Executive Summary
Transformative research and technological innovation are driving unprecedented progress against the collection of diseases we call cancer. Despite the significant barriers created by the Coronavirus Disease 2019 (COVID-19) pandemic to many aspects of medical research and cancer patient care, scientists have continued their quest to cure cancer, while responding to the challenges posed by the pandemic through innovative adaptations across the continuum of cancer science and medicine.
As the first and largest professional organization in the world with a steadfast mission to prevent and cure all cancers, American Association for Cancer Research (AACR) is dedicated to increasing public understanding of cancer and the important role of medical research in saving lives. AACR is also committed to advocating for increased annual federal funding to government entities that drive progress against cancer and improve public health, in particular, National Institutes of Health (NIH), National Cancer Institute (NCI), U.S. Food and Drug Administration (FDA), and Centers for Disease Control and Prevention (CDC).
The annual AACR Cancer Progress Report to Congress and the American public is a cornerstone of the AACR’s educational and advocacy efforts. This eleventh edition of the report highlights how research continues to transform lives, like the lives of the courageous individuals featured in the report who have shared their experiences with cancer. It also underscores how the COVID-19 pandemic has negatively affected cancer research and care, as well as how unwavering bipartisan support from Congress, in the form of robust and sustained annual increases in funding for NIH, NCI, and FDA, is vital if we are to accelerate the pace of progress against cancer for the benefit of individuals everywhere.
Cancer in 2021
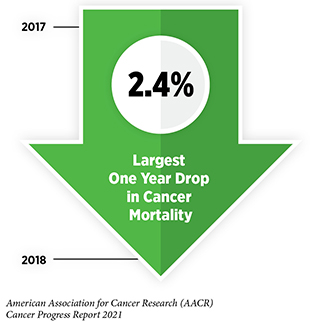
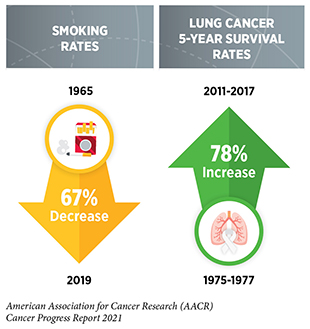
Research is the backbone of progress against cancer because it spurs the development of novel and better approaches to preventing, detecting, diagnosing, treating, and curing many of the diseases we call cancer. These advances are driving down overall U.S. cancer incidence and death rates and increasing the number of individuals who are surviving longer after a cancer diagnosis. For example, the age-adjusted overall U.S. cancer death rate declined by 31 percent from 1991 to 2018, which is the last year for which these data are available. Rapid declines in the death rates from aggressive tumors, such as lung cancer and melanoma, over the last decade have contributed significantly to this reduction in overall cancer deaths. In addition, the U.S. 5-year relative survival rate for all cancers combined has increased from 49 percent for people diagnosed in the mid-1970s to 68 percent for those diagnosed from 2011 to 2017.
Even though we are making significant progress, cancer continues to be an enormous public health challenge in the United States and around the world. One challenge is that the number of new cancer cases is projected to increase dramatically in the coming decades, rising from nearly 1.9 million in 2021 to more than 2.2 million in 2040 in the United States alone. This sharp increase is anticipated largely because of the overall population growth and because the segment of the U.S. population that accounts for most cancer diagnoses—those age 65 and older—is expanding.
Another pressing public health challenge is that the burden of cancer is shouldered disproportionately by racial and ethnic minorities and other underserved populations. Racial and ethnic minorities have also shouldered a disparate burden of the ongoing COVID-19 pandemic, laying bare stark inequities in health care. It is imperative that all stakeholders play a role in eradicating the systemic and structural injustices that are barriers to health equity.
The immense toll of cancer is felt not only through the number of lives it affects each year, but also through its significant economic impact. In the United States, an estimated $200.7 billion of total health care costs was spent on cancer-related health care in 2020. That number is projected to increase to $245.6 billion by 2030. These costs do not reflect the additional indirect economic burden due to lost earnings or lost productivity or the potential adverse impacts of COVID-19 on cancer-related health care. With the personal and economic burden of cancer predicted to rise in the next few decades, it is vital that the nation invests in the groundbreaking research that drives progress against cancer.
