Understanding Cancer Development in the Context of Cancer Health Disparities
In this section you will learn:
- Research provides our understanding of the biology of cancer, which is not one disease, but a collection of diseases characterized by the uncontrolled growth of cells.
- Genetic mutations underpin cancer biology in most cases; the mutations are inherited in only about 10 percent of cases.
- Cancer biology is strongly influenced by interactions among cancer cells and numerous factors in their environment.
- Data on cancer biology comes predominantly from mostly white individuals of Western European ancestry.
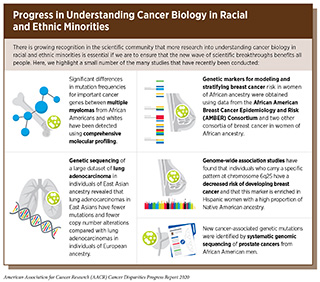
- Initiatives such as AACR Project Genomics, Evidence, Neoplasia, Information, Exchange (GENIE) and NCI-funded projects such as the African American Breast Cancer Epidemiology and Risk (AMBER) Consortium are beginning to provide insight into cancer biology in racial and ethnic minorities.
- There is an urgent need to increase research into understanding cancer biology in racial and ethnic minorities.
Decades of biomedical research have given us great insight into cancer biology. As basic research has uncovered the processes that change a normal cell to become cancerous, translational and clinical research has harnessed this information to design new and better approaches to prevent, detect, diagnose, and treat many cancers. We have learned that cancer is a collection of diseases that arise when the processes that control normal cell growth, division, and life span go awry. As a result, cells start multiplying uncontrollably, fail to die when they should, and mobilize other cells and tissues such as blood vessels, immune cells, and other types of normal cells to give the tumor a growth advantage over the surrounding tissue. In body organs and tissues, the accumulating cancer cells form masses called tumors, whereas in the blood or bone marrow they crowd out normal cells.
As the cancer grows certain cells within the cancer acquire specific changes that give them and their daughter cells the best chance to grow and survive. Those changes might include the ability to grow faster, to survive despite the presence of treatments, to invade adjacent tissues and organs, to evade the body’s immune system, and to move into the blood stream and/or lymphatic system and spread to distant parts of the body. Most advanced cancers acquire several, if not all, of these features. Cancer that has spread to other parts of the body, which is often called metastatic disease, is the main cause of most cancer deaths.
It is important to note that there are many factors, from biological to environmental to lifestyle factors, that influence cancer initiation and progression. Complex interplay among these factors can drive cancer development and may contribute to the observed disparities in cancer burden among different population groups (see Why Do Cancer Health Disparities Exist?). Therefore, understanding these interactions is especially important in the context of cancer health disparities, in particular, for those cancers that disproportionately affect racial and ethnic minorities.
Cancer Initiation: DNA at the Core of Cancer
The normal behavior of each cell in the human body is controlled by its genetic material. The genetic material comprises chains of deoxyribonucleic acid (DNA), a complex molecule made up of four building blocks called bases that is found in nearly all cells of higher organisms. In humans, the four bases that comprise DNA are organized in a very specific pattern to build two paired chains that each have 3 billion bases and represent what is often referred to as the human genome. Incredibly, the pattern of bases is 99.9 percent identical between any two individuals! However, the 0.1 percent difference is what gives each person individual characteristics. The genome is packaged together with proteins known as histones into structures called chromosomes inside the cell’s nucleus. Each person gets 23 chromosomes from each parent; thus, each normal cell has 46 chromosomes.
The order, or sequence, of the DNA bases provides the code used by a cell to produce the various proteins it needs to function. This code is at the core of what is known as Central Dogma, whereby the genetic code in DNA is converted into another form of nucleic acid called ribonucleic acid (RNA). RNA is the transcript of the original code embedded in the DNA and is used to manufacture proteins, which are the molecules that perform important functions that dictate a cell’s fate. Normal processes in cells that dictate their functions are programmed into each cell’s genome. One can generally conclude that normal DNA leads to normal proteins, which leads to normal cells, which create normal tissue. Conversely, changes in the DNA may disrupt normal protein function, which leads to altered cells, which create altered tissue and lead to cancer development. Therefore, at its basic level, cancer is a genetic disease because it is caused by some intrinsic or extrinsic factor(s) that alter the normal DNA.
