- Clinical Research
- Progress Across the Clinical Cancer Care Continuum
- Advances in Cancer Treatment with Surgery
- Advances in Radiation-based Approaches to Cancer Care
- Advances in Treatment with Cytotoxic Chemotherapy
- Advances in Treatment with Molecularly Targeted Therapeutics
- Advances in Treatment With Immunotherapeutics
- Spotlight: Research-driven Progress Against Childhood and AYA Cancers
Inspiring Science. Fueling Progress. Revolutionizing Care.
In this section, you will learn:
- Researchers are harnessing knowledge of the cellular and molecular underpinnings of cancer initiation and progression to develop safer and more effective treatments for patients.
- Advances in novel and innovative approaches to surgery, radiotherapy, chemotherapy, molecularly targeted therapy, and immunotherapy—the five pillars of cancer treatment—are saving and improving lives.
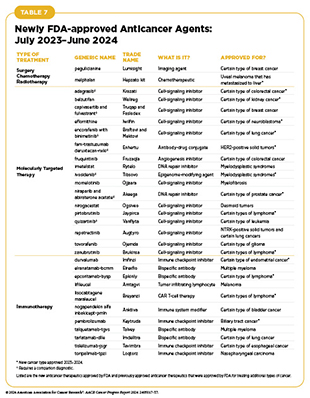
- From July 1, 2023, to June 30, 2024, the US Food and Drug Administration (FDA) has approved 15 new anticancer therapeutics, a new imaging agent to aid breast cancer surgery, and has expanded the use of 15 previously approved anticancer therapeutics to treat additional cancer types.
- Included in the FDA approvals are the first tumor-infiltrating lymphocyte-based cellular immunotherapy that will benefit patients with melanoma, a new bispecific antibody against a novel target for patients with small-cell lung cancer, the first KRAS-targeted therapy for colorectal cancer, and several new molecularly targeted therapeutics and immunotherapeutics for the treatment of patients with different types of blood cancer.
- While these exciting new advances have the potential to transform patient care, much work is needed to ensure equitable access to these treatments for all populations.
Progress across the continuum of cancer science and medicine improves survival and quality of life for people in the United States and around the world. In the United States, the overall cancer death rate is declining steadily, and more individuals are living a longer and fuller life after a cancer diagnosis (see Cancer in 2024)(3)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: March 17, 2024.. This progress is attributable, in part, to the rapid strides that we are making in cancer treatment propelled by breakthroughs in clinical research.
Clinical Research
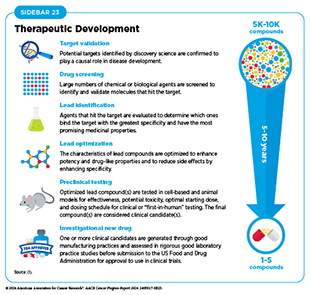
Decades of research in basic and translational sciences have deepened our understanding of the fundamental underpinnings of cancer initiation, evolution, and progression and led to the identification of numerous targets that drive cancer development (see Understanding the Path to Cancer Development). After a potential target is identified and is deemed suitable for therapeutic intervention, it takes many more years of preclinical research before a candidate agent is developed and ready for testing in clinical research, also known as clinical studies or clinical trials (see Sidebar 23).
Clinical trials evaluate the safety and efficacy of candidate agents before a therapeutic can be approved by the US Food and Drug Administration (FDA) and used as part of routine patient care. Institutional review boards critically review and approve all clinical studies before they can begin. Clinical trials are monitored throughout their duration. Patient safety and understanding of the clinical trial are prioritized through the informed consent process, which involves a discussion between the clinical research team and the patient about the trial’s purpose and what is expected of the patient, potential benefits and risks, alternative treatments, and the patient’s right to withdraw at any time.
There are several benefits to participating in a clinical trial. These include access to potentially more effective treatments with carefully standardized monitoring before they are widely available, a direct contribution to lifesaving cancer research, and an active involvement in making health care decisions (479)Abu Rous F, et al. (2024) JAMA Oncol, 10: 416. DOI: 10.1001/jamaoncol.2023.5778.. While there is some evidence that clinical trial participants may have better outcomes compared to nonparticipants, understanding whether participating in a cancer clinical trial can improve long-term survival is a topic of ongoing debate (480)Duenas JAC, et al. (2023) BMC Cancer, 23: 786. DOI: 10.1186/s12885-023-11305-3.(481)Koo KC, et al. (2018) BMC Cancer, 18: 468. DOI: 10.1186/s12885-018-4390-x..
There are several types of cancer clinical trials, including prevention trials, screening trials, treatment trials, and supportive or palliative care trials, each designed to answer different research questions (see Sidebar 24). Clinical studies in which participants are randomly assigned to receive an experimental treatment or standard of care treatment are called randomized clinical trials and are considered the most rigorous.
Clinical trials evaluating potential new therapeutics for cancer have traditionally been done in three successive phases, each with an increasing number of patients. Phase I studies are designed to determine the optimal dose of an investigational anticancer therapeutic, how humans process it, and potential toxicities. Historically, phase I trials were not designed to evaluate efficacy of a therapeutic in treating cancer. However, because of rapid progress in clinical trial design and conduct, phase I trials are increasingly incorporating a preliminary evaluation of efficacy (482)Adashek JJ, et al. (2019) Nat Rev Clin Oncol, 16: 773. DOI: 10.1038/s41571-019-0262-9.. Thanks to extraordinary advances in our understanding of cancer biology, patient responses to investigational therapies in phase I studies have also nearly doubled over the past two decades (483)Kingwell K (2022) Nat Rev Drug Discov, 21: 702. DOI: 10.1038/d41573-022-00144-9..
Phase II studies are designed to determine the initial efficacy of an investigational therapy, in addition to continually monitoring for potential toxicities. Phase III studies are large trials designed to determine therapeutic efficacy as compared to standard of care; when successful, the results of these trials can be used by the US Food and Drug Administration (FDA) to approve new therapeutics or new indications for existing therapeutics. Phase IV studies are conducted after a therapy is approved by FDA and provide additional effectiveness or “real-world” data on the therapy. Sometimes phase 0 clinical studies are performed prior to traditional clinical trials wherein low doses of potential therapeutics are administered to a small number of patients to determine whether such treatments may have the desired effect.
The multiphase clinical testing process requires many patients and takes years to complete (484)Arfe A, et al. (2023) J Natl Cancer Inst, 115: 917. DOI: 10.1093/jnci/djad082.(485)Shadbolt C, et al. (2023) JAMA Netw Open, 6: e2250996. DOI: 10.1001/jamanetworkopen.2022.50996.. Identifying and implementing more efficient clinical development strategies are areas of extensive investigation. As one example, researchers often combine different phases into one clinical trial (labeling depends on the phases combined, e.g., phase I/II or phase III/IV clinical trials), which allows research questions to be answered more quickly or with fewer patients. Additionally, a deepened grasp of the underpinnings of cancer biology has enabled researchers to develop more effective approaches to designing and conducting clinical trials such as those evaluating treatments based on a cancer’s genetic drivers rather than site of origin.
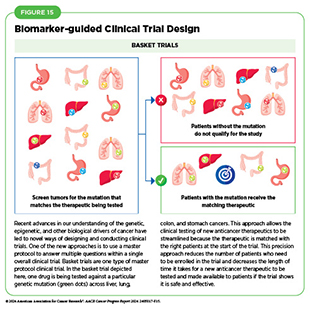
Among the new concepts and designs for clinical trials that have emerged in recent years are the basket, umbrella, and platform trials designs as part of a master protocol framework (see Figure 15) (486)Li A, et al. (2020) Cancer, 126: 4838. DOI: 10.1002/cncr.33205.. Master protocol, also known as main protocol, refers to an overarching trial design that can assess multiple clinical hypotheses with the goal of improving efficacy and streamlining therapeutic development. By allowing the evaluation of multiple new agents simultaneously and by matching the right therapeutics with the right patients earlier, master protocols reduce the number of patients who need to be enrolled in the trial and decrease the length of time it takes for a new anticancer therapeutic to be tested, approved, and made available to patients.
Basket trials allow researchers to test one anticancer therapeutic on a group of patients who all have the same type of genetic mutation, regardless of the anatomic site of the original cancer. Umbrella studies aim to identify the best therapy for different types of genetic mutations all within the same anatomic cancer type. Platform trials aim to assess multiple interventions against a disease and modify aspects of the trial design, if needed, by leveraging the accumulating data, thereby increasing the efficiency of the clinical research process. For example, this design allows researchers to terminate ineffective interventions or add new interventions during the study.
As our understanding of cancer biology continues to evolve and we uncover some of the most elusive questions in cancer medicine (see Cancer Development: Integrating Knowledge), clinical trial designs will need to evolve as well. Additionally, the design and conduct of clinical cancer research need to keep pace with the new wave of technological advances. Novel trial designs that leverage emerging approaches, such as comprehensive tumor profiling (e.g., of genome, transcriptome, proteome, microbiome, and metabolome, among others), real-world evidence and data, as well as inputs from patient advocacy communities and social media platforms, will be pivotal to advancing the frontier of cancer clinical trials (487)Subbiah V (2023) Nat Med, 29: 49. DOI: 10.1038/s41591-022-02160-z..
In addition, artificial intelligence (AI)–based strategies are being harnessed to further improve clinical research. Using data from multiple sources, including past clinical trials, tumor profiles, clinical data, and electronic health records from hospital systems, researchers are training AI algorithms to identify patients who are most likely to respond to an investigational therapeutic, simulate an investigational compound’s mechanism of action, or potentially even create virtual trial participants referred to as digital twins of human patients seen in the clinic (488)Katsoulakis E, et al. (2024) NPJ Digit Med, 7: 77. DOI: 10.1038/s41746-024-01073-0.. Researchers hope that such AI-driven approaches will help bypass the greatest barrier, the low rates of patient participation, in the conduct of clinical research. However, as with all other applications of AI, careful consideration should be given to ensure equitable benefits of these emerging approaches for all patient populations.
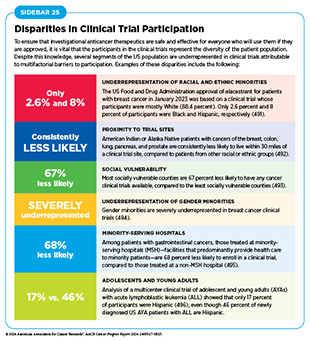
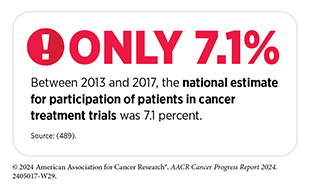
Low participation rate and a lack of sociodemographic diversity among those who do participate are two of the most pressing challenges in cancer clinical trials (see Sidebar 25). Low participation in clinical trials means that many trials fail to enroll enough patients to draw meaningful conclusions about the effectiveness of the anticancer therapeutic being tested. Lack of diversity in clinical studies means that the trial participant population does not match the actual national demographics of the cancer burden under study (490)National Academies of Sciences, Engineering, and Medicine. Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups. Accessed: June 25, 2024.. Diversity of participants is critical because the efficacy and safety of an intervention may differ among populations, e.g., among different racial and ethnic groups or between men and women. Underrepresentation in clinical trials compromises the applicability of the trial findings to the entire US patient population.
Understanding and eliminating barriers to clinical trial participation is vital if we are to accelerate the pace of progress against cancer for all patients. Numerous studies have investigated the existing barriers that limit participation of racial and ethnic minorities and other medically underserved populations in cancer clinical trials. These studies have identified a range of factors, such as lack of awareness of clinical trials, financial challenges, limited health literacy, inadequate or complete lack of insurance, medical distrust, implicit biases among health care providers, lack of trial availability, and narrow eligibility criteria, among others (497)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2022. Accessed: June 30, 2023.. Many of these barriers operate at individual, systemic, and societal levels (498)Kahn JM, et al. (2022) Cancer, 128: 216. DOI: 10.1002/cncr.33905..
As discussed in detail in AACR Cancer Disparities Progress Report 2024 (29)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2024. Accessed: June 14, 2024., increased knowledge of the barriers to clinical trial accrual is helping researchers, regulators, and policymakers design and implement evidence-based adaptations that can improve access of potential participants to clinical research. Interventions aimed at addressing social determinants of health (see Figure 3), modernizing trial design to ease patient participation, expanding eligibility criteria, improving the efficiency of data collection, including patient reported outcomes (PRO), and engaging in community outreach and patient navigation are being evaluated. Additionally, a critical area of focus for all stakeholders in medical research is fostering greater diversity, equity, and inclusion within the clinical research workforce so that it resembles the patient populations it serves.
US lawmakers and FDA are working on legislation and guidelines intended to increase the diversity of clinical trial participants (see Diversifying and Decentralizing Trials) (29)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2024. Accessed: June 14, 2024.. These include a diversity action plan that would require researchers and funders of clinical trials to submit concrete goals and needed steps for enrolling specific demographic groups in pivotal studies of new drugs (500)US Food and Drug Administration. Diversity Action Plans to Improve Enrollment of Participants from Underrepresented Populations in Clinical Studies. Accessed:.
