigenome to categorize different types of cancer at a molecular level. For example, research has shown that mutations in proteins that add or remove epigenetic modifications are characteristics of glioma, a devastating type of brain cancer in children and adults
(88)Phillips RE, et al. (2020) Cancer Cell, 38: 647. [LINK NOT AVAILABLE]. These findings can help identify new therapeutic targets in glioma and can accelerate the development of new drugs to treat this aggressive form of cancer.
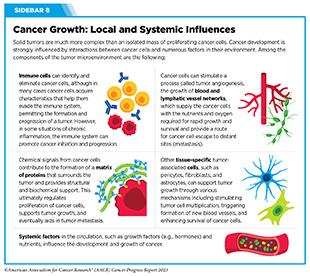
Systems That Enable Cancer Progression
A hallmark of cancer is the ability of tumor cells to break away from the primary tissue and travel to other parts of the body. Systems that enable cancer to spread from the primary tissue to other organs of the body include the blood system, the lymphatic system, and the immune system (see Sidebar 8).
The Blood System
The formation of new blood vessels, called angiogenesis, is a normal and essential process that occurs throughout life and is controlled by chemical signals in the body. The ability to promote angiogenesis toward and within a tumor is a hallmark of cancer. Tumor angiogenesis supplies high levels of oxygen and nutrients that are required to fuel rapid tumor growth. Therefore, drugs that block angiogenesis can restrict the ability of a tumor to grow, divide, and metastasize.
Several proteins play pivotal roles in angiogenesis. One such protein is vascular endothelial growth factor (VEGF), which functions in concert with its binding partner, called the VEGF receptor. There are several forms of both proteins, and both proteins are essential for the growth of cells that line the inside of blood vessels. Decades of research have shown that cancer cells can produce and release high levels of VEGF, thus directing the formation of new blood vessels (89)Apte RS, et al. (2019) Cell, 176: 1248. [LINK NOT AVAILABLE].
Advances in cancer therapeutics over the past two decades have led to the development of drugs that inhibit the function of VEGF and the VEGF receptor, thus blocking a tumor’s efforts to increase the supply of oxygen and nutrients. In 2004, FDA approved bevacizumab (Avastin), the first anti-angiogenic drug targeting a form of VEGF. Since then, FDA has approved 11 different anticancer therapeutics targeting several forms of VEGF or VEGF receptors, or other proteins that promote angiogenesis, to treat 13 different cancer types (4)American Association for Cancer Research. AACR Cancer Progress Report 2021. Accessed: June 30, 2023. .
The Lymphatic System
The lymphatics are an extensive system of vessels, called lymph vessels or lymphatic vessels, and small bean-shaped structures, called lymph nodes. Other organs such as the spleen, thymus, tonsils, and adenoids are also part of the lymphatic system. The lymphatic system runs throughout the body and is an essential component of the immune system. Key functions of the lymphatic system include maintaining total body fluid levels, removing cellular waste from tissues, detecting pathogens, absorbing fats, and producing immune cells and antibodies in the lymph nodes.
When cancer cells break away from a tumor, they can travel to other parts of the body through the blood or the lymphatic system. If cancer cells spread through the lymphatic system, they may accumulate in one or more of the nearest lymph nodes. The presence of cancer cells in lymph nodes is used to determine the stage and/or the extent of cancer (see Sidebar 5).
Tumor cells can enter the lymphatic system in several ways, including adopting mechanical changes that facilitate cancer cell entry into the lymphatic system, and releasing certain molecules that help cancer cells move toward the lymphatic vessels (90)Zhou H, et al. (2021) Cells, 10. [LINK NOT AVAILABLE]. Once inside the lymphatic system, cancer cells acquire additional properties that make them more aggressive and facilitate their spread to other parts of the body (91)Padera TP, et al. (2016) Annu Rev Biomed Eng, 18: 125. [LINK NOT AVAILABLE].
Ongoing research is focused on leveraging contributions of the lymphatic system in cancer growth and spread, including targeted delivery of anticancer therapy to lymph nodes as well as the development of drugs that block the migration of cancer cells toward lymphatic vessels (90)Zhou H, et al. (2021) Cells, 10. [LINK NOT AVAILABLE].