Genetic Changes: DNA Mutations in Cancer
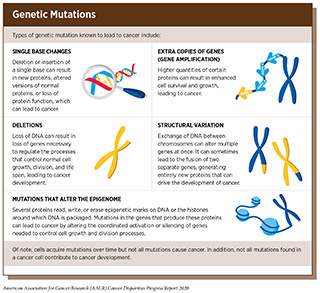
Alterations in the DNA sequence, referred to as mutations, can disrupt normal protein function, and are the leading cause of cancer development (see sidebar on Genetic Mutations). Each person’s cancer has a unique combination of mutations, and as cancer cells divide, new mutations arise in the daughter cells. Thus, a tumor is made up of a collection of cancer cells with a wide range of genetic abnormalities. This variation in cell types, also known as heterogeneity, is an important part of a cancer’s characteristics and fuels the cancer’s ability to grow faster, escape therapy, evade the immune system, and metastasize to other organs.
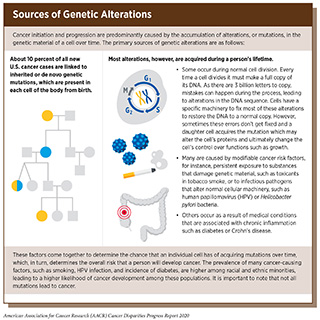
Most cancer-causing mutations are acquired over an individual’s lifetime due to errors arising during normal cell duplication or because of environmental exposures, lifestyle factors, or health conditions that fuel chronic inflammation (see sidebar on Sources of Genetic Alterations). These acquired mutations are referred to as somatic mutations. About 10 percent of cancer-causing mutations are inherited. When multiple individuals in a family carry a mutation in a gene that is important in cancer-causing processes, there is strong evidence that the mutation significantly increases risk of cancer, and mutations like these are called “pathogenic.” Decades of research have led to the identification of numerous genes that are associated with cancers as well as specific inherited mutations that are pathogenic (see Table 5).
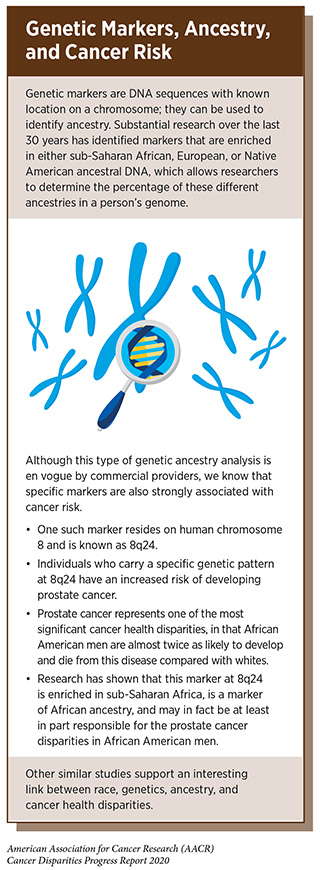
The inherited genome plays an important role in cancer risk. Each person’s inherited genome is related to their genetic ancestry, which is defined by the history of their biological family (see sidebar on Genetic Markers, Ancestry, and Cancer Risk). Among the major ancestry groups are sub-Saharan Africans, Europeans, and Native American (89)1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature [Internet]. 2015 [cited 2020 Jan 22];526:68–74.[cited 2020 Jul 15].(90)Shriver MD, Mei R, Parra EJ, Sonpar V, Halder I, Tishkoff SA, et al. Large-scale SNP analysis reveals clustered and continuous patterns of human genetic variation. Hum Genomics [Internet]. 2005 [cited 2020 Jan 22];2:81–9.[cited 2020 Jul 15].(91)Pfaff CL, Kittles RA, Shriver MD. Adjusting for population structure in admixed populations. Genet Epidemiol [Internet]. 2002 [cited 2020 Jan 22];22:196–201.