COVID-19, despite its adverse effects on all aspects of cancer research and patient care, enabled researchers to decentralize certain aspects of clinical trials, so that lifesaving therapeutics could be brought quickly to as many patients as possible (24)American Association for Cancer Research. AACR Report on the Impact of COVID-19 on Cancer Research and Patient Care. Accessed: June 30, 2022..
Adaptations implemented during the pandemic, including consenting patients remotely, permitting telehealth for routine clinical assessments, delivering experimental drugs to patients, and allowing the use of local laboratory or imaging facilities accessible to patients, have offered a blueprint of success to further revise and reform clinical trials and the drug approval process for the benefit of all patients with cancer. Ongoing research must continue to evaluate the impact of these approaches on advancing our nation’s clinical cancer research efforts (501)Vanderpool RC, et al. (2024) J Natl Cancer Inst Monogr, 2024: 51. DOI: 10.1093/jncimonographs/lgae016..
Progress Across the Clinical Cancer Care Continuum
Research discoveries made as a result of innovative cancer science are continually being translated into new medical products for cancer prevention, early detection, diagnosis, and treatment. FDA approval of new medical products, including new anticancer treatments, is not the end of a linear research process. Rather, it is an integral part of the medical research cycle (see Figure 5) because observations made during the routine use of new medical products can help to accelerate the pace at which similar products are developed and to stimulate the development of new, more effective products.
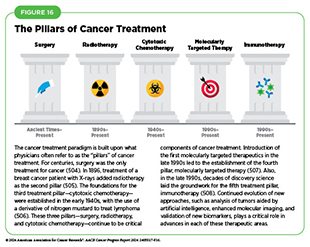
Traditionally, newly approved therapeutics are utilized alongside treatments already in use, including existing surgeries, radiotherapies, and cytotoxic chemotherapies, all of which continue to be the mainstays of clinical cancer care (see Figure 16). In recent years, there has been a rapid proliferation of molecularly targeted therapeutics and immunotherapeutics, the two newest pillars of cancer treatment, ushering in an era of personalized cancer medicine (see Figure 16). Additionally, researchers are continually evaluating new ways to refine the use of surgery, radiotherapy, and cytotoxic chemotherapeutics to improve survival and quality of life for patients.
As one example, since most prostate cancers grow slowly, active monitoring has been shown to be a safe management strategy for avoiding overtreatment and preventing undertreatment. In fact, evidence is emerging that active monitoring of the disease in patients with early-stage prostate cancer is a safe alternative to receiving immediate surgery or radiotherapy (502)Hamdy FC, et al. (2023) N Engl J Med, 388: 1547. DOI: 10.1056/NEJMoa2214122.. These findings are hopeful for patients who opt for active monitoring to avoid treatment-related adverse effects, such as sexual and incontinence problems. Similar observations have been noted among patients with certain types of thyroid cancer. Active monitoring of disease and surgery only after suspected progression has been shown to be associated with similar outcomes that are seen in patients undergoing immediate surgery (503)Levyn H, et al. (2024) JAMA Otolaryngol Head Neck Surg. DOI: 10.1001/jamaoto.2024.1699..
The following sections focus on the recent advances across the five pillars of cancer treatment, in particular, the 15 new anticancer therapeutics approved by FDA in the 12 months spanning this report, July 1, 2023, to June 30, 2024 (see Table 7 and Supplementary Table 1). During the same timeframe, FDA approved 15 previously approved anticancer therapeutics for treating additional types of cancer. Furthermore, FDA expanded the use of several previously approved therapeutics to include treatment at different timepoints during the course of clinical care or treatment of a different subtype of the same cancer. Comprehensive information on all anticancer therapeutic approvals can be found on FDA’s website. Because many of these treatments, particularly molecularly targeted therapeutics and immunotherapeutics, are relatively new to the clinic, their long-term and late effects are still unknown. The fast pace of approval and increasing clinical use of these cutting-edge therapeutics warrant close monitoring of patients receiving these novel agents.
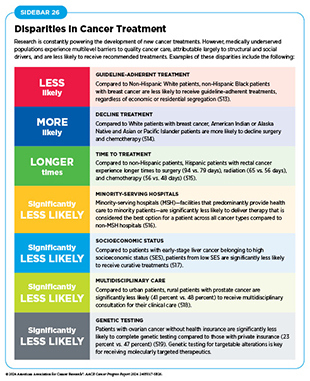
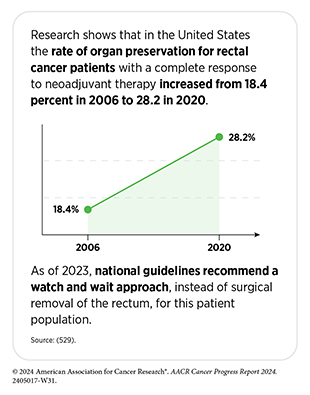
New medical products used across the continuum of clinical cancer care transform lives by extending survival and improving quality of life. However, not all patients receive the standard of care recommended for the type of cancer with which they have been diagnosed and the stage of cancer at the time of diagnosis (see Sidebar 26). Disparities in cancer treatment are driven largely by socioeconomic and structural factors such as lack of health insurance or of access to health care facilities as well as high costs of cancer care. Research has shown that racial disparities in survival for several cancer types can be eliminated when all patients have equivalent access to standard treatments (29)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2024. Accessed: June 14, 2024.. As one example, some studies have found no racial or ethnic disparities in cancer outcomes among patients who are treated at a single-payer system, such as the US Department of Veterans Affairs’ Veterans Health Administration, the nation’s largest integrated health care system (509)Kim RB, et al. (2024) J Racial Ethn Health Disparities, 00: 10.1007/s40615. DOI: 10.1007/s40615-024-02077-y..
Medicaid expansion through the Patient Protection and Affordable Care Act (ACA) has been shown to increase insured status, early diagnosis, and timely cancer treatment, and improve outcomes leading to reduced cancer disparities. As one example, a recent study evaluated the association between Medicaid expansion and time to breast cancer surgery and found that Medicaid expansion led to a significant reduction of disparity in surgery delays between White patients and patients from racial and ethnic minority populations (510)Tamirisa N, et al. (2023) Ann Surg: 00. DOI: 10.1097/SLA.0000000000006177.. Additionally, Medicaid expansion has been shown to reduce racial disparities in time to chemotherapy initiation between White patients with early-stage breast cancer and those belonging to racial and ethnic minority groups (511)Chavez-MacGregor M, et al. (2023) J Natl Cancer Inst, 115: 644. DOI: 10.1093/jnci/djad033.. It is imperative that all stakeholders committed to driving progress against cancer work together to ensure equitable access to quality cancer care.
Educating health care providers about the approval processes for relevant medical products is critical if they are to adequately advise patients about the risks and benefits associated with these treatments. Unfortunately, according to a recent national survey of physicians including oncologists, only 41 percent and 17 percent of respondents reported moderate or better understanding of FDA’s drug and medical device approval processes, respectively (512)Dhruva SS, et al. (2024) Health Aff (Millwood), 43: 27. DOI: 10.1377/hlthaff.2023.00466..
Advances in Cancer Treatment With Surgery
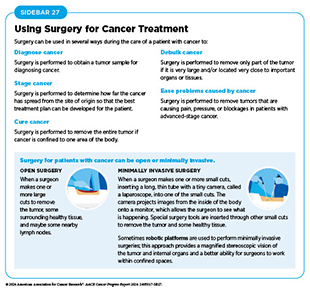
For centuries, surgery was the only pillar of cancer treatment (see Figure 16). Today, it remains the foundation of curative treatment for many patients. Surgery is used in several ways during the care of a patient with cancer (see Sidebar 27). Sometimes, additional therapy is given before, after, or around the time of surgery based on specifics of a patient’s situation (see Sidebar 28). Researchers have found that this approach not only improves the surgeon’s ability to remove the tumor (e.g., by shrinking the tumor when given before the surgery), but also increases the patient’s overall survival and/or quality of life (520)Burotto M, et al. (2019) Semin Oncol, 46: 83. DOI: 10.1053/j.seminoncol.2019.01.002..
Performing Less Invasive Cancer Surgery
Several recent studies have shown that less invasive surgeries— or avoiding surgeries altogether—may benefit certain patients by minimizing postprocedural complications without compromising and sometimes improving long-term outcomes (521)Topal H, et al. (2022) JAMA Netw Open, 5: e2248147. DOI: 10.1001/jamanetworkopen.2022.48147.(522)Son SY, et al. (2022) JAMA Surg, 157: 879. DOI: 10.1001/jamasurg.2022.2749.(523)Di Benedetto F, et al. (2023) JAMA Surg, 158: 46. DOI: 10.1001/jamasurg.2022.5697.(524)Bartels SAL, et al. (2023) J Clin Oncol, 41: 2159. DOI: 10.1200/Jco.22.01565.. A few examples of such findings are discussed below.
For breast cancer patients undergoing surgical resection, in addition to removing the breast tissue, surgeons often also remove what is called the sentinel lymph node, which is the first lymph node(s) to which the cancer is most likely to spread. Sentinel lymph node biopsy (SLNB) is a routine procedure during which the sentinel lymph node is identified, removed, and examined to determine whether cancer cells are present. Detection of cancer cells in sentinel lymph nodes through SLNB has been a standard of breast cancer care because it determines the extent of the disease and provides information that is central to the development of a patient’s treatment plan.
Historically, researchers believed that removing the axillary lymph nodes, which are the lymph nodes that run from the breast tissue into the armpit, could reduce the risk of metastases and cancer recurrence. Therefore, all axillary lymph nodes adjacent to the affected breast were removed in a surgery known as the axillary lymph node dissection (ALND). However, ALND is an invasive procedure associated with its own morbidity, particularly lymphedema, which causes swelling in the arms that can cause pain and problems in functioning (see Challenges Faced by Survivors).
More recently, studies have shown that ALND is not associated with any survival benefit compared to SLND and could thus be omitted for certain patients. Findings from a recent clinical trial suggest that patients whose breast cancers are 2 centimeters or smaller and whose axillary lymph nodes appear normal on ultrasonography can be safely spared SNLB without compromising their outcomes (525)Gentilini OD, et al. (2023) JAMA Oncol, 9: 1557. DOI: 10.1001/jamaoncol.2023.3759..
Another group for whom ALND could be omitted is patients with breast cancer who have an excellent response to chemotherapy given before surgery (neoadjuvant therapy) (see Sidebar 28). In a recent study, researchers found that patients with lymph node–positive breast cancer who no longer had any signs of cancer in their nodes following neoadjuvant chemotherapy rarely experienced cancer recurrence in their axillary nodes even without undergoing ALND (526)Montagna G, et al. (2024) JAMA Oncol. DOI: 10.1001/jamaoncol.2024.0578.. These findings support the omission of ALND in this patient population.
Additionally, certain patients with breast cancer can safely forgo ALND if they have no signs of metastasis in the axillary lymph nodes, as determined by a negative clinical examination of the axilla; have received guideline-adherent adjuvant treatments and radiation therapy, and in whom SLNB had revealed only one or two metastases. Evidence supporting this approach was obtained from a randomized clinical trial, in which breast cancer patients with the above characteristics had similar 5-year recurrence-free survival irrespective of whether they received ALND (527)de Boniface J, et al. (2024) N Engl J Med, 390: 1163. DOI: 10.1056/NEJMoa2313487..
Another patient population for whom less extensive surgery could be a safe and effective alternative are those with early-stage cervical cancer. Traditionally, most patients are treated with a radical hysterectomy, which involves removing the uterus, cervix, part of the vagina, and ligaments and tissues around the uterus. In contrast, a simple hysterectomy is a limited surgical procedure involving the removal of the uterus and the cervix.
Results of a recent clinical study among patients with early-stage cervical cancer who underwent either a radical hysterectomy or a simple hysterectomy showed that rates of cancer recurrence were low (less than 3 percent), regardless of the procedure the participant received (528)Plante M, et al. (2024) N Engl J Med, 390: 819. DOI: 10.1056/NEJMoa2308900.. However, those who had a simple hysterectomy experienced fewer side effects, such as urinary incontinence and urinary retention, and a better quality of life, supporting the use of the less extensive procedure.
While less invasive approaches to surgery are promising, it is vital that their benefits, as well as any adverse effects on long-term patient survival, are tested in rigorous, well-designed, larger and diverse clinical trials before they can become standard of care.
Visualizing Breast Cancer Cells More Precisely During Surgery
Breast cancer is the most common cancer and the second leading cause of cancer death in women in the United States. Many patients with breast cancer are treated with lumpectomy, also called breast-conserving surgery, a procedure performed to remove cancerous tissue and some normal tissue around it, but not the breast itself. Residual tumor cells left behind after surgery may pose a risk for breast cancer recurrence. Currently, surgeons use pathologic tests that identify tumor cells at or near the lumpectomy-derived tissue margin to determine residual tumor. However, these approaches are flawed, since a proportion of patients do experience local recurrence necessitating a second surgery.
In April 2024, FDA approved the imaging molecule pegulicianine (Lumisight) and the Lumicell Direct Visualization System for adult patients with breast cancer undergoing lumpectomy to help detect residual cancerous tissue within the breast following removal of the main tumor. Pegulicianine is injected into the patients 2 to 6 hours prior to surgery. The molecule reacts with enzymes that are found at high levels in and around tumor cells, which leads to a fluorescent signal that can be detected by a handheld probe and a tumor detection algorithm (Lumicell). The surgeon can thereby identify suspicious areas in the breast where residual cancer may remain after the main resection and perform a targeted removal of the suspicious tissue to avoid future surgeries.