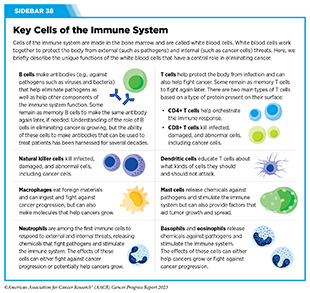
The Immune System
The immune system is a complex network of cells, tissues, organs, and the substances they make that help the body fight infections and other diseases, including cancer (see Sidebar 8 and Sidebar 38). The immune system actively detects and eliminates abnormal or damaged cells from the body. However, because of the heterogeneous nature of the changes cancer cells acquire over time, some cancer cells obtain properties that help them evade the immune system. The properties of cancer cells that facilitate evasion from the immune system are one of the hallmarks of cancer.
Extensive research over the last three decades has revealed some of the ways cancer cells evade the immune system (92)Spranger S, et al. (2018) Annual Review of Cancer Biology, 2: 213. [LINK NOT AVAILABLE]. These include disruption of the cellular machinery that helps immune cells recognize damaged or abnormal cells; increasing levels of proteins in tumor cells that function as brakes on the immune system; and release of molecules that prevent the immune cells from becoming fully functional (93)Kim SK, et al. (2022) Front Pharmacol, 13: 868695. [LINK NOT AVAILABLE]. Research has also shown that certain immune cells present in the tumor microenvironment (see Tumor Microenvironment) can promote tumor growth (94)Binnewies M, et al. (2018) Nat Med, 24: 541. [LINK NOT AVAILABLE]. This issue of the AACR Cancer Progress Report includes a spotlight on advances in immunotherapy that highlights the tremendous progress that has been made in recent decades to harness the potential of the immune system to treat cancer (see Immunotherapy: Pushing the Frontier of Cancer Medicine).
Processes That Promote Cancer Growth and Metastasis
Cancer metastasis refers to the spread of cancer cells from the tissue where they first originated to another part of the body. During metastasis, cancer cells break away from the original (primary) tumor, travel through the blood or lymph system, and form a new tumor in other organs or tissues of the body. Although the new, metastatic tumor acquires many additional alterations during the course of cancer development, it remains the same type of cancer as the primary tumor. For example, if prostate cancer spreads to the bone, the cancer cells in the bone are prostate cancer cells, not bone cancer cells.
Patients with metastatic cancer have considerably lower 5-year survival rates than those with localized cancer (28)American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . More than 90 percent of cancer-related deaths result from metastatic disease (95)Mittal V (2018) Annu Rev Pathol, 13: 395. [LINK NOT AVAILABLE]. Furthermore, chances of a cure are limited in patients with metastatic cancer, and thus researchers are continually uncovering additional complex processes that facilitate cancer metastasis.
Tumor Heterogeneity
During the course of disease, as cancer cells divide, they continue to acquire new alterations in their genomes, epigenomes, transcriptomes, and proteomes in various combinations and become more heterogeneous. Researchers use the term ‘tumor heterogeneity’ to describe the differences between cancer cells within a single tumor, the differences between tumors of the same type in different patients, or the differences between a primary (original) tumor and the metastatic tumor.
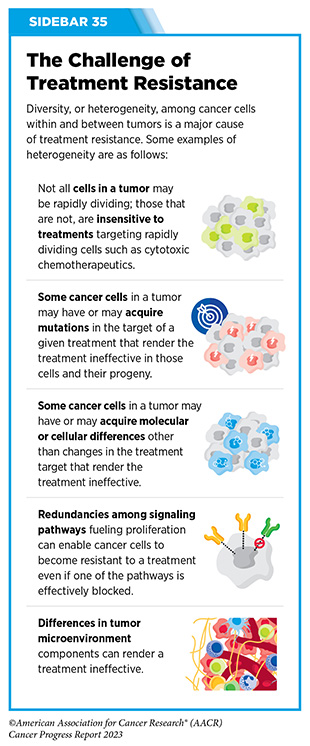
Tumor heterogeneity plays a crucial role in cancer development and influences how cancer spreads, and how it responds to treatment. The heterogeneity of cancer cells in the primary tumor enables some tumor cells to acquire properties that facilitate their spread to other parts of the body. Tumor heterogeneity is also one of the major reasons why certain patients with cancer are resistant to treatments (96)Dagogo-Jack I, et al. (2018) Nat Rev Clin Oncol, 15: 81. [LINK NOT AVAILABLE] (see Sidebar 35).