[cited 2020 Jul 15].. Much of the world’s population is made up of subgroups that have high rates of a single major ancestral group, or subgroups that have a combination of these major ancestries. For example, many people in the United States who self-identify as non-Hispanic white have greater than 95 percent European ancestry. However, many people in the United States who self-identify as African American or Hispanic have varying degrees of ancestral contributions from any or all three of these major ancestral groups (89)1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature [Internet]. 2015 [cited 2020 Jan 22];526:68–74.[cited 2020 Jul 15].(90)Shriver MD, Mei R, Parra EJ, Sonpar V, Halder I, Tishkoff SA, et al. Large-scale SNP analysis reveals clustered and continuous patterns of human genetic variation. Hum Genomics [Internet]. 2005 [cited 2020 Jan 22];2:81–9.[cited 2020 Jul 15].(91)Pfaff CL, Kittles RA, Shriver MD. Adjusting for population structure in admixed populations. Genet Epidemiol [Internet]. 2002 [cited 2020 Jan 22];22:196–201.[cited 2020 Jul 15].. Ultimately, there is growing recognition of the extensive genetic diversity across the human population.
Unfortunately, the preponderance of research studies into inherited cancer risks have focused on individuals of high European genetic ancestry, namely, non-Hispanic whites in the United States. Thus, there is growing concern in the scientific community regarding the inadequate representation and lack of data from other racial and ethnic minorities who may have lower amounts of European genetic ancestry, such as many African Americans and Hispanics (92)Sirugo G, Williams SM, Tishkoff SA. The Missing Diversity in Human Genetic Studies. Cell [Internet]. Elsevier Inc.; 2019;177:26–31.[cited 2020 Jul 15].(93)Popejoy, Alice B., Fullerton SM. Genomics is falling. Nature. 2016;538:161–4.[cited 2020 Jul 15].. In other words, the most data on the genetics of cancer risk is derived from mostly white individuals of western European ancestry. Given these limitations, our current knowledge of cancer genetics cannot be applied to all populations, limiting our knowledge of inherited cancer risks in racial and ethnic minorities. For instance, because of limited information from racial and ethnic minorities, we often have insufficient evidence to determine with statistical strength whether a mutation is truly cancer causing, and these mutations are often categorized as variants of undetermined significance (VUS). Consequently, genetic counseling for racial and ethnic minority individuals becomes less precise and less informative than it is for those of high European ancestry. Therefore, there is an urgent need to increase research into understanding the genes and specific mutations that are associated with hereditary cancer in racial and ethnic minorities.