Read the Full Section: Cancer in 2021Understanding How Cancer Develops
Discoveries across the spectrum of cancer research from basic science to translational, clinical, and population research have led to our current understanding of how cancer arises and develops. We now understand that cancer is a collection of diseases that arise when the processes that control normal cell growth, cell division, and cellular life span go awry. This happens primarily because of changes, or mutations, in the genetic material of normal cells. The identity of genetic mutations and the order and speed at which a cell acquires them determine the length of time it takes a given cancer to develop. Inherited mutations play a role in about 10 percent of cancer cases, but most cancers are caused by mutations acquired over an individual’s lifetime. Some mutations are acquired during normal cell division; others are acquired because of persistent exposure to substances that damage genetic material, such as carcinogens in tobacco smoke and ultraviolet radiation (UV) from the sun, among other cancer risk factors and yet other mutations are associated with underlying medical conditions such as chronic inflammation.
Although genetic alterations underpin cancer initiation in most cases, interactions between cancer cells and their environment—known as the tumor microenvironment—play an important role in disease progression.
Read the Full Section: Understanding How Cancer DevelopsPreventing Cancer: Identifying Risk Factors
Decades of research have led to the identification of numerous factors that increase a person’s risk of developing cancer. Given that exposure to many of these factors can be eliminated or reduced, many cases of cancer can be prevented. In fact, it is estimated that about 40 percent of cancer cases in the United States are attributable to preventable causes.
The main preventable causes of cancer are tobacco use, obesity, poor diet, lack of physical activity, alcohol consumption, exposure to UV light from the sun or tanning devices, and failure to use interventions that treat or prevent infection with cancer-associated pathogens, such as cancer-causing strains of the human papillomavirus (HPV).
The development and implementation of public education and policy initiatives designed to eliminate or reduce exposure to preventable causes of cancer have reduced cancer incidence, morbidity, and mortality in the United States. Thanks to such initiatives, cigarette smoking rates among U.S. adults have declined steadily from 42 percent in 1965 to 14 percent in 2019. However, the current popularity of electronic cigarettes (e-cigarettes) among U.S. youth and young adults threatens to reverse our significant progress against tobacco use. Recent legislation that raises the federal minimum age of sale of all tobacco products, including e-cigarettes, to 21 years, and impose restrictions on kid-friendly e-cigarette flavors have the potential to accelerate future progress against tobacco-related diseases.
The prevalence of obesity, another major risk factor that is linked to 15 types of cancer, continues to rise among U.S. adults and children. These trends threaten to slow the rapid decline in overall cancer death rates that we have experienced in recent years.
Therefore, it is essential that all stakeholders work together to enhance the dissemination of our current knowledge of cancer risk prevention and implement evidence-based policies to minimize the incidence, morbidity, and mortality of cancers attributable to preventable causes.
Read the Full Section: Identifying Risk FactorsScreening for Early Detection
Cancer screening refers to checking for precancerous lesions or cancer in people who have no signs or symptoms of the cancer for which they are being checked. Research discoveries that have deepened our understanding of cancer initiation and progression are the foundation of screening strategies to detect precancerous lesions or cancer at an early stage of development. Finding precancerous lesions or cancer at an early stage of development makes it more likely that a cancer can be intercepted, and a patient can be treated successfully.
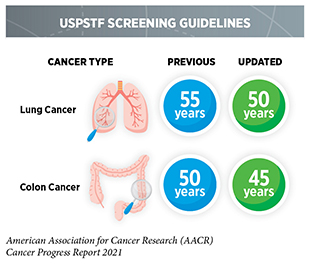
Determining whether broad implementation of a cancer screening test across a defined population can decrease deaths from the screened cancer and provide benefits that outweigh the potential risks of undergoing the test requires extensive research and careful analysis of the data generated. Currently, there are five types of cancer—breast, cervical, colorectal, lung, and prostate cancer—for which screening tests have been used to monitor large segments of the U.S. population. During the 12 months covered in this report, U.S. Preventive Services Task Force (USPSTF), an independent volunteer panel of experts in prevention and evidence-based medicine, updated their guidelines for colorectal and lung cancer screening by expanding the age-based eligibility to a broader population.
Every person has a unique risk for each type of cancer based on genetic, molecular, and cellular makeup, lifetime exposures to cancer risk factors, and general health, as well as the person’s own tolerance of the potential risks of a screening test. Therefore, individuals should consult with their health care practitioners to develop a personalized cancer prevention and early detection plan.
