Imprecision of Precision Medicine
In this section you will learn:
- Precision medicine is a more personalized approach to medicine that uses information about a person’s genes, proteins, and environment to prevent, diagnose, and treat disease.
- In cancer care, genomics is the predominant factor influencing precision medicine, but other biological factors, environmental exposures, and lifestyle also contribute to the uniqueness of each person’s cancer.
- Research identifying the genetic mutations associated with certain cancers has led to numerous new treatments called molecularly targeted therapeutics; these treatments are the backbone of precision medicine in cancer care.
- Our limited knowledge of cancer biology in racial and ethnic minorities diminishes the potential of precision medicine in these populations.
- Research initiatives like AACR Project Genomics, Evidence, Neoplasia, Information, Exchange (GENIE) are beginning to provide more information about cancer in all populations, which will allow us to develop and implement precision medicine for everyone.
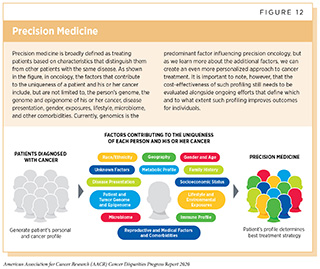
Over the past decade, we have made significant progress in how we understand and treat the complex group of diseases we call cancer. We have learned that each person’s cancer is unique, in part because it is influenced by a patient’s biological characteristics, environmental exposures, and lifestyle. As a result, we have seen a major shift from a “one size fits all” approach to cancer treatment to a more personalized approach called precision medicine. Precision medicine, also referred to as personalized medicine, is defined by the NCI as a form of medicine that uses information about a person’s genes, proteins, and environment to prevent, diagnose, and treat disease (see Figure 12). Precision medicine has the potential to revolutionize cancer care and if used optimally, it may help address the challenges of cancer health disparities.
Precision Medicine: The Promise
In cancer care, precision medicine aims to use genetic and other information about a patient and their tumor, to help diagnose the patient, plan that patient’s treatment, determine how well the treatment is working, and/or make a prognosis. The factors that contribute to the uniqueness of a patient and their cancer include, but are not limited to, the person’s genome, the genome and epigenome of the cancer, disease presentation, gender, exposures, lifestyle, microbiome, and other comorbidities. Currently, genomics is the predominant factor influencing precision oncology. Comprehensive analyses of cancer genomes have revealed numerous genetic mutations associated with various cancers. These discoveries have led to the development and FDA approval of numerous therapeutics targeted to specific molecules with the aim of rectifying the cellular changes that arise due to the mutations. For example, as of January 31, 2020, there are five therapeutics targeting ALK approved for use in the treatment of NSCLC driven by mutations in the ALK gene. Nevertheless, our current knowledge of cancer-causing genetic, lifestyle, and environmental risks is incomplete, and ongoing research will continue to uncover additional cellular and molecular alterations that lead to cancer development.
The development of molecularly targeted therapeutics often relies on the presence of specific biomarkers, such as a genetic mutation, within tumors to identify those patients who are most likely to benefit from these treatments. Genetic biomarkers are detected using tests that frequently utilize cutting-edge technologies such as next-generation sequencing techniques. Notably, recent reports show that using biomarkers, such as the presence of a specific mutation, can increase the efficiency of the clinical development of new therapeutics (340)Wong CHIH, Siah KWEI, Lo AW. Estimation of clinical trial success rates and related. 2018;1–14.[cited 2020 Jul 15].(341)Clinical Development Success Rates. 2016;[cited 2020 Jul 15].. A study looking at anticancer therapeutics estimated that the chance of FDA approval was 10.7 percent for candidate agents that were matched to patients using biomarkers, compared with merely 1.6 percent for unmatched candidates (340)Wong CHIH, Siah KWEI, Lo AW. Estimation of clinical trial success rates and related. 2018;1–14.[cited 2020 Jul 15].. These data emphasize the value of identifying cancer-specific alterations in the development of effective therapies.