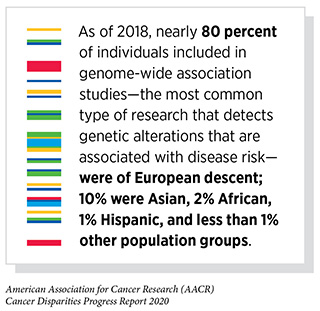
As mentioned earlier, most cancer mutations are not inherited, but rather are DNA changes that occur in cells during cell duplication or as a result of an assault on the cell by an extrinsic factor such as carcinogens or other environmental exposures. Comprehensive analyses of human cancer genomes (the DNA that is present specifically in cancer cells) over the past three decades have revealed numerous cancer-causing genetic alterations. For instance, The Cancer Genome Atlas, an initiative supported by the NCI and National Human Genome Research Institute, looked at the genetic content of about 11,000 tumors across 33 different cancer types. Notably, within the 11,000 tumors, racial and ethnic minorities are significantly underrepresented (99)Wang X, Steensma JT, Bailey MH, Feng Q, Padda H, Johnson KJ. Characteristics of The Cancer Genome Atlas cases relative to U.S. general population cancer cases. Br J Cancer [Internet]. Nature Publishing Group; 2018 [cited 2019 Dec 16];119:885–92.[cited 2020 Jul 15].(100)Spratt DE, Chan T, Waldron L, Speers C, Feng FY, Ogunwobi OO, et al. Racial/Ethnic Disparities in Genomic Sequencing. JAMA Oncol [Internet]. 2016 [cited 2019 Dec 16];2:1070.[cited 2020 Jul 15].. This is concerning because we know that the incidence and mortality of many cancers are higher among racial and ethnic minorities. It is extremely important to determine whether cancer patients from different racial and ethnic minority groups have different mutational patterns and if these patterns are associated with worse outcomes. In fact, there is mounting evidence that the cancer-associated mutation patterns may be different in racial and ethnic minority patients (101)Dearden S, Stevens J, Wu Y-L, Blowers D. Mutation incidence and coincidence in non small-cell lung cancer: meta-analyses by ethnicity and histology (mutMap). Ann Oncol Off J Eur Soc Med Oncol [Internet]. Oxford University Press; 2013 [cited 2019 Dec 16];24:2371–6.[cited 2020 Jul 15]., but the overall body of data is still too limited to permit a true understanding of the actual magnitude of these differences.
Discoveries uncovering cancer-causing genetic alterations have also led to the development of a new class of therapeutics, molecularly targeted therapeutics, which aim to rectify the cellular changes that arise due to cancer-causing mutations. A better understanding of the mutational patterns in racial and ethnic minorities is critical for the development of such therapeutics that will be effective in treating racial and ethnic minority cancer patients.
Epigenetic Changes: DNA’s Third Dimension
Epigenetics refers to modifications to DNA that do not involve a change in the DNA base sequence. The epigenetically modified genome is referred to as the “epigenome”. One major epigenetic process is involved in packaging the DNA of a cell inside of a nucleus, which is a small compartment inside of the cell. The epigenetic process of packaging DNA in the nucleus involves the wrapping of DNA around proteins called histones. This process results in condensing DNA so that it can fit inside of the nucleus. This condensed DNA, called chromatin, also functions to allow for proper cell division, protects the DNA strands from breaking, and controls how genes are turned on or off. A second epigenetic change, called DNA methylation, involves a chemical modification of adding a methyl molecule to a DNA base. This does not change the actual DNA sequence, but it does change the composition of the DNA.
Epigenetic modifications play a very important role in cells because they regulate how and when genes are turned “on” or “off” and they are made by specialized proteins that “add” or “erase” unique chemical modifications on DNA and/or histones (102)Dawson MA. Therapeutic Opportunities. 2017;1:379–85.[cited 2020 Jul 15].. If the normal epigenetic processes go awry, there can be significant changes in the cells normal growth machinery leading to cancer. In fact, epigenetic alterations of DNA repair genes or cell growth control genes are commonly seen in cancers (103)Lahtz C, Pfeifer GP. Epigenetic changes of DNA repair genes in cancer. J Mol Cell Biol [Internet]. Oxford University Press; 2011 [cited 2019 Dec 16];3:51–8.[cited 2020 Jul 15].(104)Kanwal R, Gupta S. Epigenetic modifications in cancer. Clin Genet [Internet]. NIH Public Access; 2012 [cited 2019 Dec 16];81:303–11.[cited 2020 Jul 15].. Research has also led to the identification of a significant number of cancer-causing mutations in genes that control these epigenetic processes.