Technological advances in sequencing and molecular imaging are enabling researchers to decode the heterogeneous nature of tumors at the single cell level. As one example, an analysis of transcriptomes of 1,163 tumor samples across 24 cancer types identified 41 different types of mRNA expression patterns that were common among the cancer types tested. Although there were common genes involved in cancer development across different cancer types, researchers found that individual cells within a tumor were highly heterogeneous in terms of the types of mutations they carried (98)Gavish A, et al. (2023) Nature, 618: 598. [LINK NOT AVAILABLE], which can yield both underlying resistance to therapy as well as distinct vulnerabilities. Understanding and therapeutically targeting tumor heterogeneity is the next frontier in cancer science and medicine.
Epithelial-to-mesenchymal Transition
Epithelial cells are the cells that tightly connect with each other to form the covering of all body surfaces, line body cavities and hollow organs, and are the major tissue in glands. Roughly 90 percent of cancers develop in epithelial cells (99)Holly JM, et al. (2013) Cancer Metastasis Rev, 32: 673. [LINK NOT AVAILABLE].
Cancers that develop in epithelial cells acquire properties of another type of cells, called mesenchymal cells, which form the connective tissue, blood vessels, and lymphatic tissue, and have the ability to migrate within the body. Cancer cells acquire the mesenchymal characteristic of moving within the body by hijacking pathways fundamental for epithelial-to-mesenchymal cell transition, or EMT, which is an essential process for the formation of organs during normal embryonic development (100)Castaneda M, et al. (2022) Semin Cancer Biol, 87: 17. [LINK NOT AVAILABLE]. Hijacking of EMT pathways by cancer cells is one of the hallmarks of cancer.
Research has established that EMT is regulated by several proteins that promote cancer cell division, survival, and mobility, and enable metastasis (102)Fischer KR, et al. (2015) Nature, 527: 472. [LINK NOT AVAILABLE](103)Wang G, et al. (2021) NPJ Precis Oncol, 5: 56. [LINK NOT AVAILABLE]. Recent studies have found that EMT also plays a critical role in the ability of cancer cells to evade the immune system (103)Wang G, et al. (2021) NPJ Precis Oncol, 5: 56. [LINK NOT AVAILABLE]. Ongoing research is exploring whether therapeutically targeting EMT could improve clinical outcomes.
Tumor Microenvironment
Cancer cells interact with and modify surrounding cells and tissues to sustain their ability to multiply unchecked and accumulate in the primary tissue of origin. The cells, molecules, and blood vessels that surround and sustain cancer cells collectively form the tumor microenvironment.
The tumor microenvironment can affect how a tumor grows and spreads, and cancer cells can reciprocally influence the tumor microenvironment (see Sidebar 8). For instance, cancer cells can release molecules that shape their surrounding environment to provide them with nutrients, oxygen, and a supportive structure. The tumor microenvironment, in turn, can adapt to make it difficult for the immune cells or anticancer drugs to reach and eliminate tumor cells (104)Jin MZ, et al. (2020) Signal Transduct Target Ther, 5: 166. [LINK NOT AVAILABLE](105)Anderson NM, et al. (2020) Curr Biol, 30: R921. [LINK NOT AVAILABLE].
The vital role of tumor microenvironment in cancer initiation, progression, and metastasis has made it a key target for therapeutic development. For example, researchers are working to modify certain types of immune cells such that these cells can infiltrate the tumor microenvironment and destroy cancer cells (106)Bejarano L, et al. (2021) Cancer Discov, 11: 933. [LINK NOT AVAILABLE] (see Immunotherapy: Pushing the Frontier of Cancer Medicine). Blocking the supply of oxygen and nutrients with anticancer therapeutics that inhibit tumor angiogenesis has also shown great promise in therapeutically targeting the tumor microenvironment (106)Bejarano L, et al. (2021) Cancer Discov, 11: 933. [LINK NOT AVAILABLE].
Cancer Development: Integrating Knowledge
We are making remarkable strides toward understanding how cancer develops, grows, and spreads, thanks to contributions of countless researchers across the spectrum of cancer science and medicine. Knowledge gleaned from medical research has already enabled the development of effective anticancer therapies that offer hope to many patients with cancer (see Advancing the Frontiers of Cancer Science and Medicine). In parallel, technological advances in our ability to investigate characteristics of cancer cells at single cell and single molecule levels are providing an in-depth knowledge of the complexities of cancer.
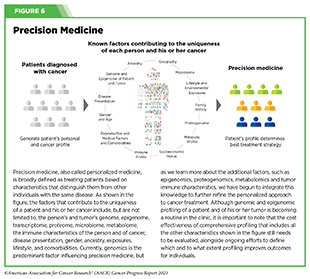
One of the most important insights gleaned from this knowledge is that each patient’s cancer is unique at the molecular level. Together, these insights have provided the basis for precision medicine, also called personalized medicine. Precision medicine is broadly defined as treating patients based on characteristics that distinguish them from other individuals with the same disease (see Figure 6).