The rapid expansion in our knowledge of the genetic mutations that drive individual cancers has led to a number of precision medicine clinical trials designed to streamline the clinical development of new molecularly targeted therapeutics by matching the right therapeutics with the right patients earlier. Among these clinical trials are the NCI’s Molecular Analysis for Therapy Choice trial (NCI-MATCH), the NCI’s Molecular Profiling-Based Assignment of Cancer Therapy trial (NCI-MPACT), and the Biomarker-integrated Approaches of Targeted Therapy for Lung Cancer Elimination (BATTLE) trial (342)NCI-MATCH: More Labs, New Arms, Initial Findings – National Cancer Institute [Internet]. [cited 2019 Dec 18].[cited 2020 Jul 15].(343)Kim ES, Herbst RS, Wistuba II, Lee JJ, Blumenschein GR, Tsao A, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov [Internet]. American Association for Cancer Research; 2011 [cited 2019 Dec 18];1:44–53.[cited 2020 Jul 15].. In NCI-MATCH and NCI-MPACT, patients with a wide array of cancer types are assigned to receive a certain treatment only if genomic sequencing and other tests on their cancers reveal the presence of the matching genetic abnormality. In BATTLE, a similar approach is used for patients with lung cancer. Early data from these as well as other precision medicine trials indicate that patients benefit when treated with therapeutics chosen based on their tumors’ specific genetic features, highlighting the promise of precision medicine (343)Kim ES, Herbst RS, Wistuba II, Lee JJ, Blumenschein GR, Tsao A, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov [Internet]. American Association for Cancer Research; 2011 [cited 2019 Dec 18];1:44–53.[cited 2020 Jul 15].(344)Von Hoff DD, Stephenson JJ, Rosen P, Loesch DM, Borad MJ, Anthony S, et al. Pilot study using molecular profiling of patients’ tumors to find potential targets and select treatments for their refractory cancers. J Clin Oncol [Internet]. 2010 [cited 2019 Dec 18];28:4877–83.[cited 2020 Jul 15].(342)NCI-MATCH: More Labs, New Arms, Initial Findings – National Cancer Institute [Internet]. [cited 2019 Dec 18].[cited 2020 Jul 15].(345)Sicklick JK, Kato S, Okamura R, Schwaederle M, Hahn ME, Williams CB, et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat Med [Internet]. Nature Publishing Group; 2019 [cited 2019 Jun 19];25:744–50.[cited 2020 Jul 15]..
Precision Medicine: The Challenges
To achieve the full potential of precision medicine every cancer patient’s tumor should be tested for the presence of mutations for which there is a matching molecularly targeted therapeutic, and every patient with such a mutation should be treated with the matching anticancer therapeutic. Therefore, the delivery of precision medicine mandates that the right care is delivered to the right patient at the right time. Unfortunately, many recent reports indicate that in real-world settings, this is not the case and only a fraction of patients have their tumors tested for genetic mutations (346)Kehl KL, Lathan CS, Johnson BE, Schrag D. Race, Poverty, and Initial Implementation of Precision Medicine for Lung Cancer. JNCI J Natl Cancer Inst [Internet]. 2019 [cited 2019 Dec 18];111:431–4.[cited 2020 Jul 15].. Moreover, even among patients for whom genetic testing reveals biomarkers associated with a targeted therapeutic, not everyone receives treatment with the targeted therapeutic. For instance, only 64 and 70 percent of NSCLC patients with EGFR and ALK mutations, respectively, received one of the matching molecularly targeted therapeutics (347)Singal G, Miller PG, Agarwala V, Li G, Kaushik G, Backenroth D, et al. Association of Patient Characteristics and Tumor Genomics With Clinical Outcomes Among Patients With Non-Small Cell Lung Cancer Using a Clinicogenomic Database. Jama. 2019;321:1391–9.[cited 2020 Jul 15]..