Epigenetic changes can be a part of normal development or may result from external or environmental factors such as chemical/biochemical/biological exposures, threats to food security, diet, exercise, and physiological and psychological stress (105)Martin EM, Fry RC. Environmental Influences on the Epigenome: Exposure- Associated DNA Methylation in Human Populations. Annu Rev Public Health [Internet]. Annual Reviews ; 2018 [cited 2019 Dec 16];39:309–33.[cited 2020 Jul 15].(106)Alegría-Torres JA, Baccarelli A, Bollati V. Epigenetics and lifestyle. Epigenomics [Internet]. NIH Public Access; 2011 [cited 2019 Dec 16];3:267–77.[cited 2020 Jul 15].. In contrast to genetic mutations, epigenetic changes are often reversible, providing an opportunity for therapeutic intervention. Our understanding of the role of epigenetics in cancer and cancer health disparities is, however, incomplete, and continued research is needed to fulfill the real potential of the epigenome in cancer science and medicine.
Cancer Progression: Role of the Tumor Microenvironment
As cancer cells grow and divide in tissue, a mass of cells, or tumor, develops. The tumor is not just made up of cancer cells; rather, it is made up of both cancerous and noncancerous cells. Research has shown that the rate at which tumors grow and progress is largely dependent upon complex interactions between the cancer cells and the other cells and factors in their surrounding tissue, which is known as the tumor microenvironment (see sidebar on Cancer Progression: Local and Systemic Influences). Bidirectional communication between cancer cells and the tumor microenvironment has a profound influence on cancer progression (107)Mcgranahan N, Swanton C. Review Clonal Heterogeneity and Tumor Evolution : Past , Present , and the Future. Cell [Internet]. Elsevier Inc.; 2017;168:613–28.[cited 2020 Jul 15] LINK NOT AVAILABLE.(108)Hawkins ED, Duarte D, Akinduro O, Khorshed RA, Passaro D, Nowicka M, et al. T-cell acute leukaemia exhibits dynamic interactions with bone marrow microenvironments. Nature. 2016;[cited 2020 Jul 15].(109)Binnewies M, Roberts EW, Kersten K, Chan V, Fearon DF, Merad M, et al. Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat Med [Internet]. 2018 [cited 2019 Dec 16];24:541–50.[cited 2020 Jul 15].. Moreover, the tumor microenvironment can shelter cancer cells from the effects of some cancer treatments, including radiation, chemotherapy, and immunotherapy, and thereby modify a patient’s response to treatment (110)Sun Y. Tumor microenvironment and cancer therapy resistance. Cancer Lett [Internet]. Elsevier Ireland Ltd; 2016;380:205–15.[cited 2020 Jul 15]..
Recent research suggests that several components of the tumor microenvironment are different among diverse racial and ethnic patient populations. These differences among racial and ethnic populations may result from factors associated with inherited genetic ancestry, increased levels of chronic inflammation, innate differences in immune response, or a combination of these (74)Wallace TA, Prueitt RL, Yi M, Howe TM, Gillespie JW, Yfantis HG, et al. Tumor Immunobiological Differences in Prostate Cancer between African-American and European-American Men. Cancer Res [Internet]. 2008 [cited 2019 Dec 16];68:927–36.[cited 2020 Jul 15].(111)Deshmukh SK, Srivastava SK, Tyagi N, Ahmad A, Singh AP, Ghadhban AAL, et al. Emerging evidence for the role of differential tumor microenvironment in breast cancer racial disparity: a closer look at the surroundings. Carcinogenesis [Internet]. Oxford University Press; 2017 [cited 2019 Oct 31];38:757–65.[cited 2020 Jul 15].(113)Kinseth MA, Jia Z, Rahmatpanah F, Sawyers A, Sutton M, Wang-Rodriguez J, et al. Expression differences between African American and Caucasian prostate cancer tissue reveals that stroma is the site of aggressive changes. Int J Cancer [Internet]. 2014 [cited 2019 Dec 16];134:81–91.[cited 2020 Jul 15].(114)Tang W, Wallace TA, Yi M, Magi-Galluzzi C, Dorsey TH, Onabajo OO, et al. IFNL4 -ΔG Allele Is Associated with an Interferon Signature in Tumors and Survival of African-American Men with Prostate Cancer. Clin Cancer Res [Internet]. 2018 [cited 2019 Dec 16];24:5471–81.[cited 2020 Jul 15].. A better understanding of how cancer cells and the tumor microenvironment differ among diverse patient populations will help to better identify causes of cancer health disparities and, more importantly, provide information on how to eliminate them.