The approval was based on findings of a clinical trial which showed that the imaging system helped detect and remove tumors left behind after standard lumpectomy in nearly 8 percent (27 out of 357) of patients who received the agent. In 19 of these 27 patients, standard pathology evaluation did not find any cancers, and the residual cancer would have been missed without Lumisight (530)Ghilardi G, et al. (2024) NEJM Evid, 3: EVIDoa2300213. DOI: 10.1056/EVIDoa2300213.. Further research is needed to overcome current limitations of this technology, including low sensitivity—not all patients who receive a negative result are free of residual cancer—as well as severe life-threatening allergic reactions in certain patients.
Advances in Radiation-based Approaches to Cancer Care
Radiotherapy is the use of high-energy rays (e.g., gamma rays and X-rays) or particles (e.g., electrons, protons, and carbon nuclei) to control or eradicate cancer. Discovery of X-rays in 1895 allowed visualization of internal organs at low doses, and the effective use of X-rays at high doses to treat a breast cancer patient a year later established radiotherapy as the second pillar of cancer treatment (see Figure 16). Radiotherapy plays a central role in the management of cancer and works primarily by damaging DNA, leading to cancer cell death.
There are many types and uses of radiotherapy (see Sidebar 29). However, it is important to note that radiotherapy may also have harmful side effects, partly because of the radiation-induced damage to healthy cells surrounding the tumor tissue (531)Wang K, et al. (2021) CA Cancer J Clin, 71: 437. DOI: 10.3322/caac.21689.. Because of the central role of radiotherapy in the treatment and management of cancer, researchers are continually innovating radiotherapeutic approaches to maximize the benefit for patients, while minimizing potential harms associated with the use of radiation (see A New Age of Radiation Therapy).
Despite the immense benefits of radiotherapy, the long-term effects can negatively impact a patient’s quality of life. Researchers are evaluating approaches to make radiotherapy safer and more effective and identify when radiotherapy can be reduced or even avoided without affecting the outcomes for patients. As one example, several recent studies have shown that patients with very low-risk early-stage breast cancer with certain molecular characteristics who received lumpectomy can forgo radiation therapy without any excess risk of cancer recurrence, as long as they receive guideline-adherent treatment with hormone therapies (532)Jagsi R, et al. (2024) J Clin Oncol, 42: 390. DOI: 10.1200/JCO.23.02270.(533)Whelan TJ, et al. (2023) N Engl J Med, 389: 612. DOI: 10.1056/NEJMoa2302344.(534)Mann GB, et al. (2024) Lancet, 403: 261. DOI: 10.1016/S0140-6736(23)02476-5..
Stereotactic body radiotherapy (SBRT) is an advanced approach to radiotherapy that can target radiation to tumors more precisely than traditional radiotherapy. Greater precision of the procedure means that higher doses of radiation can be used compared with traditional radiotherapy and that healthy tissues surrounding a tumor are spared from damage caused by the radiation, which can reduce the long-term adverse effects of radiotherapy. Given the potential benefits of SBRT, there are many clinical trials testing ways to incorporate these treatments into clinical cancer care. As one example, for certain patients with localized kidney cancer for whom surgery is not an option, SBRT was shown to be highly effective in keeping their cancers at bay and improving survival (535)Siva S, et al. (2024) Lancet Oncol, 25: 308. DOI: 10.1016/S1470-2045(24)00020-2.. This finding provides new hope to patients, especially those with large kidney tumors for whom surgery is not a viable option because of comorbidities such as obesity, cardiovascular disease, or chronic kidney disease.
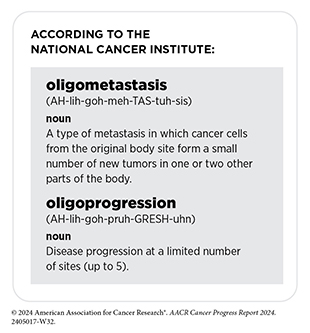
Historically, the main use of radiotherapy in the treatment of patients with metastatic cancer has been to reduce or control symptoms of disease. However, recent studies have shown that radiotherapy targeted to the initial cancer site from which tumors have metastasized can improve survival for patients who have metastatic tumors at a limited number of sites and are said to have oligometastatic disease (536)van Moorselaar RJA, et al. (2022) Eur Urol Open Sci, 35: 70. DOI: 10.1016/j.euros.2021.11.004.. Additionally, studies have shown that stereotactic radiotherapy targeted to oligometastatic or oligoprogressive tumors can reduce the chances of disease progression and increase survival for patients who have solid tumors, such as prostate cancer, lung cancer, or gynecologic cancers (537)Palma DA, et al. (2020) J Clin Oncol, 38: 2830. DOI: 10.1200/JCO.20.00818.(538)Donovan EK, et al. (2024) JAMA Oncol, 00: e241796. DOI: 10.1001/jamaoncol.2024.1796.(539)Chinniah S, et al. (2022) Int J Radiat Oncol Biol Phys, 114: 684. DOI: 10.1016/j.ijrobp.2022.07.014.. For example, according to findings from a new clinical trial, adding SBRT targeted at oligoprogressive sites to standard treatment for patients with lung cancer led to more than a four-fold increase in progression-free survival compared to standard treatment (540)Tsai CJ, et al. (2024) Lancet, 403: 171. DOI: 10.1016/S0140-6736(23)01857-3..
Another recent advance in radiotherapy is the emergence of hypofractionated radiotherapy, whereby patients receive fewer but higher doses of radiotherapy compared to the traditional regimen (541)Cho WK, et al. (2024) JAMA Oncol, 10: 737. DOI: 10.1001/jamaoncol.2024.0565.. Thus, patients who have hypofractionated radiotherapy complete their radiotherapy over a shorter period and in fewer treatment sessions. Researchers are also testing whether lowering the dose of radiotherapy, which may spare patients from many of the adverse effects of treatment, can still manage cancer effectively. As one example, a recent study suggests that an individualized radiation therapy regimen, including doses lower than those routinely administered for patients with non–small cell lung cancer (NSCLC), can still prevent tumor recurrence (542)Gensheimer MF, et al. (2023) JAMA Oncol, 9: 1525. DOI: 10.1001/jamaoncol.2023.3495..
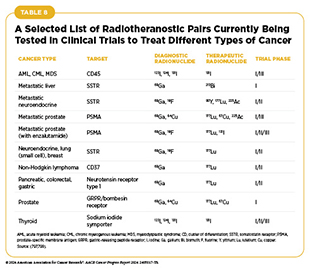
One of the most exciting new areas in radiation oncology is the use of molecularly targeted radiotherapeutics—radiation-emitting molecules that are linked to targeting molecules which steer the radiation specifically to cancer cells (see Emergence of Radiotheranostics). Several such therapeutics have been approved by FDA in recent years for the treatment of a variety of cancer types (1)American Association for Cancer Research. AACR Cancer Progress Report 2023. Accessed: Feb 29, 2024.(73)American Association for Cancer Research. AACR Cancer Progress Report 2021. Accessed: June 30, 2023.(543)American Association for Cancer Research. AACR Cancer Progress Report 2018. Accessed: June 30, 2022., and several more are at various stage of clinical testing (see Table 8). As one example, in January 2018, Lu-177 dotatate was approved for treating patients with gastroenteropancreatic neuroendocrine tumors whose cancer had progressed after prior treatments (543)American Association for Cancer Research. AACR Cancer Progress Report 2018. Accessed: June 30, 2022..
Research has shown that most neuroendocrine tumors have the protein somatostatin receptor on their cell surface. In Lu-177 dotatate, the radionuclide Lu-177 is linked to a molecule that is analogous to somatostatin which targets the radiation to somatostatin receptor–positive cancer cells. More recently, a clinical study showed that Lu-177 dotatate can be a promising option even as the initial therapy for certain patients with neuroendocrine tumors (544)Singh S, et al. (2024) Lancet, 403: 2807. DOI: 10.1016/S0140-6736(24)00701-3.. In the trial, patients who received Lu-177 dotatate lived nearly three times as long without their cancer getting worse, compared to the control group. These findings bring hope to many more patients with this rare but aggressive cancer.
Advances in Treatment With Cytotoxic Chemotherapy
Cytotoxic chemotherapy—use of chemicals to kill cancer cells—was first introduced as a pillar of cancer treatment in the early to mid-20th century (506)DeVita VT, Jr., et al. (2008) Cancer Res, 68: 8643.. Chemotherapy remains a backbone of cancer treatment and its use is continually evolving to minimize potential harm to patients, while maximizing its benefits.
As with surgery and radiotherapy, chemotherapy is more commonly used to treat cancer in combination with one or more additional types of treatments. Newer and more effective chemotherapeutics continue to be evaluated in clinical research. In addition, FDA routinely expands the use of previously approved chemotherapeutics for additional cancer types through review of new clinical trials, as well as by monitoring of current real-world use of such agents. The FDA Project Renewal leverages expertise of clinical researchers to review existing published literature on drug utilization and maintain updated labeling of older, commonly prescribed anticancer therapeutics. For instance, in September 2023, FDA approved updated labeling for the chemotherapeutic temozolomide (Temodar), which included new indications and dosing regimen.
Treatment with cytotoxic chemotherapeutics can have adverse effects. These can occur during treatment and continue in the long term, or they can appear months or even years later. Researchers are investigating different approaches to make chemotherapeutics safer for patients. Areas of ongoing investigation include designing modifiable chemotherapeutics, e.g., with “on” and “off ” switches, that are selectively delivered to tumors while sparing healthy tissue; evaluating less aggressive chemotherapy regimens that can allow patients the chance of an improved quality of life without compromising survival; identifying patients for whom chemotherapy has no added benefit; and identifying biomarkers such as circulating tumor DNA to correctly predict which patients will or will not benefit from chemotherapy.
As one example, data from a recent retrospective analysis showed that patients with a certain subtype of breast cancer, known as estrogen receptor (ER)–positive, human epidermal growth factor receptor 2 (HER2)–negative invasive lobular carcinoma, who are treated with hormone therapy do not derive any additional benefit from chemotherapy (545)Oztekin S, et al. (2024) Cancer, 130: 927. DOI: 10.1002/cncr.35125.. Data from a separate clinical trial showed that circulating tumor DNA can be a promising way to identify patients with colorectal cancer who can safely forgo postsurgical chemotherapy without a risk of cancer recurrence (546)Kasi PM, et al. (2024) J Clin Oncol, 42: 9. DOI: 10.1200/JCO.2024.42.3_suppl.9..
Another recent development in cancer chemotherapy was FDA approval of the chemotherapeutic melphalan as the first liver-directed treatment for uveal melanoma that has metastasized to the liver. Uveal melanoma is a rare cancer that develops in the eye and has a high tendency to metastasize. Liver metastases occur in up to 95 percent of patients with metastatic disease, and these lesions are often inoperable. The newly approved Hepzato kit includes melphalan and a device through which the chemotherapeutic is infused into the hepatic artery. This administration method allows for delivery of a higher dose of chemotherapy directly to the liver while avoiding toxicity to other tissues.
Advances in Treatment With Molecularly Targeted Therapeutics
Remarkable advances in our understanding of the biology of cancer, including the identification of numerous genetic mutations that fuel tumor growth, have set the stage for a new era of precision medicine, an era in which the standard of care for many patients is changing from a one-size-fits-all approach to one in which greater understanding of the individual patient and the characteristics of that patient’s cancer dictates the best treatment option for the patient (see Understanding the Path to Cancer Development).
Therapeutics directed to molecules influencing cancer cell multiplication and survival target tumor cells more precisely than cytotoxic chemotherapeutics, which generally target all rapidly dividing cells, and thereby limit damage to healthy tissues. The greater precision of these molecularly targeted therapeutics tends to make them more effective and less toxic than cytotoxic chemotherapeutics (see Sidebar 30). As a result, they are not only saving lives but also allowing patients with cancer to have a higher quality of life. Unfortunately, because of multilevel barriers to health care, including inadequate health insurance and lack of access to quality cancer care, there are disparities in the utilization of molecularly targeted treatments among patients from racial and ethnic minorities and other medically underserved populations (29)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2024. Accessed: June 14, 2024.(497)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2022. Accessed: June 30, 2023.. It is vital that ongoing research and future public health policies are aimed to ensure equitable access to precision cancer medicine, including tumor genetic testing and the receipt of molecularly targeted therapeutics for all patients.
In the 12 months spanning July 1, 2023, to June 30, 2024, FDA approved eight new molecularly targeted anticancer therapeutics (see Table 7). During this period, FDA also expanded the use of 11 previously approved molecularly targeted anticancer therapeutics for treating additional types of cancer.
Expanding Precision Treatments Against Common Cancer Types
Common cancer types are those that are diagnosed with the highest frequency in the United States. Cancers of the breast, colon and rectum, lung, and prostate are the most common cancers diagnosed, with an estimated 313,510, 152,810, 234,580, and 299,010 new cases, respectively, expected in the United States in 2024 (3)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: March 17, 2024.. Together, these cancers will be attributable to more than 40 percent of all cancer deaths in the United States in 2024. While researchers are continually evaluating new and improved treatments for these cancers and mortality from these diseases has been declining steadily, additional research and innovation are urgently needed considering the ongoing burden of these four cancers, especially in selected population groups, e.g., colorectal cancer in people younger than 50 and lung cancers among women without a history of smoking. FDA decisions made during the 12 months covered in the report are providing new and expanded therapeutic options for patients with breast, colorectal, lung, and prostate cancers.