Challenges with suboptimal implementation of precision medicine are exacerbated in medically underserved populations, including racial and ethnic minorities. For example, a recent analysis of genetic testing rates among lung cancer patients showed that only 14 percent of African Americans received testing compared with 26 percent of white patients (346)Kehl KL, Lathan CS, Johnson BE, Schrag D. Race, Poverty, and Initial Implementation of Precision Medicine for Lung Cancer. JNCI J Natl Cancer Inst [Internet]. 2019 [cited 2019 Dec 18];111:431–4.[cited 2020 Jul 15].. Similar trends have been noted in other types of cancer, including ovarian cancer (348)Kurian AW, Ward KC, Howlader N, Deapen D, Hamilton AS, Mariotto A, et al. Genetic Testing and Results in a Population-Based Cohort of Breast Cancer Patients and Ovarian Cancer Patients. J Clin Oncol [Internet]. 2019 [cited 2019 Dec 18];37:1305–15.[cited 2020 Jul 15]..
Taken together these data highlight the need to identify the current barriers to broad utilization of precision medicine as well as physician- and patient-education programs for effective dissemination of the current knowledge to ensure guidelines-concordant care for every cancer patient.
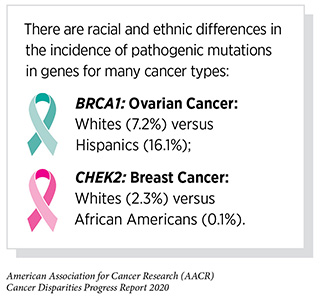
While precision medicine has transformed cancer care for many patients, it has also brought attention to a lack of racial and ethnic diversity in human genomic studies. Our limited knowledge of cancer biology, including inherited cancer predisposition and the genomic underpinnings of cancer initiation and progression, in racial and ethnic minorities diminishes the potential of precision medicine in these populations. For example, past efforts relied on investigators to obtain biospecimens from institutional or local biobanks, which fell short from the perspective of racial and ethnic diversity. While the cumulative benefits of these efforts have been important to developing resources such as TGCA, a recent report that examined the racial diversity of 5,729 samples in TCGA found that whites were over-represented with respect to their proportion in the U.S. population while Asian and Hispanic patients were underrepresented (100)Spratt DE, Chan T, Waldron L, Speers C, Feng FY, Ogunwobi OO, et al. Racial/Ethnic Disparities in Genomic Sequencing. JAMA Oncol [Internet]. 2016 [cited 2019 Dec 16];2:1070.[cited 2020 Jul 15].. In addition, there were only enough samples from white patients to detect mutations present in a given type of cancer at a five percent frequency; there were insufficient samples from any type of cancer in any racial and ethnic minority group to detect mutations present at that frequency. Other reports are consistent with these findings. A recent analysis found that 81 percent of samples included in genome-wide association studies—a tool for discovering the genetic factors involved in common diseases such as cancer—have been from individuals of European ancestry and that individuals of other ancestries have been seriously underrepresented (93)Popejoy, Alice B., Fullerton SM. Genomics is falling. Nature. 2016;538:161–4.[cited 2020 Jul 15].. Rectifying these issues is an area of active research investigation (see Integrating Our Knowledge: Charting the Path Forward).
The underrepresentation of racial and ethnic minorities in cancer clinical trials and prevention research, and the documented challenges to enrolling these individuals into studies, pose another barrier for the implementation of precision medicine among these medically underserved populations. A consistent observation in some of the recent precision medicine trials mentioned above is the lack of diversity in patients. For example, it has been reported that in BATTLE, 82 percent of participants were Caucasian, and only 6 percent African American and 6 percent Hispanics (343)Kim ES, Herbst RS, Wistuba II, Lee JJ, Blumenschein GR, Tsao A, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov [Internet]. American Association for Cancer Research; 2011 [cited 2019 Dec 18];1:44–53.[cited 2020 Jul 15].. For the NCI-MATCH trial, preliminary numbers show that African Americans accounted for about 10 percent of patients who have had their tumors profiled, but only about 8 percent of those who have been treated on study (342)NCI-MATCH: More Labs, New Arms, Initial Findings – National Cancer Institute [Internet]. [cited 2019 Dec 18].[cited 2020 Jul 15].. Therefore, it should not be assumed that the response rates or clinical effectiveness of the therapeutics tested in these trials will generalize to all racial and ethnic groups. Collectively, these challenges can slow the pace of medical innovation, decrease the generalizability of research findings, lead to incorrect interpretations, and limit our full understanding of the effectiveness of precision medicine.