Read the Full Section: Screening for Early DetectionDiscovery Science Driving Clinical Breakthroughs
The dedicated efforts of individuals working throughout the continuum of cancer science and medicine are constantly powering the translation of new research discoveries into lifesaving advances for people in the United States and around the world. Between August 1, 2020 and July 31, 2021—the 12-month period covered in this report—FDA approved 16 new anticancer therapeutics and expanded the use of 11 previously approved anticancer therapeutics for treating new cancer types.
Several of these approvals are groundbreaking advances.
In May 2021, FDA approved the first molecularly targeted therapeutic against the protein KRAS, which has long been considered an undruggable target, for the treatment of certain patients with lung cancer. The therapeutic, sotorasib (Lumakras), targets an altered form of the protein, known as KRAS G12C, and is providing a new treatment option and new hope for patients with non–small cell lung cancer such as Steve Castellaw.
In March 2021, FDA approved the first CAR T-cell therapy for the treatment of patients with multiple myeloma such as David Wellenstein, MD. In April 2021, FDA approved a new immune-checkpoint inhibitor, dostarlimab-gxly (Jemperli), for treatment of patients with endometrial cancer whose tumors have a specific genetic feature. Both CAR T cells and immune-checkpoint inhibitors are types of immunotherapeutics, a class of revolutionary anticancer agents that have been shown to yield remarkable responses for many patients with advanced cancers.
Read the Full Section: Science Driving Clinical BreakthroughsSupporting Cancer Patients and Survivors
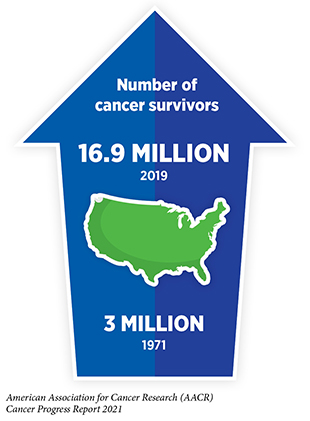
Research-fueled advances in cancer detection, diagnosis, and treatment are helping more and more people to survive longer and lead fuller lives after a cancer diagnosis. According to the latest estimates, more than 16.9 million U.S. adults and children with a history of cancer were alive on Jan. 1, 2019, compared to just 3 million in 1971.
Rapid advances across the continuum of cancer research and care have also highlighted the current gaps in our knowledge that require additional research. We have learned that survivors of cancer still face serious and persistent adverse outcomes, including physical, emotional, and psychosocial challenges, because of their disease and treatment. Each person diagnosed with cancer faces his or her own unique set of challenges. For example, an estimated 25 percent of cancer survivors report poor physical health and 10 percent report poor mental health, both adversely affecting quality of life. Researchers are exploring ways to utilize healthy behaviors, palliative care, psycho-oncology, and other evidence-based strategies to improve survival and quality of life for patients with cancer and survivors of cancer. For example, research has shown that an active lifestyle is especially beneficial to cancer survivors because it can help mitigate the numerous physical, mental, and emotional challenges they experience.
Ongoing research is investigating the potential of new technologies and innovative intervention strategies for coordinated care that improves the quality of life and meets the personalized needs of cancer survivors and caregivers from different age groups.
Read the Full Section: Supporting Cancer Patients and SurvivorsLooking to the Future
Research drives progress against cancer because it provides us with a deeper understanding of cancer biology.
As we look to the future, many researchers, including AACR President (2020–2021) David A. Tuveson, MD, PhD, FAACR, are confident that we can accelerate the pace of progress against cancer by facilitating synergistic collaborations across disciplines and by assembling and supporting a diverse workforce. The new wave of innovation driven by advances in discovery science will enable researchers to gain a deeper insight into the mechanisms underlying cancer development and identify novel ways to target and eradicate cancer cells. In addition, incorporation of cutting-edge technologies, such as liquid biopsies and artificial intelligence (AI), will allow us to achieve the full potential of precision medicine by addressing a wide range of unsolved clinical questions across the spectrum of cancer research and care.
Combating Cancer Through Science-based, Patient-centered Policies
Federal investments in NIH, NCI, FDA, and CDC have fueled tremendous advances against cancer by catalyzing scientific discoveries and facilitating the translation of these discoveries into new and better anticancer medical products and community-based programs to save lives and improve public health.