Cancer and the Immune System
One of the most significant advances in cancer research over the past two decades has been our increased understanding of the body’s ability to mount an immune response against tumor cells, and the processes by which tumor cells suppress a patient’s immune response. This knowledge forms the basis of one of the most exciting new approaches to cancer treatment—cancer immunotherapy—which utilizes patients’ own immune system to fight cancer. Cancer immunotherapy holds tremendous promise for improving outcomes for patients diagnosed with many types of cancer, including those types of cancer with a higher burden among racial and ethnic minorities. For instance, the incidence of triple-negative breast cancer, a particularly aggressive form of breast cancer, is twice as high among African American women compared to white women. In March 2019, the FDA approved an immunotherapeutic for the treatment of triple-negative breast cancer, bringing hope to many patients like Eva Joseph whose story was featured in the AACR Cancer Progress Report 2019 (115)AACR Cancer Progress Report 2019.[cited 2020 Jul 15]..
Like genetic studies, however, research in cancer immunology has focused on individuals of largely European ancestry, with a significant lack of data from racial and ethnic minorities. Researchers are now beginning to understand the differences in immune biology among individuals with different ancestry. For example, studies have shown that the African genome has evolved over hundreds of generations to effectively combat infectious pathogens in the environment (116)Rotimi CN, Bentley AR, Doumatey AP, Chen G, Shriner D, Adeyemo A. The genomic landscape of African populations in health and disease. Hum Mol Genet [Internet]. 2017 [cited 2019 Dec 16];26:R225–36.[cited 2020 Jul 15].(117)Rotimi CN, Tekola-Ayele F, Baker JL, Shriner D. The African Diaspora: History, Adaptation and Health. Curr Opin Genet Dev [Internet]. NIH Public Access; 2016 [cited 2019 Dec 16];41:77.[cited 2020 Jul 15].. These evolutionary selections may have altered genes associated with inflammation, immune response, and wound repair. Genetic alterations that protect against infectious pathogens, however, are sometimes associated with inherited diseases. Early preclinical studies have also shown that genetic alterations that are specific to African ancestry correlate with distinct immune characteristics in tumors that may affect responses to immunotherapy. These data highlight the urgent need for comprehensive immune profiling of cancer patients from diverse racial and ethnic backgrounds in order to develop precise therapeutic interventions that are effective for these populations.
Cancer Metastasis
Cancer metastasis is a multistep process in which the tumor cells break away from the original mass, gain access to the blood stream and/or lymphatic system in nearby tissue, and travel to distant organs. One important question that remains to be answered about metastasis is why some cancers invade more often depending on the patient’s race or genetic ancestry (118)Schootman M, Jeffe DB, Gillanders WE, Aft R. Racial disparities in the development of breast cancer metastases among older women: a multilevel study. Cancer [Internet]. 2009 [cited 2020 Jan 22];115:731–40.[cited 2020 Jul 15].(119)Tsodikov A, Gulati R, de Carvalho TM, Heijnsdijk EAM, Hunter-Merrill RA, Mariotto AB, et al. Is prostate cancer different in black men? Answers from 3 natural history models. Cancer [Internet]. 2017 [cited 2020 Jan 22];123:2312–9.[cited 2020 Jul 15].(120)Akinyemiju T, Sakhuja S, Waterbor J, Pisu M, Altekruse SF. Racial/ethnic disparities in de novo metastases sites and survival outcomes for patients with primary breast, colorectal, and prostate cancer. Cancer Med [Internet]. 2018 [cited 2020 Jan 22];7:1183–93.[cited 2020 Jul 15].. This remains a complicated question to address, but the answer may lie in differences in the interactions of the tumor and the tumor microenvironment, which play a critical role in the cascade of steps that lead to metastasis (121)Smith CJ, Minas TZ, Ambs S. Analysis of Tumor Biology to Advance Cancer Health Disparity Research. Am J Pathol [Internet]. 2018 [cited 2019 Dec 16];188:304–16.[cited 2020 Jul 15].(122)Wallace TA, Martin DN, Ambs S. Interactions among genes, tumor biology and the environment in cancer health disparities: examining the evidence on a national and global scale. Carcinogenesis [Internet]. 2011 [cited 2019 Dec 16];32:1107–21.[cited 2020 Jul 15].. Genetic ancestry–associated differences in intrinsic tumor biology and the immune microenvironment of tumors are expected to provide broader insights into factors that might influence steps in the metastatic cascade.