Despite major advances in the treatment of breast cancer, this disease is the second leading cause of cancer-related death for women in the United States (2)American Cancer Society. Cancer Facts and Figures 2024. Accessed: July 10, 2024.. A recent FDA decision has the potential to further accelerate progress against breast cancer because it has provided a new molecularly targeted treatment option for certain patients with the disease.
For patients with breast cancer, one factor determining what treatment options should be considered is the presence or absence of three tumor biomarkers, two hormone receptor (HR) proteins and the HER2 protein. About 70 percent of breast cancers diagnosed in the United States are characterized as HR-positive and HER2-negative (547)Giaquinto AN, et al. (2022) CA Cancer J Clin, 72: 524. DOI: 10.3322/caac.21754.. Potential treatment options for these patients include an antihormone therapeutic, such as tamoxifen, which works by preventing the hormone estrogen from attaching to its receptor; or letrozole, which works by lowering the level of estrogen in the body; or fulvestrant, which works by destroying estrogen receptors (ER), alongside a cyclin-dependent kinase 4/6 inhibitor. Treatment with anti-hormone therapeutics is also called endocrine therapy.
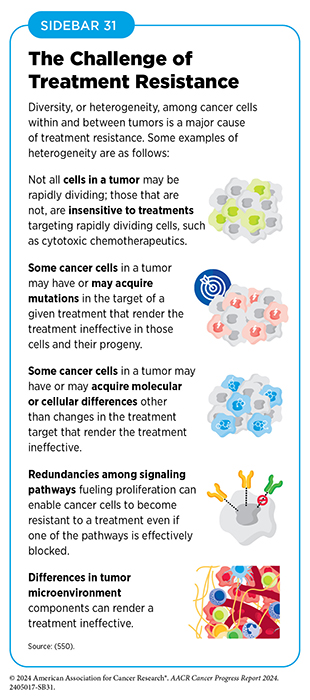
Unfortunately, most advanced, HR-positive breast cancers that initially respond to endocrine therapy eventually progress because they have become treatment resistant (see Sidebar 31). Research has shown that a signaling pathway that is vital for driving cell multiplication and survival, and involves PI3K, AKT, and PTEN proteins, is overactivated in approximately half of HR-positive, HER2-negative breast cancers through activating mutations in PI3K or AKT and/or inactivating mutations in PTEN (548)Turner NC, et al. (2023) N Engl J Med, 388: 2058. DOI: 10.1056/NEJMoa2214131.. Overactivation of PI3K–AKT–PTEN signaling has been implicated in the development of endocrine therapy resistance. A molecularly targeted therapeutic, alpelisib (Piqray), which blocks the function of PI3K, was approved for patients with breast cancer in 2019.
The protein AKT, also known as protein kinase B, plays a central role in PI3K–AKT–PTEN signaling. In November 2023, FDA approved capivasertib (Truqap), the first molecularly targeted therapeutic that acts by blocking the function of AKT. Together with alpelisib, capivasertib is now the second molecularly targeted therapeutic against the PI3K–AKT–PTEN pathway and is benefiting patients with invasive lobular breast cancer such as Julia K. Levine.
Capivasertib was approved for use in combination with fulvestrant for adult patients with HR-positive, HER2-negative locally advanced or metastatic breast cancer with one or more alterations in PI3K, AKT, or PTEN genes, as detected by an FDA-approved test, following progression on or after endocrine therapy. At the same time, FDA also approved the FoundationOne CDx assay as a companion diagnostic test (see Sidebar 32) to identify patients with breast cancer who are eligible for treatment with capivasertib. FDA approval was based on results from a phase III clinical trial showing that patients who received capivasertib along with fulvestrant had a 40 percent reduction in the risk of disease progression or death compared to patients who received fulvestrant alone and that adding capivasertib to fulvestrant almost doubled the time before disease progression (548)Turner NC, et al. (2023) N Engl J Med, 388: 2058. DOI: 10.1056/NEJMoa2214131..
Colorectal cancer is the fourth most common cancer and the second most common cause of cancer mortality in the United States. Notably, over the past few decades, there has been an increase in the incidence of early-onset (those cases diagnosed in patients younger than 50 years) colorectal cancer. Additionally, the incidence and mortality are higher in certain racial and ethnic minority groups, such as American Indian or Alaska Native and Non-Hispanic Black populations (3)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: March 17, 2024..
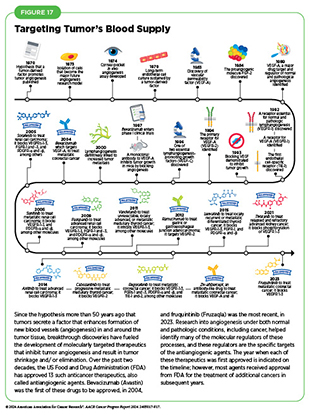
Solid tumors such as colorectal cancers can be highly dependent on the growth of new blood and lymphatic vessels, a phenomenon referred to as angiogenesis, to grow and survive (see Systems That Enable Cancer Progression). Thus, targeting these key components of the tumor microenvironment (see Sidebar 8) provides an ideal avenue for therapeutic intervention. In fact, researchers have developed many molecules, called antiangiogenic drugs or angiogenesis inhibitors, that work in similar ways to impede the growth of the new blood and lymphatic vessel networks that enable cancer cells to thrive, and as of June 30, 2024, FDA has approved 13 such therapeutics (see Figure 17).
Antiangiogenic drugs mainly function by stopping members of a family of growth-promoting proteins called VEGFs from activating the molecules they attach to, VEGF receptors, which are abundant on the cells that make up blood and lymphatic vessel walls and are vital for blood vessel formation. Antiangiogenic therapeutics have had the biggest impact for adult patients with the most common type of kidney cancer, renal cell carcinoma. However, they also greatly benefit patients with the most aggressive form of liver cancer, as well as those with some forms of pancreatic cancer; some gastrointestinal stromal tumors and soft-tissue sarcomas; and some thyroid, lung, and colorectal cancers.
A new therapeutic option in this growing class of drugs is fruquintinib (Fruzaqla), which was approved by FDA in November 2023 for the treatment of patients with metastatic colorectal cancer who received prior treatments with chemotherapy, an anti-VEGF therapeutic, and for certain patients an EGFR-targeted therapy. The approval was based on findings from two phase III clinical trials, both of which showed superior survival among patients treated with fruquintinib compared to their respective control groups (552)Li J, et al. (2018) JAMA, 319: 2486. DOI: 10.1001/jama.2018.7855.(553)Dasari A, et al. (2023) Lancet, 402: 41. DOI: 10.1016/S0140-6736(23)00772-9.. This new treatment is taken orally and offers a survival benefit for patients who have received multiple prior therapies and may be out of options, thereby fulfilling a critical unmet need in metastatic colorectal cancer.
Nearly 80 percent of lung cancers diagnosed in the United States are classified as non–small cell lung cancers (NSCLC). In the past decade, researchers have significantly increased our understanding of the genetic changes that fuel NSCLC growth, which has led to the development of therapeutics that target many of these changes (see Figure 1). Despite the emergence of numerous molecularly targeted therapeutics as groundbreaking new treatments for NSCLC, and the evidence showing that targeted treatments guided by molecular testing of the tumor yield superior outcomes for patients with NSCLC (554)Scott JA, et al. (2024) JCO Oncology Practice, 20: 145. DOI: 10.1200/op.22.00611., molecular testing rates and targeted therapy use remain low and there are wide variations across health care practices (555)Roberts TJ, et al. (2023) JAMA Netw Open, 6: e2310809. DOI: 10.1001/jamanetworkopen.2023.10809.. Broad implementation of cutting-edge molecular testing to simultaneously identify all genetic alterations driving NSCLC that could be therapeutically targeted offers meaningful benefits to patients and is estimated to be cost-effective (556)Lemmon CA, et al. (2023) JCO Precis Oncol, 7: e2200294. DOI: 10.1200/PO.22.00294..
About 2 percent of NSCLC cases are fueled by genetic alterations known as chromosomal translocations that involve the ROS1 gene and lead to the production of ROS1 fusion proteins (557)Drilon A, et al. (2024) N Engl J Med, 390: 118. DOI: 10.1056/NEJMoa2302299.. The ROS1-targeted therapeutic repotrectinib (Augtyro) was approved by FDA in November 2023 for patients with advanced or metastatic NSCLC with ROS1 fusions as an initial treatment or as the second treatment in those who previously received another ROS1-targeted drug. FDA approval was based on findings from a clinical trial that showed tumor shrinkage in nearly 80 percent of the study participants who had not previously received a ROS1-targeted drug, and in nearly 40 percent of participants who had already received another ROS1-targeted drug, such as crizotinib (Xalkori) or entrectinib (Rozlytrek) (557)Drilon A, et al. (2024) N Engl J Med, 390: 118. DOI: 10.1056/NEJMoa2302299.. The median time before the disease worsened was nearly 36 months among participants who had not previously received a ROS1-targeted drug and 9 months among those who had previously received a ROS1-targeted drug.
Notably, repotrectinib was able to shrink tumors that had spread to the brain, a common location for lung metastases. Another advantage of treatment with repotrectinib is that it is effective against tumors expressing certain mutated forms of ROS1, including one called G2032R, that render other ROS1-targeted drugs, crizotinib and entrectinib, ineffective. In the clinical trial, nearly 60 percent of patients whose tumors had the G2032R mutation responded to repotrectinib.
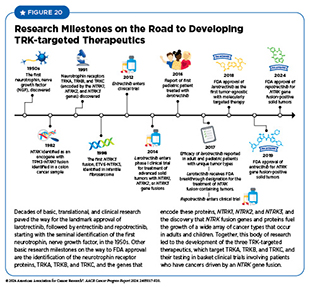
In addition to ROS1 fusion protein, repotrectinib also targets three other related proteins called TRKA, TRKB, and TRKC. The genes NTRK1, NTRK2, and NTRK3 provide the code that cells use to make these proteins. Research has shown that chromosomal translocations that involve the three NTRK genes and lead to the production of TRK fusion proteins drive the growth of up to 1 percent of all solid tumors. A significant advance in precision medicine during the 12 months spanning this report was the FDA approval of repotrectinib to treat adult and pediatric patients aged 12 years or older (see Research-driven Progress Against Childhood and AYA Cancers) who have solid tumors that test positive for the NTRK gene fusions.
Although in this section we focus on the approval of new anticancer therapeutics, it should be noted that several previously approved molecularly targeted therapeutics received expanded approval by FDA for the treatment of additional cancer types in the 12 months covered by the report. As one example, in August 2023, FDA approved the molecularly targeted therapeutic niraparib (Zejula) in combination with the antihormone therapy abiraterone acetate (Zytiga), for certain adult patients with metastatic prostate cancer that is fueled by a mutation in the BRCA gene, as determined by an FDA-approved test.
This was the first FDA approval of niraparib for the treatment of prostate cancer. However, it was previously approved for treatment of certain patients with cancers of the ovary, fallopian tube, and peritoneum. Along with olaparib (Lynparza) and rucaparib (Rubraca), two additional molecularly targeted therapeutics that work in the same way and have already been approved by FDA, the recent approval of niraparib expands available treatment options for metastatic prostate cancer harboring a mutation in the BRCA gene.
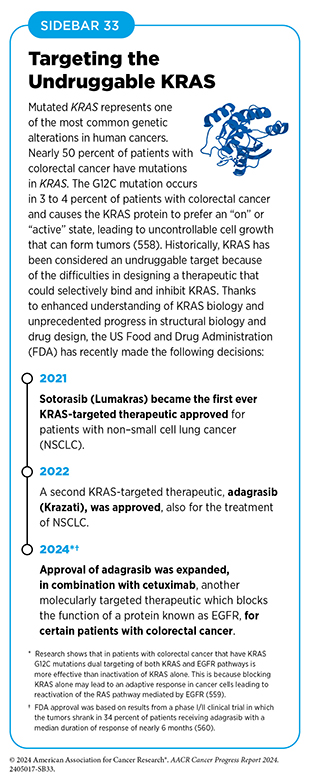
Another significant expansion of a previously approved therapeutic that occurred in the 12 months covered in this report was the June 2024 FDA approval of the KRAS-targeted therapeutic adagrasib (Krazati) in combination with cetuximab (Erbitux) for adults with locally advanced or metastatic colorectal cancer that has a mutation known as KRAS G12C, as determined by an FDA-approved test (see Sidebar 33). Adagrasib was previously approved for certain patients with NSCLC and ongoing research is evaluating its efficacy in patients with pancreatic cancer, such as Dr. Humberto M. Guiot.
Personalizing Treatment for Patients With a Rare Solid Tumor
Rare cancer is defined by the National Cancer Institute (NCI) as cancer that occurs in fewer than 15 out of 100,000 people each year. Rare cancers can be challenging for researchers to study and for physicians to treat (see Sidebar 34). During the 12 months covered by this report (July 1, 2023, to June 3, 2024), FDA approved molecularly targeted therapeutics and immunotherapeutics for treating a number of rare cancers, bringing the promise of precision medicine to patients who often have few treatment options.