To continue to make strides against cancer we need robust, sustained, and predictable annual budget increases for NIH and NCI. We also need ongoing congressional commitment to support the important role of FDA to ensure the safety and efficacy of anticancer therapeutics, and to support the cancer prevention and control programs at CDC. These vital investments will help diversify the research workforce, advance regulatory science initiatives, and allow us to pursue policies that improve cancer prevention, early detection, and control for individuals, families, and communities.
Read the Full Section: Combating Cancer through PolicyThe AACR Call to Action
The extraordinary advances against cancer detailed in this report were made possible by the dedicated efforts of a broad coalition of researchers, clinicians, cancer survivors, patient advocates, and policy makers. Decades of investment in medical research have fueled new discoveries, making it possible to prevent, detect, diagnose, treat, and cure many types of cancer that previously lacked effective treatment options. These advances are driving down overall U.S. cancer incidence and death rates and increasing the number of individuals who are surviving longer after a cancer diagnosis.
Thanks to the remarkable bipartisan efforts of Congress, NIH funding has increased by nearly $13 billion or 42 percent from FY 2015 to FY 2021. These significant investments make it possible for researchers across the country to continue making advances against cancer and many other diseases.
Despite this progress, much more work needs to be done on behalf of those living with cancer and those who will be diagnosed in the future. For example, there are still no effective treatments for many of the over 200 known types of cancer. Furthermore, the COVID-19 pandemic has had a profoundly negative impact on medical research and cancer care, bringing many critical projects to a halt, delaying screening and treatments, and diverting resources to the immediate need of responding to COVID-19. The adverse consequences of the COVID-19 pandemic will be felt for years and perhaps decades to come.
As the United States recovers from the devastating toll of the COVID-19 pandemic, we are reminded of the enormous value of medical research in overall public health. Decades of investment in basic, translational, and clinical research have enabled scientists to develop diagnostics, treatments, and vaccines for this novel disease at a pace never seen before. This robust approach to medical research has already saved hundreds of thousands of lives from COVID-19 in the United States alone. Cancer researchers were uniquely positioned to respond to the challenges posed by COVID-19 and have played a vital role in combatting the pandemic while continuing their quest to cure cancer. With so many promising opportunities ahead of us it is critical that we maintain our momentum of progress against cancer.
AACR deeply appreciates the commitment of Congress to expediting progress against cancer and other diseases through robust funding increases for NIH, as well as to supporting the critical regulatory science work at FDA and public health programs of CDC.
Therefore, AACR urges Congress to:
- Continue to support robust, sustained, and predictable growth for NIH and NCI by providing increases in their FY 2022 base budgets of at least $3.2 billion and $1.1 billion, respectively, for a total funding level of $46.4 billion for NIH and $7.6 billion for NCI.
- Ensure that the funding designated through the 21st Century Cures Act for targeted initiatives, including the National Cancer Moonshot, is fully appropriated in FY 2022 and is supplemental to the overall increase in the NIH base budget.
- Provide at least $10 billion for NIH in emergency supplemental funding to restart research and clinical trials that have been put on hold due to the pandemic, as proposed in the Research Investment to Spark the Economy (RISE) Act of 2021.
- Provide $50 million for the third year of the Childhood Cancer Data Initiative and no less than $30 million for the continued implementation of the Childhood Cancer STAR Act.
- Support the creation of an Advanced Research Projects Agency for Health (ARPA-H) designed to prioritize high-risk, high-reward approaches to prevent, diagnose, and cure diseases such as cancer.
- Support FDA’s critical regulatory science initiatives and advance the development and regulation of oncology products by providing an increase of at least $343 million in discretionary budget authority in FY 2022, as recommended in President Biden’s budget proposal.
- Support vital CDC Cancer Prevention and Control Programs with total funding of at least $559 million. This includes funding for comprehensive cancer control, cancer registries, and screening and awareness programs for specific cancers.
If we hope to reach the day when cancer is no longer a major health threat to our nation’s citizens, Congress must provide robust, sustained, and predictable annual funding increases for NIH, NCI, FDA, and CDC in FY 2022 and beyond. These investments will help us transform cancer care, increase survivorship, spur economic growth, and maintain the position of the United States as a global leader in scientific and medical research and specifically in cancer research. Most importantly this will continue to bring lifesaving cures to the millions of people worldwide whole lives are touched by cancer.
Read the Full Section: The AACR Call to Action