In cancer, precision medicine means using specific information about a patient’s tumor, such as the genome sequence of cancer cells, to help make a diagnosis, plan treatment, evaluate whether treatment is working, and/ or predict prognosis. In recent years, FDA has approved an increasing number of anticancer therapeutics that are developed on the basis of genomic characteristics, and many molecularly targeted drugs are being used to treat cancers that originate from different organs but share similar genomic characteristics (see Advancing the Frontiers of Cancer Science and Medicine) (107)Malone ER, et al. (2020) Genome Med, 12: 8. [LINK NOT AVAILABLE](108)Adashek JJ, et al. (2021) Trends Cancer, 7: 15. [LINK NOT AVAILABLE].
The next frontier of precision medicine is to integrate our ever-growing knowledge of the genome, epigenome, proteome, transcriptome, microbiome (see Targeting the Microbiome in Cancer Treatment), immune system and other information about a patient to create a treatment that is tailored to his or her tumor. In fact, researchers are already integrating multiple molecular aspects of a patient’s tumor to determine characteristics that can improve cancer diagnosis; to identify drug targets that can treat cancer precisely; and to establish features that can predict treatment responses and outcomes accurately (111)Mani DR, et al. (2022) Nat Rev Cancer, 22: 298. [LINK NOT AVAILABLE].
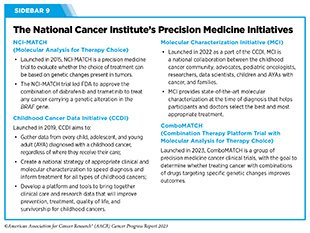
In the United States, NCI is playing a vital role in supporting research in precision cancer medicine (see Sidebar 9). For example, results from NCI’s initial investments in the Clinical Proteomic Tumor Analysis Consortium (CPTAC) are laying the foundation for the next horizon in precision medicine, called proteogenomics (112)Rodriguez H, et al. The next horizon in precision oncology: Proteogenomics to inform cancer diagnosis and treatment. Cell. Volume 184: Elsevier Inc.; 2021. p 1661. [LINK NOT AVAILABLE].
Proteogenomics is the study of how information about the genome of a cancer cell relates to the proteins made by that cell. This includes understanding how genes control when proteins are produced and what changes occur to proteins after they are made that may switch them on and off. Proteogenomics research can help us learn more about which proteins are involved in certain diseases, such as cancer, and may also be used to help develop new drugs that block these proteins.
In a recent study, researchers performed proteogenomic profiling of tumor samples from patients with early esophageal cancer—an aggressive type of cancer, whose underlying biology is not well known. Findings of the study uncovered six distinct molecular signatures that may be related to the development of esophageal cancer, and identified a protein involved in energy production by cells as a novel drug target (113)Li L, et al. (2023) Nat Commun, 14: 1666. [LINK NOT AVAILABLE]. Several recent studies have carried out similar analyses for other diseases such as gastric cancer (114)Shi W, et al. (2023) Nat Commun, 14: 835. [LINK NOT AVAILABLE], glioma (115)Wang Y, et al. (2023) Nat Commun, 14: 505. [LINK NOT AVAILABLE], leukemia (116)Herbst SA, et al. (2022) Nat Commun, 13: 6226. [LINK NOT AVAILABLE], breast cancer (117)Lei JT, et al. (2023) Cold Spring Harb Perspect Med. [LINK NOT AVAILABLE], and bladder cancer (118)Dong L, et al. (2022) Cancer Cell, 40: 70. [LINK NOT AVAILABLE], among others. Researchers can now use this information to further refine approaches to diagnose and treat these cancers.
Precision medicine holds immense promise to deliver better outcomes with reduced toxicity for patients with cancer. However, many questions remain unanswered such as the cost effectiveness of such multidimensional profiling and the extent to which such profiling improves outcomes for individuals (119)Wahida A, et al. (2023) Nat Rev Cancer, 23: 43. [LINK NOT AVAILABLE] It is vital that stakeholders across cancer science, medicine, and public health work together to ensure that all patients with cancer can equitably benefit from breakthroughs being made in cancer care by precision medicine approaches (120)Mateo J, et al. (2022) Nat Med, 28: 658. [LINK NOT AVAILABLE].
Next Section: Reducing the Risk of Cancer Development Previous Section: Cancer in 2023