NCI has launched several initiatives with the goal of accelerating the pace of basic, translational, and clinical research in rare cancers. As one example, the My Pediatric and Adult Rare Tumor (MyPART) Network is a group of scientists, patients, family members, advocates, and health care providers working together to find treatments for rare cancers in childhood, teen, and young adults faster.
Desmoid tumors are an extremely rare and potentially debilitating condition that affects an estimated 1,650 people in the United States each year. Also known as aggressive fibromatosis, desmoid tumors mainly affect young individuals but can also develop in people of any age. Those with the inherited condition familial adenomatous polyposis are at a particularly high risk (see Table 4) (561)Bektas M, et al. (2023) Adv Ther, 40: 3697. DOI: 10.1007/s12325-023-02592-0.. While desmoid tumors do not have the ability to metastasize, they grow fast and can invade locally, causing debilitating pain and deformity and, in extreme cases, life-threatening organ damage.
Currently, there is no standard treatment for desmoid tumors. Surgery and chemotherapy are the most common interventions, but the disease comes back often after treatment. Therefore, the first-ever FDA approval of a therapeutic for adults with desmoid tumors, nirogacestat (Ogsiveo), in November 2023 is a major breakthrough for these patients. Nirogacestat blocks the activity of an enzyme called gamma secretase, which is involved in driving desmoid tumor growth through the activation of a signaling protein called Notch. Researchers have hypothesized that desmoid tumors produce high amounts of Notch protein, which is thought to drive their growth.
FDA approval was based on results of a phase III clinical trial in which 41 percent of patients treated with nirogacestat had tumor shrinkage, compared to only 8 percent of those in the control group (562)Gounder M, et al. (2023) N Engl J Med, 388: 898. DOI: 10.1056/NEJMoa2210140.. Among patients who had tumor shrinkage with nirogacestat, tumors completely disappeared in 7 percent of people, compared to none in the control group. After 2 years of treatment, there was no evidence of tumors getting worse in 75 percent of patients who received nirogacestat, compared to just 44 percent of patients in the control group.
Adding Precision to the Treatment of Blood Cancers
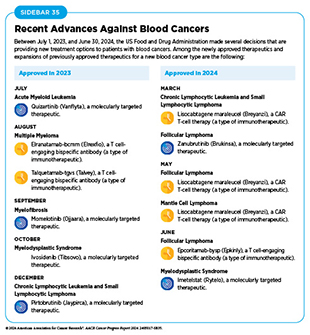
Cancers that arise in blood-forming tissues, such as the bone marrow, or in cells of the immune system, are called blood cancers, or hematologic cancers. In the 12 months covered by this report, FDA has made numerous decisions that are transforming the lives of patients with a wide array of hematologic cancers (see Sidebar 35).
Acute myeloid leukemia (AML) is the most commonly diagnosed leukemia in the United States, with 20,800 new cases anticipated in 2024 (2)American Cancer Society. Cancer Facts and Figures 2024. Accessed: July 10, 2024.. AML has only 32 percent overall 5-year relative survival rate, the lowest among leukemias (3)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: March 17, 2024.. Research has substantially increased our understanding of the biology of AML, in particular the different types of genetic mutations that promote AML development. This knowledge is fueling the emergence of molecularly targeted therapeutics for defined groups of patients with the disease.
Mutations in the FLT3 gene promote the multiplication and survival of AML cells in 25 to 30 percent of cases, and patients with this type of AML have particularly poor outcomes (563)Yanada M, et al. (2005) Leukemia, 19: 1345. DOI: 10.1038/sj.leu.2403838.. In July 2023, FDA approved a new molecularly targeted therapeutic, quizartinib (Vanflyta), for treating adults who have newly diagnosed AML that tests positive for a mutation in the FLT3 gene known as FLT3 internal tandem duplication (ITD). The approval was based on results from a phase III clinical trial showing that patients who received quizartinib along with standard chemotherapy lived more than twice as long as those who received standard treatment alone (564)Erba HP, et al. (2023) Lancet, 401: 1571. DOI: 10.1016/S0140-6736(23)00464-6.. Quizartinib can cause several cardiac side effects and is therefore available only through a restricted program.
At the same time that FDA made the decision about quizartinib, it expanded the use of the LeukoStrat CDx FLT3 Mutation Assay as a companion diagnostic to identify patients with FLT3 ITD mutation–positive AML who are eligible for treatment with the new molecularly targeted therapeutic. Quizartinib is the third FLT3-targeted drug approved for the treatment of patients with AML and, along with midostaurin (Rydapt) and gilteritinib (Xospata) previously approved by FDA, expands treatment options for the subset of AML patients with the FLT3 alteration.
Myelodysplastic syndromes (MDS) are defined by the National Cancer Institute (NCI) as a diverse group of cancers in which immature blood cells in the bone marrow do not mature or become healthy blood cells. A third of patients diagnosed with MDS may progress to AML. Healthy bone marrow produces immature blood cells called stem cells, which develop into three types of mature blood cells: red blood cells, white blood cells, and platelets. In the case of MDS, the stem cells may not mature, or have a shorter life span, resulting in fewer than normal mature blood cells in the circulation.
Patients with MDS who have symptoms such as anemia, caused by their low blood cell counts, may receive curative treatments, including chemotherapy followed by stem cell transplant, or supportive care using molecularly targeted therapeutics and immune modulating agents as well as blood transfusion and erythropoiesis-stimulating agents to improve their quality of life. Unfortunately, MDS patients frequently become dependent on red blood cell transfusions, which can be associated with long-term adverse health consequences. There is an urgent need to develop better treatments that can provide patients with long-term independence from continuously receiving red blood cell transfusions.
Telomeres are protective caps at the end of chromosomal DNA that prevent damage to the inner protein-coding sequences of DNA. Telomeres naturally shorten each time a cell divides and eventually become too short to protect the DNA. This signals a normal cell to stop multiplying or to initiate cell death, thereby preventing unregulated multiplication that is a characteristic of cancer. Most cancer cells, including abnormal bone marrow cells in low-risk MDS, express telomerase, a protein that restores telomere length. By maintaining telomere length, cancer cells avoid telomere shortening and circumvent limitations to DNA replication. This allows cancer cells to multiply indefinitely.
In June 2024, FDA approved imetelstat (Rytelo) for the treatment of adult patients with lower-risk MDS with transfusion-dependent anemia who require four or more red blood cell units over 8 weeks and for whom erythropoiesis-stimulating agents are not an option. This is the first approval of a molecularly targeted therapeutic that works by blocking telomerase.
As a telomerase inhibitor, imetelstat works by preventing telomerase from performing its telomere-restoring function, thereby killing cancerous cells in the bone marrow that cause MDS. However, research indicates that the anticancer effect of imetelstat may also be driven by a novel cell death–promoting mechanism independent of telomere shortening (565)Bruedigam C, et al. (2024) Nat Cancer, 5: 47. DOI: 10.1038/s43018-023-00653-5.. FDA approval was based on the findings of a phase III clinical trial that showed significantly improved red blood cell transfusion independence among certain MDS patients treated with imetelstat compared to the control group (566)Platzbecker U, et al. (2024) Lancet, 403: 249. DOI: 10.1016/S0140-6736(23)01724-5..
Myelofibrosis is a rare type of blood cancer with an incidence rate of 1.5 cases per 100,000 people in the United States (567)National Organization for Rare Diseases. Primary Myelofibrosis – Symptoms, Causes, Treatment. Accessed: July 31, 2024.. In more than 50 percent of cases, myelofibrosis is driven by mutations in the JAK2 gene. In September 2023, FDA approved a new JAK2-targeted therapeutic, momelotinib (Ojjaara), for treating certain patients who have myelofibrosis.
Myelofibrosis is one of a group of six blood cancers called chronic myeloproliferative neoplasms: chronic myelogenous leukemia, polycythemia vera, primary myelofibrosis, essential thrombocythemia, chronic neutrophilic leukemia, and chronic eosinophilic leukemia. In some cases, polycythemia vera and essential thrombocythemia progress to become myelofibrosis. In this situation, the disease is referred to as secondary myelofibrosis.
Myelofibrosis usually develops slowly. Abnormal blood cells and fibers build up inside the bone marrow, which is where blood cells are made, leading to low levels of red blood cells (anemia). This causes tiredness, weakness, and shortness of breath. In addition, to make up for the low number of blood cells, the spleen begins to make blood cells, which causes the spleen to enlarge dramatically, a condition known as splenomegaly.
The likely outcome for patients diagnosed with myelofibrosis is estimated based on several risk factors. Patients with one to four risk factors—including being aged 65 or older; having anemia; experiencing fever, night sweats, or weight loss; having high white blood cell counts; and having at least 1 percent of blood cells being cancerous—are classified as having intermediate risk disease. Patients with four or more risk factors are classified as high risk.
Momelotinib was approved for treating intermediate- or high-risk myelofibrosis, including secondary myelofibrosis in adults with anemia. The approval was based on results from a phase III clinical trial that showed that treatment with momelotinib significantly reduced spleen volume and reduced myelofibrosis-related symptoms compared to placebo (568)Verstovsek S, et al. (2023) Lancet, 401: 269. DOI: 10.1016/S0140-6736(22)02036-0..
Advances in Treatment With Immunotherapeutics
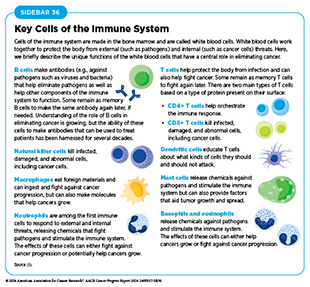
The immune system is a complex network of cells (called white blood cells; see Sidebar 36), tissues (e.g., bone marrow), organs (e.g., thymus), and the substances they make that help the body fight infections and other diseases, including cancer. The immune system actively detects threats from external (such as viruses and bacteria) and internal sources (such as abnormal or damaged cells) and works to eliminate them from the body.
The immune system is highly effective in detecting and eliminating cancer cells, a process also known as cancer immune surveillance (112)Hiam-Galvez KJ, et al. (2021) Nat Rev Cancer, 21: 345. DOI: 10.1038/s41568-021-00347-z.. However, as cancer cells acquire new properties during the course of cancer development (see Understanding the Path to Cancer Development), some cells find ways to “hide” from the immune system, such as by decreasing or eliminating the numbers and/or amounts of proteins on the surface of tumor cells that are used by the immune system to recognize cancer cells; triggering certain brakes on immune cells that prevent them from eradicating cancer cells; and releasing molecules that weaken the ability of immune cells to detect and destroy cancer cells (569)Mishra AK, et al. (2022) Diseases, 10: 60. DOI: 10.3390/diseases10030060.. Ongoing research is focused on better understanding how tumor cells evade the immune system and leveraging this knowledge to develop novel cancer treatments.
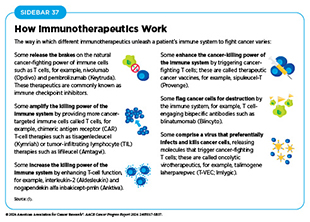
Advances in understanding of how the immune system detects and destroys cancer cells in the human body has invigorated the field of cancer immunology and has firmly established immunotherapy as the fifth pillar of cancer medicine (570)Kaufmann SHE (2019) Front Immunol, 10: 684. DOI: 10.3389/fimmu.2019.00684.. Cancer immunotherapy refers to any treatment that works by using the immune system to fight cancer. There are various ways in which different immunotherapeutics unleash the immune system to fight cancer (see Sidebar 37).
Releasing the Brakes on the Immune System
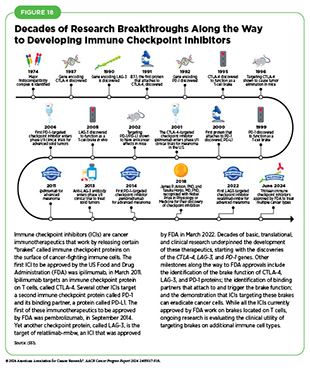
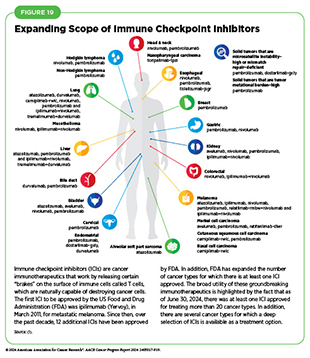
Decades of research have revealed that some tumor cells have increased levels of certain proteins on their surface that attach to and activate “brakes” on T cells, thus stopping them from attacking cancer cells (see Figure 18). These brakes are proteins on the surface of T cells and are called immune checkpoint proteins. Immune checkpoint inhibitors (ICIs) are a class of transformative new therapeutics that can release the brakes on T cells and trigger previously restrained T cells to attack and destroy cancer cells (83)Marin-Acevedo JA, et al. (2021) J Hematol Oncol, 14: 45. DOI: 10.1186/s13045-021-01056-8..