Precision Medicine: The Future Provides Opportunities to Achieve Health Equity
New insights obtained through investigations that incorporate research models and biospecimens that are representative of all populations and the inclusion of all segments of the U.S. population in cancer clinical trials are critical if we are to develop and implement precision medicine that will eliminate cancer for everyone. Many research initiatives are underway to tackle these challenges head-on. To enhance the potential for precision medicine to be effective at reducing disparities in health care and outcomes among minorities, strategic efforts and investments have been made by the NIMDH and NCI to fund centers in Precision Medicine and Minority Health. These transdisciplinary centers are working to understand the combined contribution of genetic, environmental, and lifestyle factors to racial and ethnic disparities in health care and outcomes. This approach is distinct from previous efforts that examined these factors in isolation. The objectives are to advance the science of minority health and health disparities research by exploring the contribution of understudied determinants of minority health and establish the infrastructure to integrate basic, genomic, and population sciences within the context of health disparities research.
Funded by the NIH, the All of Us Research Program is another initiative to address the lack of racial and ethnic inclusivity in biomedical research. The scientific goals of these major prospective, retrospective, and cross-sectional analyses are to improve the understanding of health disparities, discover new disease biomarkers, support clinical trials, and develop new targeted therapeutics for a range of diseases including cancer. The program plans to enroll a diverse group of at least 1 million individuals in the United States with the aim of accelerating the pace of biomedical research and improving public health. The participants are asked to share their electronic health record data, donate biospecimens for genomic and other laboratory analyses, respond to surveys, and have standardized physical measurements taken. Participants can also contribute data from digital health devices. Researchers are currently developing tools to collect additional information that includes surveys regarding social and behavioral determinants of health as well as geospatial and environmental data such as air quality and pollutant levels.
Active community engagement has been one of the foundational pillars of the All of Us Research Program. All of Us has provided funding to health care providers and organizations (for example, federally qualified health centers) to identify best practices for recruiting and enrolling medically underserved patients into this precision medicine initiative. Similarly, organizations in the All of Us Engagement Partners are funded to motivate individuals from diverse communities to participate. As of July 2019, the program has enrolled more than 175,000 participants at more than 340 recruitment sites. More than half of the participants are nonwhite, and more than 80 percent are from groups that have been historically underrepresented in biomedical research (349) Special Report: The “All of Us” Research Program. 2019 [cited 2020 Jul 15]..
Other clinical research initiatives that are aimed toward reducing cancer health disparities among minority patients and survivors include NCI-led studies such as the Research on Prostate Cancer in Men of African Ancestry: Defining the Roles of Genetics, Tumor Markers, and Social Stress (RESPOND) (350)NIH, PCF launch study on prostate cancer in black men – National Cancer Institute [Internet]. [cited 2019 Dec 18].[cited 2020 Jul 15]. and the Detroit Research on Cancer Survivors (ROCS) (323)Beebe-Dimmer JL, Albrecht TL, Baird TE, Ruterbusch JJ, Hastert T, Harper FWK, et al. The Detroit Research on Cancer Survivors (ROCS) Pilot Study: A focus on outcomes after cancer in a racially-diverse patient population. Cancer Epidemiol Biomarkers Prev [Internet]. American Association for Cancer Research; 2018 [cited 2019 Dec 17];28:cebp.0123.2018.[cited 2020 Jul 15].. These coordinated research efforts are designed to understand the role of environmental, genetic, social, and behavioral determinants of health that contribute to the higher burden among African American patients with prostate and breast cancer.