A current idea is that there may be subtle differences in how tissue repairs itself across different racial and ethnic minority groups (123)Byun JS, Park S, Caban A, Jones A, Gardner K. Linking Race, Cancer Outcomes, and Tissue Repair. Am J Pathol [Internet]. 2018 [cited 2019 Dec 16];188:317–28.[cited 2020 Jul 15].(124)Byun JS, Gardner K. Wounds That Will Not Heal. Am J Pathol [Internet]. 2013 [cited 2019 Dec 16];182:1055–64.[cited 2020 Jul 15].(125)Gardner K. The Science of Cancer Health Disparities. Am J Pathol [Internet]. 2018 [cited 2019 Dec 16];188:268–70.[cited 2020 Jul 15].. Other data suggest that cells derived from individuals of African ancestry might be more vulnerable to becoming cancerous and gaining invasive characteristics (126)Mackey LC, Annab LA, Yang J, Rao B, Kissling GE, Schurman SH, et al. Epigenetic Enzymes, Age, and Ancestry Regulate the Efficiency of Human iPSC Reprogramming. Stem Cells [Internet]. 2018 [cited 2019 Dec 16];36:1697–708.[cited 2020 Jul 15]..
Integrating Our Knowledge: Charting the Path Forward
Over the past decade, we have made significant progress in how we understand and treat the complex group of diseases we call cancer. We have learned that cancer development is influenced by many factors including a patient’s biological characteristics, social and environmental exposures, and lifestyle. Therefore, we are beginning to see a major shift from a “one size fits all” approach to cancer prevention, screening, and treatment to a more personalized approach called precision medicine. The aim of precision medicine is to use information about an individual’s biology as well as other factors to prevent, diagnose, and treat disease. Precision medicine has the potential to revolutionize cancer care. However, lack of relevant data from racial and ethnic minorities has really hampered the development and implementation of precision medicine for these individuals (see Imprecision of Precision Medicine).
To further improve our understanding of cancer development in the broadest sense we must study the genetic and biological mechanisms that underpin cancer initiation and progression in all populations. Several studies and initiatives such as AACR Project Genomics, Evidence, Neoplasia, Information, Exchange (GENIE) and the NCI-funded African American Breast Cancer Epidemiology and Risk (AMBER) Consortium designed to address gaps in our knowledge about cancer biology in all populations are underway and complement those studies already conducted (see sidebar on Progress in Understanding Cancer Biology in Racial and Ethnic Minorities). To continue enhancing our knowledge of biological and genetic contributors to cancer disparities additional resources are needed, including:
- biospecimens collected and analyzed from patients representing a diverse array of racial and ethnic groups;
- patient-derived cancer models generated from patients representing a diverse array of racial and ethnic groups, including both cell-based and animal models (e.g. cell lines, organoids, PDXs);
- biological information that might be unique to patients from specific racial, ethnic, or ancestral populations; and
- racially diverse research consortia.
A concerted effort is needed from all sectors of the biomedical research community to ensure that the new wave of scientific breakthroughs benefits every cancer patient including individuals from racial and ethnic minorities and other underserved populations.