The use of ICIs in the treatment of cancer has rapidly expanded over the past decade and these therapeutics are considered one of the most exciting approaches to cancer treatment. This is in part because some patients with metastatic disease who have been treated with these therapeutics have had remarkable and durable responses. As one example, long-term results from a clinical trial testing the ICI pembrolizumab in patients with advanced NSCLC showed that 23 percent of patients lived 5 or more years after the treatment, which stands in stark contrast to the historically low 5-year relative survival rate for these patients of just about 5 percent (571)Garon EB, et al. (2019) J Clin Oncol, 37: 2518. DOI: 10.1200/Jco.19.00934.. Recent analysis suggests that the use of ICIs is also favorably associated with patients’ quality of life (572)Pala L, et al. (2022) JAMA Netw Open, 5: e2226252. DOI: 10.1001/jamanetworkopen.2022.26252..
During the 12 months spanning this report (July 1, 2023–June 30, 2024), FDA approved two new ICIs—tislelizumab-jsgr (Tevimbra) and toripalimab-tpzi (Loqtorz)—and expanded the uses of two of the previously approved ICIs—durvalumab (Imfinzi) and pembrolizumab (Keytruda)—to treat additional cancer types. These approvals mean that, as of June 30, 2024, FDA has approved 13 ICIs, targeting one of three different
T-cell brakes, CTLA-4, PD-1/PD-L1, or LAG-3. Additionally, these groundbreaking treatments are now approved for treating more than 20 cancer types as well as for treating any type of solid tumor characterized by the presence of certain molecular characteristics (see Figure 19).
In October 2023, FDA approved a new PD-1–targeted ICI, toripalimab-tpzi, for the treatment of patients with nasopharyngeal carcinoma, a rare form of head and neck cancer with a high prevalence in certain parts of Asia. This is the first approval of an immunotherapeutic for this cancer. Toripalimab-tpzi was approved as an initial treatment for people with nasopharyngeal carcinoma that has come back or metastasized as well as for patients with recurrent or metastatic disease that has gotten worse despite standard chemotherapy.
FDA approval was based on the findings from two clinical trials that evaluated toripalimab-tpzi in patients with advanced nasopharyngeal carcinoma. In one trial, toripalimab-tpzi treatment shrank tumors or prevented them from growing in certain patients whose cancer had gotten worse despite previous treatment with standard chemotherapy. The second trial was a phase III clinical study which showed that patients treated with toripalimab-tpzi and chemotherapy lived for a median of 21.4 months without their cancer getting worse, compared to 8.2 months for those treated with chemotherapy alone (573)Mai HQ, et al. (2023) JAMA, 330: 1961. DOI: 10.1001/jama.2023.20181.. Additionally, overall survival after 3 years of starting treatment was 64 percent among patients treated with toripalimab-tpzi and chemotherapy compared to 49 percent for those treated with chemotherapy alone.
The approval of toripalimab-tpzi is a significant advance for patients with nasopharyngeal carcinoma, a cancer for which surgery is generally not a good option due to the location of the tumors and for which there is no standard treatment once the cancer has progressed after chemotherapy.
The second new ICI approved in the 12 months covered in this report is tislelizumab-jsgr (Tevimbra). In March 2024, it was approved for treating patients who have surgically inoperable or metastatic squamous cell carcinoma of the esophagus that has progressed despite cytotoxic chemotherapy. Although esophageal cancer is rare—22,370 new cases expected in 2024 in the United States—it is one of the deadliest; the 5-year relative survival rate for patients diagnosed with the disease is just 22 percent (3)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: March 17, 2024.. The approval of tislelizumab-jsgr was based on results from a phase III clinical trial in which it was shown that the ICI significantly improved overall survival compared with standard cytotoxic chemotherapy (574)Xu J, et al. (2023) Lancet Oncol, 24: 483. DOI: 10.1016/S1470-2045(23)00108-0..
ICIs have yielded extraordinary benefits for many patients, but they can also have adverse effects, particularly the induction of autoimmune-like conditions. This occurs because ICIs release the brakes not only on cancer-fighting immune cells but also on some that recognize and injure normal tissues. To predict which patients are likely to experience adverse events and design treatments to combat these events without compromising the anticancer efficacy of the ICI, researchers must understand better why and how the adverse effects arise.
Identifying cellular and molecular markers that can predict whether ICIs are likely to work in a patient is an area of extensive research investigation (575)Westcott PMK, et al. (2023) Nat Genet, 55: 1686. DOI: 10.1038/s41588-023-01499-4.. Such biomarkers can help patients avoid unnecessary treatments and ICI-related toxicities including potential financial toxicities arising from high costs of these treatments (see Challenges Faced by Survivors), and can also help avoid delaying potentially more effective treatments. Another important area of scientific inquiry is to identify behavioral and clinical factors, such as diet, physical activity, gut microbiome composition, and optimal combinations with other therapeutic modalities that can boost the efficacy of ICIs and increase the number of patients who respond favorably to these lifesaving treatments.
ICIs have transformed the clinical care of patients with a diverse array of cancer types, including historically intractable diseases, such as metastatic melanoma, lung cancer, and kidney cancer (576)Topalian SL, et al. (2019) JAMA Oncol, 5: 1411. DOI: 10.1001/jamaoncol.2019.2187.. While their use was initially limited to people with very advanced cancers that were no longer responding to standard treatments, ICIs are increasingly being approved as first-line, or initial, treatments for patients. Researchers are also evaluating how to best integrate the use of ICIs in combination with standard treatments such as surgery, radiation therapy, and/or chemotherapy in patients with early-stage cancers (577)Schmid P, et al. (2020) N Engl J Med, 382: 810. DOI: 10.1056/NEJMoa1910549.(578)Altorki NK, et al. (2021) Lancet Oncol, 22: 824. DOI: 10.1016/S1470-2045(21)00149-2.. One area of extensive research is the use of these therapeutics before initial surgery, known as neoadjuvant treatment, in people with locally advanced cancers that are largely restricted to the tissue of origin.
Enhancing Immune Cell Function
Immune cells communicate with each other and with their surrounding cells through direct contact as well as through the release of a class of molecules called cytokines. Cytokines are also produced by nonimmune cells and play an essential role in rapidly activating the immune system in response to cellular stresses, such as infection, inflammation, and cancer (579)Liu C, et al. (2021) Adv Sci (Weinh), 8: e2004433. DOI: 10.1002/advs.202004433..
For many decades, researchers have been investigating the natural ability of cytokines, such as interferons and interleukins, to boost the cancer-killing function of the immune system (580)Conlon KC, et al. (2019) J Interferon Cytokine Res, 39: 6. DOI: 10.1089/jir.2018.0019.. Although cytokines have shown some promise, their success has been limited. One limitation is that cytokines do not persist very long in the body, so ongoing research is developing more stable versions of cytokines (581)Xue D, et al. (2021) Antib Ther, 4: 123. DOI: 10.1093/abt/tbab014.. Another challenge is the significant adverse effects when cytokines are given as a systemic treatment—treatment that is administered through the bloodstream and affects cells all over the body. Researchers are exploring ways to enhance the efficacy of cytokines while minimizing their side effects, for example, by delivering them in or near tumors (581)Xue D, et al. (2021) Antib Ther, 4: 123. DOI: 10.1093/abt/tbab014..
FDA approval of nogapendekin alfa inbakicept-pmln (Anktiva) in April 2024 was a major advance in the field of interleukin-based cancer immunotherapy. Nogapendekin alfa inbakicept-pmln was approved for the treatment of adult patients with non–muscle-invasive bladder cancer (NMIBC) that did not respond to Bacillus Calmette-Guérin (BCG) treatment. The treatment is intended for patients with carcinoma in situ, which refers to very early cancer cells in the inner layer of the bladder lining, who may or may not also present with papillary tumors, which are unusual growths that start in the bladder lining and extend into the center of the bladder.
More than 83,000 new cases of bladder cancer will be diagnosed in the United States in 2024 (2)American Cancer Society. Cancer Facts and Figures 2024. Accessed: July 10, 2024.. NMIBC—a type of bladder cancer that has grown through the lining of the bladder but has not yet invaded the muscle layer—makes up around 75 percent of all new cases of bladder cancer (583)Mond HG, et al. (1981) Pacing Clin Electrophysiol, 4: 304. DOI: 10.1111/j.1540-8159.1981.tb03699.x.. Patients with high-risk NMIBC are usually treated with BCG—an immunotherapeutic that was originally developed as a vaccine against tuberculosis— which is instilled directly into the bladder. Although 80 percent of patients initially respond to BCG, over half of patients with an initial response experience recurrence and progression of cancer within a year, and many develop disease that no longer responds to BCG (584)Bree KK, et al. (2021) Hematol Oncol Clin North Am, 35: 513. DOI: 10.1016/j.hoc.2021.02.003.(585)Boorjian SA, et al. (2021) Lancet Oncol, 22: 107. DOI: 10.1016/S1470-2045(20)30540-4..
Patients who do not respond to BCG have very few treatment options other than surgical removal of the bladder. Although potentially curative, surgery is associated with high rates of complications. Additionally, many patients with underlying health conditions may be unwilling or unable to undergo surgery (585)Boorjian SA, et al. (2021) Lancet Oncol, 22: 107. DOI: 10.1016/S1470-2045(20)30540-4.. Therefore, alternative treatments for patients with bladder cancer are an urgent medical need.
Nogapendekin alfa inbakicept-pmln is a mutated form of the cytokine interleukin (IL)-15 with potential immunomodulating and antitumor properties. It binds to the IL-15 receptor on immune cells, such as natural killer (NK) cells and CD8+ T cells (see Sidebar 36), which activates the NK cells and T cells and boosts their multiplication, so they are better able to eradicate tumor cells. FDA approval was based on findings from a phase II/III clinical trial in which 62 percent of patients treated with nogapendekin alfa inbakicept-pmln experienced a complete response, which means no cancer cells could be detected in their urine and in the urinary bladder tissue. Among patients who had a complete response, the response lasted for at least 12 months in 58 percent of patients and at least 24 months in 40 percent of patients (586)Chamie K, et al. (2022) J Clin Oncol, 40: 4508. DOI: 10.1200/JCO.2022.40.16_suppl.4508..
Boosting the Cancer-killing Power of Immune Cells
Research has shown that immune cells, such as T cells, are naturally capable of destroying cancer cells. It has also shown that in patients with cancer, there are often insufficient numbers of cancer-killing T cells, and that the cancer-killing T cells that are present are unable to find or destroy the cancer cells for one of several reasons. This knowledge has led researchers to identify several ways to boost the ability of T cells to eliminate cancer cells.
Adoptive cell therapy (ACT), also called cellular immunotherapy, is designed to dramatically increase the number of cancer-killing immune cells a patient has, thereby boosting the immune system’s ability to seek and destroy cancer cells (587)Rohaan MW, et al. (2019) Virchows Arch, 474: 449. DOI: 10.1007/s00428-018-2484-0.. While many of the adoptive cell therapies currently in late-stage clinical development and all that are approved by FDA utilize patient-derived T cells (see Sidebar 38), ongoing research is looking to harness the cancer-killing power of other types of immune cells, including NK cells and macrophages. Chimeric antigen receptor (CAR) T-cell therapy is one type of ACT that has generated enormous excitement in cancer medicine in recent years. This is because treatment with CAR T cells has demonstrated unprecedented efficacy in certain patients with very advanced leukemia or lymphoma.
Like ICIs, CAR T-cell therapy is the culmination of decades of basic, translational, and clinical research utilizing knowledge of the cellular and molecular components of the immune system, genetic engineering, and the biological underpinnings of blood cancers. As of June 30, 2024, six CAR T-cell therapies have been approved by FDA, all for the treatment of blood cancers, including lymphoma, leukemia, and, most recently, multiple myeloma (see Sidebar 39). Collectively, these treatments have transformed the lives of adult and pediatric patients with blood cancers (589)Dana H, et al. (2021) Acta Pharm Sin B, 11: 1129. DOI: 10.1016/j.apsb.2020.10.020.. As one example, based on a recent analysis, axicabtagene ciloleucel (Yescarta), which was also approved by FDA in 2017, is the first treatment in nearly three decades to improve overall survival in relapsed or refractory large B-cell lymphoma (590)Westin JR, et al. (2023) N Engl J Med, 389: 148. DOI: 10.1056/NEJMoa2301665..
Generating CAR T cells is a complex procedure that can only be performed at specially certified health care facilities by highly trained medical professionals, which may reduce access to this therapy. Additionally, like other cancer treatments, CAR T-cell therapies can cause side effects, some of which can be potentially life-threatening. Moreover, recent studies show that certain CAR T-cell therapies may even be linked to secondary cancers (591)Hamilton MP, et al. (2024) N Engl J Med, 390: 2047. DOI: 10.1056/NEJMoa2401361.(592)Ozdemirli M, et al. (2024) N Engl J Med, 390: 2074. DOI: 10.1056/NEJMoa2401530.. Another issue that researchers are trying to address is the fact that CAR T-cell therapies have so far proven less successful against solid tumors. Developing simpler and safer ways to bring the promise of this class of immunotherapeutics to more patients with different cancer types is an area of ongoing investigation. One area of active research is using T cells collected from healthy donors instead of patients so that these “off-the-shelf ” CAR T cells would be readily available for use rather than manufactured for each patient.