As we move deeper into the era of precision cancer medicine it is clear that the genomic characteristics of a patient’s cancer will need to be considered along with other factors, including the patient’s epigenome, microbiome, metabolome, lifestyle, and environmental exposures, which are emerging as important influences on cancer initiation and progression. To deepen our understanding of these factors we need to first generate and gather real-world data, including patient history, results from diagnostic and genetic tests, treatment decisions, and measured and patient-reported outcomes from large numbers of cancer patients from all backgrounds including racial and ethnic minorities. One way to accelerate the pace at which we gather patient-derived information is through data sharing, and several cancer organizations as well as multi-institutional teams have already launched initiatives to catalyze these efforts. A few examples of these cross-institutional projects are AACR Project Genomics, Evidence, Neoplasia, Information, Exchange (GENIE), ASCO CancerLinQ, BRCA Exchange, NCI Genomic Data Commons, and Oncology Research Information Exchange Network (ORIEN).
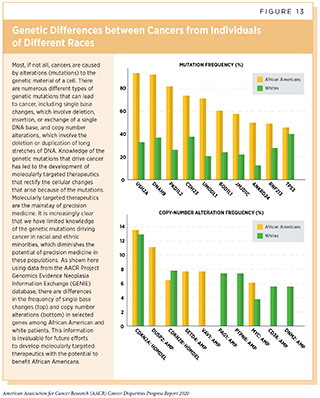
By using these “big data” sets researchers can answer many of cancer’s most elusive questions. Physicians may be able to match existing FDA-approved molecularly targeted therapeutics to novel cancer types or identify subgroups of patients who are most or least likely to benefit from a certain treatment. Researchers may also be able to uncover important information related to cancer health disparities. For example, using the AACR Project GENIE database, which currently includes genomic and other information on nearly 4000 African American cancer patients, we can infer that there are significant differences in the frequency of alterations in many genes between African American and white patients (Figure 13). These data are invaluable regarding ongoing and future endeavors for the development of targeted therapeutics.
For precision medicine to be precise, we must increase the resolution of our measurements. Precision medicine must rely on accuracy for personalizing prevention, diagnosis, and treatment; however, with our current limitations in the diversity of patient cohorts in biomedical research, the precision and accuracy of this paradigm remains critically limited. As discussed throughout this report, most clinical trials as well as precision oncology studies are largely limited to patients of European descent. Because of the lack of diversity, the measurements and results do not describe the full story. We must ensure that the next wave of breakthroughs in cancer science and medicine is not limited to a select few, but benefits all patients. We also need to ensure that the latest tools in medical innovation, such as digital health or artificial intelligence, are racially unbiased and are used for the benefit of all patients (351)Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science [Internet]. American Association for the Advancement of Science; 2019 [cited 2019 Dec 18];366:447–53.[cited 2020 Jul 15].(352)Shcherbina A, Mattsson C, Waggott D, Salisbury H, Christle J, Hastie T, et al. Accuracy in Wrist-Worn, Sensor-Based Measurements of Heart Rate and Energy Expenditure in a Diverse Cohort. J Pers Med [Internet]. Multidisciplinary Digital Publishing Institute; 2017 [cited 2019 Dec 18];7:3.[cited 2020 Jul 15].. It is therefore encouraging that efforts such as the All of Us program are in progress. Nevertheless, there needs to be a stronger, concerted, and continued push from all stakeholders including the government, industry, and the community to develop new guiding principles and policies to understand why these limitations in racial and ethnic diversity persist, and what approaches can be developed to promote and incentivize inclusion in studies and trials. Only when we make complete measurements of the etiology of cancer across all populations will we see precision medicine truly become precise.