While clinical research has been long evaluating the efficacy of three different types of adoptive T-cell therapy (see Sidebar 38) prior to 2024 only CAR T-cell therapy was approved by FDA. This changed in February 2024 with FDA approval of lifileucel (Amtagvi), the first cancer immunotherapeutic that uses tumor-infiltrating lymphocytes (TILs).
Like CAR T cells, TILs are manufactured using a patient’s own T cells (see Sidebar 38). However, a key difference is that unlike CAR T cells, which are derived from T cells in the patient’s blood, TILs are collected from a patient’s tumor. The second major difference is that unlike CAR T cells, which are engineered to recognize and kill cancer cells, TILs are not genetically modified in the laboratory because these cells are already capable of recognizing and finding their way to tumors as evident from the fact that they were isolated from the tumors themselves. TILs recognize cancer cells based on the presence of specific abnormal proteins, or antigens, on their surface. Once collected from a patient’s tumor, TILs are treated with the cytokine IL-2 to expand them in numbers before they are infused back into the patient.
Lifileucel was approved for patients with advanced melanoma that has gotten worse after treatment with ICIs or molecularly targeted therapeutics and is the first cellular immunotherapy to be approved for a solid tumor. FDA approval was based on a clinical trial in which the tumors shrank or disappeared in more than 31 percent of patients after treatment with lifileucel (593)Chesney J, et al. (2022) J Immunother Cancer, 10: e005755. DOI: 10.1136/jitc-2022-005755.(594)Medina T, et al. (2023) IOTECH, 20: 100591. DOI: 10.1016/j.iotech.2023.100591.. Of the patients who responded to lifileucel, more than half lived at least a year without any evidence of their cancer getting worse. Following the treatment, some patients experienced adverse events, which were mostly transient and manageable by their clinical care team.
Up until now, patients with advanced melanoma, such as Jennifer Ficko, whose disease progressed after treatments with ICI and/or molecularly targeted therapeutics, such as BRAF/MEK inhibitors, had no effective therapeutic options. The FDA approval of lifileucel in this subset of patients fulfills a major unmet medical need.
Directing the Immune System to Cancer Cells
An immune cell must find a cancer cell before it can attack and eliminate it. Many therapeutic antibodies approved by FDA for the treatment of cancer work, at least in part, by helping immune cells find cancer cells. Because of the effectiveness and promise of antibody-based immunotherapeutics, researchers have been working to develop new and improved versions of this important class of anticancer therapeutics.
T cell-engaging bispecific antibodies are one class of anticancer therapeutics that are moving rapidly from the laboratory to clinical practice. Using two or more arms that are engineered into these antibody particles, these immunotherapeutics bind to immune cells and cancer cells simultaneously. By acting as a connector, T cell-engaging bispecific antibodies bring cancer cells into close proximity with T cells, which are then activated and eliminate the cancer cells.
The first of these agents, blinatumomab (Blincyto), was approved by FDA in December 2014 for treating certain patients with a type of acute lymphoblastic leukemia (ALL) called B-cell ALL (595)Baselga J, et al. (2015) Clin Cancer Res, 21: S1.. Unprecedented advances in genetic engineering, molecular biology, and immunology over the past decade have led to a rapid proliferation in this innovative new area of cancer medicine. Between July 1, 2023, and June 30, 2024, FDA approved three new T cell-engaging bispecific antibodies for the treatment of multiple myeloma and lung cancer.
Two of the T cell-engaging bispecific antibodies approved during the 12 months covered in this report were for treatment of patients with multiple myeloma, one of the most common blood cancers in the United States. An estimated 35,780 new cases are expected to be diagnosed in 2024 and 12,540 people will succumb to the disease (3)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: March 17, 2024.. The burden is disproportionally higher in the Black population (3)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: March 17, 2024.. In recent years, the development and FDA approval of new therapeutics—including proteasome inhibitors like bortezomib (Velcade) and carfilzomib (Kyprolis), immunomodulatory agents like lenalidomide (Revlimid) and pomalidomide (Pomalyst), and immunotherapeutics like the CD38-targeted daratumumab (Darzalex)—have improved outcomes for patients. Despite these advances, unfortunately, many patients whose disease initially responds to the new therapeutics eventually experience relapse owing to treatment resistance.
In August 2023, FDA approved two T cell-engaging bispecific antibodies, talquetamab-tgvs (Talvey) and elranatamab-bcmm (Elrexfio), for adult patients with multiple myeloma that have relapsed after, or never responded to, at least four prior lines of therapy. Both antibodies attach to a molecule called CD3 on T cells with one arm. With the second arm, talquetamab-tgvs attaches to a protein, G protein–coupled receptor, family C, group 5, member D (GPRC5D), and elranatamab-bcmm attaches to a protein called B-cell maturation antigen (BCMA). Both GPRC5D and BCMA are present at high levels on the surface of most multiple myeloma cells. By attaching to these molecules on T cells and myeloma cells, the T cell-engaging bispecific antibodies bring the two cell types together, directing the T cells to home in on the myeloma cells. As a result, T cells are activated, and they destroy the adjacent myeloma cells.
Talquetamab-tgvs is the first FDA-approved therapeutic that targets GPRC5D and has been transformative for patients such as Vicki W. Jones. The approval was based on a clinical trial in which patients received either 0.4 mg/kg or 0.8 mg/kg of the therapeutic subcutaneously, following step-up doses, until disease progression or unacceptable toxicity. Step-up dosing is an approach used in the treatment with T cell-engaging bispecific antibodies whereby the dose administered to a patient is raised incrementally before reaching the target dose level. This helps the body’s immune system to be primed gradually, thereby reducing the risk of severe immune-related adverse events. Among both groups, receiving either 0.4 mg/kg or 0.8 mg/kg of the therapeutic, more than 70 percent of patients responded to the treatment (597)US Food and Drug Administration. FDA Grants Accelerated Approval to talquetamab-tgvs for Relapsed or Refractory Multiple Myeloma. Accessed:.
The approval of elranatamab-bcmm was based on a clinical trial in which nearly 58 percent of patients responded to the therapeutic. This is the second FDA approval of a BCMA-targeted T cell-engaging bispecific antibody for patients with multiple myeloma who have received at least four prior lines of therapy. The first, teclistamab-cqyv (Tecvayli), was approved in October 2022 (1). Historically, multiple myeloma that has progressed following multiple classes of treatment has been extremely challenging to treat. Therefore, recent approvals of talquetamab-tgvs (Talvey) and elranatamab-bcmm (Elrexfio) bring hope to patients by providing them with new and effective treatment options.
About 10 to 15 percent of all lung cancer cases are small-cell lung cancer (SCLC). SCLC is a fast-growing, aggressive disease associated with poor outcomes. In the United States, overall incidence of SCLC has been declining since its peak in the 1980s (598)Cittolin-Santos GF, et al. (2024) Cancer, 130: 2453. DOI: 10.1002/cncr.35281.. However, incidence has increased among women and there has been little improvement in survival outcomes.
Most patients with SCLC are diagnosed with extensive-stage disease, which means the cancer has spread beyond the lung and the area between the lungs to other lymph nodes or other parts of the body. Although most patients may respond to treatments initially, their cancers usually progress within months. Patients at this stage have limited options, and their overall survival rarely exceeds 8 months (599)Ahn MJ, et al. (2023) N Engl J Med, 389: 2063. DOI: 10.1056/NEJMoa2307980.. Therefore, FDA approval of tarlatamab-dlle (Imdelltra) in May 2024 for extensive-stage SCLC with disease progression on or after chemotherapy is a major clinical advance for this patient population.
Tarlatamab-dlle is a T cell-engaging bispecific antibody that binds CD3 on T cells and the protein delta-like ligand 3 (DLL3) on lung cancer cells, leading to T-cell–mediated killing of cancer cells. DLL3 is abnormally expressed on the surface of SCLC cells in 85 percent to 94 percent of patients with SCLC, making it a suitable target for therapeutic intervention (599)Ahn MJ, et al. (2023) N Engl J Med, 389: 2063. DOI: 10.1056/NEJMoa2307980.. FDA approval was based on a phase II clinical trial in which tarlatamab-dlle shrank tumors in 40 percent of the patients who received the treatment (599)Ahn MJ, et al. (2023) N Engl J Med, 389: 2063. DOI: 10.1056/NEJMoa2307980.. In more than 50 percent of patients whose tumors shrank with tarlatamab, the therapeutic kept the cancer at bay for at least 6 months.
It is important to note that immunotherapeutics, including CAR T cells, ICIs, and T cell-engaging bispecific antibodies, may cause serious adverse side effects, some of which could be life-threatening if not managed immediately and appropriately by trained medical professionals. In fact, FDA approvals of all three T cell-engaging bispecific antibodies discussed above come with a warning of life-threatening adverse events, such as cytokine release syndrome and neurologic toxicity. Because of these risks, talquetamab-tgvs and elranatamab-bcmm are available only through a restricted program, called the Risk Evaluation and Mitigation Strategy (REMS).

Research-driven Progress Against Childhood and AYA Cancers
In the United States, an estimated 9,620 children (ages 0 to 14 years) and 84,100 adolescents and young adults (AYA) (ages 15 to 39 years) will be diagnosed with cancer in 2024. There has been enormous progress in the treatment of childhood and AYA cancers over the past several decades, as reflected in the greater than 85 percent 5-year relative survival rates for all cancers combined for both populations. However, some cancers such as bone sarcomas or certain brain tumors have been difficult to treat and continue to have poor survival.
Many of the initial advances in the treatment of childhood cancers were made through intensification of cytotoxic chemotherapeutics, which, while effective, were associated with significant toxicities, including short- and long-term adverse effects (600)Butler E, et al. (2021) CA Cancer J Clin, 71: 315. DOI: 10.3322/caac.21665.. With greater understanding of the biology of childhood and AYA cancers and innovations in technology, there is an increasing focus on identifying therapeutic vulnerabilities in historically intractable cancers and utilizing personalized approaches to target these diseases as well as on reducing treatment intensities among patients with curable cancers who have a favorable prognosis, to improve their quality of lives (601)Helms L, et al. (2023) Pediatrics, 152: e2023061539. DOI: 10.1542/peds.2023-061539.. Research shows that cutting-edge technologies such as gene sequencing can improve clinical care of children with cancer by informing personalized treatment options (602)Hodder A, et al. (2024) Nat Med: s41591. DOI: 10.1038/s41591-024-03056-w.. Researchers are also refining the use of traditional treatments, such as using novel formulations or newer delivery methods for cytotoxic chemotherapeutics to reduce toxicities.
One of the drivers of progress against cancer in children is that the enrollment of children (14 and younger) in clinical trials has been historically much higher compared to that of adult patients (603)Unger JM, et al. (2016) Am Soc Clin Oncol Educ Book, 35: 185. DOI: 10.1200/EDBK_156686.. Enrollment rates of childhood cancer patients from racial and ethnic minority groups in clinical trials supporting FDA approval of cancer treatments are also higher than that of their adult counterparts (604)Fashoyin-Aje LA, et al. (2024) JAMA Oncol, 10: 380. DOI: 10.1001/jamaoncol.2023.5781.. These differences are partly attributable to the fact that most children with cancer are treated at specialized pediatric cancer centers that offer access to clinical trials. However, recruitment of AYA patients with cancer has been an ongoing challenge (605)Wyatt KD, et al. (2024) JCO Oncol Pract, 20: 603. DOI: 10.1200/OP.23.00826. and needs additional efforts.
Similar to what has been highlighted in the prior 13 editions of the annual AACR Cancer Progress Report, FDA has made numerous decisions in the 12 months covered in this report that will continue the momentum of progress against pediatric and AYA cancers (606)US Food & Drug Administration. Pediatric Oncology Drug Approvals. Accessed: June 26, 2024..
Advances in the Treatment of Leukemia
Leukemias are the most common cancers among US children and adolescents. B-cell acute lymphocytic leukemia (ALL) is the most common cancer diagnosed among children ages 0 to 14 in the United States. The 5-year survival for children and adolescents is greater than 90 percent, attributable to spectacular advances in risk stratification at diagnosis with treatment escalation for those with high risk of relapse as well as to the new and improved treatment options that are now available in the clinic. Decades of basic, translational, and clinical research have enhanced our knowledge of the underpinnings of leukemia as well as that of the immune system. Researchers are harnessing this knowledge to develop personalized treatments including immunotherapeutics and molecularly targeted therapeutics that target ALL.
As one example, in 2017, the first CAR T-cell therapy tisagenlecleucel (Kymriah) was approved by FDA for the treatment of children and young adults with B-cell ALL that had not responded to standard treatments or had relapsed at least twice. This revolutionary immunotherapeutic has allowed some patients whose leukemia had returned or stopped responding to other treatments to experience complete remission. A long-term follow-up of patients treated with tisagenlecleucel showed that more than 60 percent were living 3 years or longer after their first infusion of CAR T cells (607)Laetsch TW, et al. (2023) J Clin Oncol, 41: 1664. DOI: 10.1200/Jco.22.00642.. Additionally, more than 50 percent of patients were living without their disease coming back at the end of three years after treatment completion, suggesting that CAR T cells can lead to durable cancer control.
The T cell-engaging bispecific antibody blinatumomab, which was initially approved in 2014 for the treatment of adults with B-cell ALL, received expanded approval by FDA in 2017 for the treatment of children whose ALL had returned following at least one course of treatment. Clinical studies show that in children and AYAs with B-cell ALL that had relapsed, treatment with blinatumomab led to better outcomes and fewer side effects compared to chemotherapy alone (608)Brown PA, et al. (2021) JAMA, 325: 833. DOI: 10.1001/jama.2021.0669.(609)Locatelli F, et al. (2021) JAMA, 325: 843. DOI: 10.1001/jama.2021.0987.. Moreover, the addition of blinatumomab to chemotherapy appears to be highly efficacious for infants with newly diagnosed ALL with certain genetic alterations known as KMT2A-rearrangement, a disease historically associated with poor outcomes (610)van der Sluis IM, et al. (2023) N Engl J Med, 388: 1572. DOI: 10.1056/NEJMoa2214171..
Antibody-drug conjugates are an emerging class of molecularly targeted therapeutics that use an antibody to deliver an attached cytotoxic chemotherapeutic directly to the cancer cells that have the antibody’s target on their surfaces. Once the antibody attaches to its target on the surface of a cancer cell, the antibody-drug conjugate is internalized by the cells. This leads to the chemotherapeutic being released from the antibody and killing the cancer cell. The precision of antibody targeting reduces the side effects of the chemotherapeutic compared with traditional systemic delivery.
Inotuzumab ozogamicin (Besponsa) is an antibody-drug conjugate comprising a CD22-targeted antibody linked to the chemotherapeutic calicheamicin. It was approved for treating adults with B-cell ALL in August 2017. Subsequent studies have shown that inotuzumab ozogamicin is also effective in children and adolescents. Based on findings from a clinical trial in which 42 percent of patients who received inotuzumab ozogamicin achieved a complete remission, meaning they had no evidence of cancer, in March 2024, FDA approved the therapeutic for pediatric patients 1 year and older with CD22-positive B-cell ALL that has relapsed or stopped responding to treatments.
Patients who receive inotuzumab ozogamicin may need a stem cell transplant to ensure durable cancer remission. While treatment with inotuzumab ozogamicin increases the risk of developing serious liver toxicities in certain patients, it increases treatment options for a group of ALL patients who may be ineligible for CAR T-cell therapy and have no remaining options.
Advances in the Treatment of Brain Tumors
Brain and other nervous system tumors are the second most diagnosed cancers in children. Low-grade glioma is the most common type of brain tumor in children. These are slow-growing tumors that can often be cured with surgery alone. However, depending on their location in the brain, some low-grade gliomas cannot be fully removed, for example, if they are adjacent to vital structures in the brain. Additionally, in some cases low-grade gliomas may grow back even after a complete surgical removal. Traditionally, most children whose tumors are not surgically removable or have come back after surgery receive chemotherapeutics. While chemotherapies can be effective, they are associated with substantial side effects. Therefore, alternative treatments for these children are an urgent need in cancer medicine.
Research has demonstrated that alterations in the BRAF gene leading to aberrant activation of the BRAF protein signaling pathway are common in pediatric low-grade gliomas. The BRAF protein has a critical role in controlling cell growth. The BRAF gene is altered in approximately 6 percent of all human cancers (402)Stone BV, et al. (2024) Eur Urol Oncol, 7: 563. DOI: 10.1016/j.euo.2023.11.020.. Most cancer-related changes in the BRAF gene cause the protein to continuously stay active, thus helping cancer cells grow faster than normal cells. Common cancer-related changes in the BRAF gene include structural variations such as BRAF gene fusions or rearrangements and/or single base changes such as the BRAF V600E mutation (see Table 2). BRAF structural variations are more common than BRAF V600E mutations in children and adolescents with low-grade gliomas.
A combination of two molecularly targeted therapeutics that target the BRAF pathway, dabrafenib (Tafinlar) and trametinib (Mekinist), was approved by FDA in March 2023 for children with low-grade glioma that has a BRAF V600E mutation. The combination therapy, however, does not work in patients such as Michael Methner who have BRAF gene fusions or rearrangements. Therefore, FDA approval of tovorafenib (Ojemda) in April 2024 for patients 6 months and older with relapsed or treatment-unresponsive low-grade glioma that has a BRAF fusion or rearrangement or BRAF V600 mutation brings hope to many more parents and families whose children are diagnosed with the disease. The approval was based on a clinical trial in which tumors shrank or disappeared entirely in almost 70 percent of children treated with tovorafenib (611)Kilburn LB, et al. (2024) Nat Med, 30: 207. DOI: 10.1038/s41591-023-02668-y..
Researchers are now investigating whether tovorafenib in combination with chemotherapy could be used earlier on during the course of treatment as the initial therapy for children with low-grade gliomas that have fusions, rearrangements, or other mutations in the BRAF gene. Additionally, ongoing investigation is evaluating a separate molecularly targeted therapy, selumetinib (Koselugo), as the initial treatment after surgery for children with low-grade glioma regardless of their BRAF status. Selumetinib blocks the function of a protein called MEK, which is part of the same growth-promoting signaling pathway as BRAF. The therapeutic was approved by FDA in 2020 for the treatment of a different childhood cancer known as NF1–related plexiform neurofibroma.
Researchers are also exploring new and improved therapeutic options for children with more aggressive forms of brain tumors. As one example, clinical studies are examining CAR T-cell therapy in some children and young adults with a fast-growing, highly aggressive brain tumor called diffuse intrinsic pontine glioma (DIPG) (612)Majzner RG, et al. (2022) Nature, 603: 934. DOI: 10.1038/s41586-022-04489-4.. The CAR T cells which, in this case, target a tumor-associated glycolipid (lipid attached to a carbohydrate) on the surface of DIPG cells, called GD2, are administered in small doses and infused directly into the brain. Initial findings from the study reported positive responses in terms of reductions in tumor size as well as improvements in cancer-related symptoms.
Advances in the Treatment of Solid Tumors Outside the Brain
Neuroblastoma is the most common extracranial solid tumor in children. Researchers use patient factors (age at diagnosis, disease stage) and tumor genetics to predict the likelihood of a child with neuroblastoma to be cured and decide treatments accordingly. While children with high-risk disease have had poorer outcomes historically, research-driven clinical breakthroughs in recent years have made major strides in the clinical care for these patients.
As one example, decades of basic, translational, and clinical research, starting from the recognition of the molecule GD2 as a tumor-associated glycolipid in 1984, led to the development of dinutuximab (Unituxin), an immunotherapeutic, that was approved in 2015 for treating children with high-risk neuroblastoma. Dinutuximab works by attaching to GD2 on neuroblastoma cells and flagging them for destruction by immune cells, using a natural process called antibody-dependent cellular cytotoxicity. Recent data demonstrate that dinutuximab is extending lives for many children with high-risk neuroblastoma (613)Desai AV, et al. (2022) J Clin Oncol, 40: 4107. DOI: 10.1200/Jco.21.02478.. Since the approval of dinutuximab in 2015, FDA has approved a second therapeutic, naxitamab-gqgk (Danyelza), which works similarly to dinutuximab, for the treatment of patients with neuroblastoma.
Despite recent advances, only around 50 percent of children with high-risk neuroblastoma survive 5 years or longer. Patients whose cancer has come back have a poor outcome, with a 5-year overall survival of less than 10 percent (614)Oesterheld J, et al. (2024) J Clin Oncol, 42: 90. DOI: 10.1200/JCO.22.02875.. Therefore, additional treatment options are still needed. In this regard, in December 2023, FDA approved the first therapeutic intended to reduce the risk of relapse in children with high-risk neuroblastoma such as Parker Shaw. The treatment, eflornithine (Iwilfin), was approved for adult and pediatric patients with high-risk neuroblastoma with at least a partial response to prior therapies, including anti-GD2 immunotherapy.
Eflornithine blocks the function of a protein named ornithine decarboxylase, which has a high activity in tumor cells and promotes tumor cell multiplication. FDA approval was based on findings from a clinical trial that compared outcomes of patients who were treated with either eflornithine along with current standards of care or the standard of care alone as maintenance treatment after the initial therapy for high-risk neuroblastoma. The data from the trial showed a reduction in the risk of cancer relapse and improved overall survival in patients who received the eflornithine regimen (614)Oesterheld J, et al. (2024) J Clin Oncol, 42: 90. DOI: 10.1200/JCO.22.02875..
Advances in cancer treatment are also benefiting childhood and AYA patients with rarer forms of solid tumors. As one example, in December 2022, the ICI atezolizumab (Tecentriq) became the first treatment to be approved by FDA for the treatment of patients with alveolar soft part sarcoma (ASPS), an extremely rare cancer that mainly affects AYAs. According to NCI, about 80 people are diagnosed with the disease in the United States each year. ASPS is a slow-growing cancer that forms in soft tissues such as muscle, fat, or nerves. Although the tumor grows slowly, once metastatic, ASPS has poor outcomes.
Chemotherapeutics have limited benefit and molecularly targeted therapeutics do not have lasting effectiveness against ASPS. FDA approved atezolizumab for the treatment of ASPS that has spread to other parts of the body or cannot be removed by surgery. The approval was based on findings from a clinical trial which showed that treatment with atezolizumab either led to tumor shrinkage or kept the disease at bay (615)Chen AP, et al. (2023) N Engl J Med, 389: 911. DOI: 10.1056/NEJMoa2303383. and represents a significant advance for a rare disease.
Advances in Biomarker-based Treatments
Characterization of genetic alterations that drive tumor growth has been instrumental in understanding tumor biology and conducting genetically informed clinical trials such as basket, umbrella, and platform clinical trials (see Clinical Research). These advances have accelerated the pace of development and FDA approval of molecularly targeted therapeutics and immunotherapeutics that are effective against cancers originating at different sites in the body but share biological underpinnings. In fact, one of the most notable achievements in precision medicine was the first FDA approval of a molecularly targeted therapeutic to treat cancer based on the presence of a specific genetic biomarker in the tumor irrespective of the site at which the tumor originated. The therapeutic, larotrectinib (Vitrakvi), was approved by FDA in 2018 for treating children and adults who have solid tumors that test positive for the NTRK gene fusions.
Since the approval of larotrectinib, two additional molecularly targeted therapeutics, entrectinib and repotrectinib, have been approved by FDA for treating children with solid tumors based on the same NTRK gene fusion biomarker (see Figure 20). The approvals of larotrectinib, entrectinib, and repotrectinib for use in a tissue-agnostic way followed several decades of basic, translational, and clinical research (see Figure 20).
These therapeutics work by targeting three related proteins called TRKA, TRKB, and TRKC. The genes NTRK1, NTRK2, and NTRK3 provide the code that cells use to make these proteins. Genetic alterations known as structural variations that involve the three NTRK genes and lead to the production of NTRK gene fusions, and subsequently to TRK fusion proteins, drive the growth of several cancer types that occur in adults and children, including rare cancers such as soft tissue sarcomas. Overall, researchers estimate that NTRK gene fusions fuel the growth of up to 1 percent of all solid tumors.
The recent approval of repotrectinib in June 2024 for children 12 years and older and adults was based on findings from a clinical trial that evaluated the therapeutic in patients who had or had not received a prior TRK-targeted therapy. The study showed that the tumors shrank in nearly 60 percent of patients who had not received a prior TRK-targeted therapy and in half of patients who had received a prior TRK-targeted therapy.
Mutations in the RET gene, including single base changes, structural variations, and deletions that lead to abnormal activation of the RET protein, are rare alterations observed mostly in patients with certain types of thyroid cancer and lung cancer (616)Duke ES, et al. (2023) Clin Cancer Res, 29: 3573.. In children and AYA patients, RET mutations are frequently reported in papillary thyroid carcinomas and medullary thyroid cancers and less frequently in glioma, lipofibromatosis, inflammatory myofibroblastic tumor, and infantile myofibromatosis (617)Ortiz MV, et al. (2020) JCO Precis Oncol, 4: 341. DOI: 10.1200/po.19.00401..
A RET-targeted therapeutic, selpercatinib, was approved by FDA first in 2020, for lung and thyroid cancers with RET mutations and then in 2022 in a tumor-agnostic manner for the treatment of adult patients with advanced solid tumors with a RET mutation. Most recently, in May 2024, FDA has approved selpercatinib for the treatment of pediatric patients 2 years and older with metastatic thyroid cancer or any solid tumor with a RET gene alteration, as detected by an FDA-approved test. The approval of selpercatinib was based on the findings of a clinical trial in which nearly 50 percent of patients treated with the molecularly targeted therapeutic saw their tumors shrink.
Despite the significant progress in treatment, cancers remain one of the leading causes of death among US children and AYAs. With the rapid expansion of novel molecularly targeted therapeutics and immunotherapeutics, there is also an increasing need to monitor patients over time to better understand treatment-related long-term effects and morbidities. Additionally, there are disparities in clinical care experienced by children from racial and ethnic minority groups and other medically underserved populations. Continued progress against childhood and AYA cancers necessitates increasing collaboration among all stakeholders in medical research so that the new wave of innovation in cancer science and technologies, coupled with advances in regulatory policies and legislation (see Accelerating Progress Against Childhood Cancer), can drive new breakthroughs that benefit all patients.
Next Section: Supporting Cancer Patients and Survivors Previous Section: Screening for Early Detection