Understanding How Cancer Develops
In this section, you will learn:
- Research provides our understanding of the biology of cancer, which is not one disease, but a collection of diseases characterized by the uncontrolled growth of cells.
- Genetic mutations underpin cancer initiation and development in most cases; cancers in about 10 percent of patients contain inherited mutations.
- Cancer initiation and progression are strongly influenced by interactions among cancer cells and cellular and molecular factors in their environment, referred to as the tumor microenvironment.
- Each person’s cancer is unique and so is the response to treatments; understanding why certain patients respond exceptionally well to treatments that are not effective for others is an elusive question in cancer medicine and an area of extensive ongoing investigation.
- The more we know about the contributions of the numerous individual genetic and other factors and their interplay in influencing cancer development among all populations, the more precisely and effectively we can prevent, diagnose, and treat cancer.
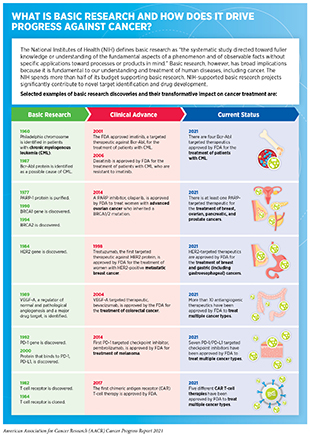
Discoveries across the breadth of medical research, from basic research to translational, clinical, and population science, have fueled our current understanding of cancer initiation and progression (see sidebar on What Is Basic Research and How Does It Drive Progress against Cancer?).
We now understand that cancer is not one disease but a collection of diseases that arise when the processes which control normal cell growth, division, and life span go awry. As a result, cells start to multiply uncontrollably, fail to die, acquire unique ways to obtain nutrients for survival, and begin to accumulate. In body organs and tissues, the accumulating cancer cells form masses called tumors, whereas in the blood or bone marrow they crowd out normal cells. Over time, some cancer cells may invade distant tissues, a process termed metastasis, by entering the bloodstream or the lymphatic system and form secondary tumors at remote sites. Most deaths from cancer are due to metastasis. Therefore, understanding the biological underpinnings of metastatic dissemination and identifying ways to prevent this process are key focus areas of ongoing research (127)Quinn JJ, Jones MG, Okimoto RA, et al. Single-cell lineages reveal the rates, routes, and drivers of metastasis in cancer xenografts. Science (80- ). 2021;371(6532).(128)Ubellacker JM, Tasdogan A, Ramesh V, et al. Lymph protects metastasizing melanoma cells from ferroptosis. Nature. 2020;585(7823)..
Cancer Development: Influences Inside the Cell
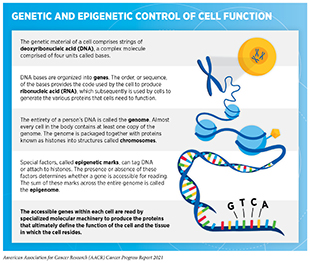
The normal behavior of each cell in the human body is controlled by its genetic material. The genetic material comprises chains of deoxyribonucleic acid (DNA), a complex molecule made up of four building blocks called bases. The four bases are organized in a very specific pattern to build two paired chains of the DNA. The DNA is packaged together with proteins called histones into condensed structures called chromosomes that are contained within a cell’s nucleus (see sidebar on Genetic and Epigenetic Control of Cell Function). Each person gets 23 chromosomes from each parent; thus, each normal cell has 46 chromosomes. The DNA is first converted into another complex molecule called ribonucleic acid (RNA), which is subsequently used by the cell to manufacture proteins. The order of the DNA bases and the way the DNA chains are packaged into chromosomes dictate which proteins and how much of them are made by each cell. Proteins are the molecules that perform important functions that dictate a cell’s fate.
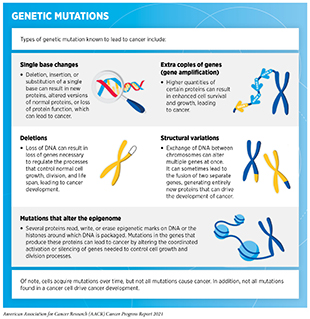
Alterations in the DNA sequence, referred to as mutations, can disrupt normal protein function, and are the leading cause of cancer development (see sidebar on Genetic Mutations). Each person’s cancer has a unique combination of mutations, and as cancer cells divide, new mutations arise in the daughter cells. Thus, a tumor is made up of a collection of cancer cells with a wide range of genetic abnormalities. This variation in cell types, also known as tumor heterogeneity, is evident in most tumors. Tumor heterogeneity fuels the cancer’s ability to grow faster and metastasize, escape therapy, and evade destruction by the immune system. Therefore, characterization of tumor heterogeneity is an area of extensive research investigation (130)Lee Y, Bogdanoff D, Wang Y, et al. Xyzeq: Spatially resolved single-cell RNA sequencing reveals expression heterogeneity in the tumor microenvironment. Sci Adv. 2021;7(17).(131)Dentro SC, Leshchiner I, Haase K, et al. Characterizing genetic intra-tumor heterogeneity across 2,658 human cancer genomes. Cell. 2021;184(8)..
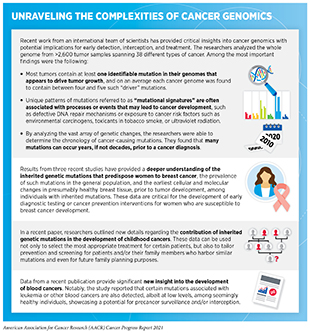
While inherited genetic mutations, often associated with cancer predisposing syndromes, play a role in about 10 percent of all cancer cases (see Table 2), most mutations are acquired over an individual’s lifetime due to errors arising during normal cell multiplication or because of environmental exposures, lifestyle factors, or coexisting health conditions (see sidebar on Sources of Genetic Mutations). Ongoing research continues to uncover the mutational landscape of specific cancer types providing new insights into the genetic basis of cancer (130)Lee Y, Bogdanoff D, Wang Y, et al. Xyzeq: Spatially resolved single-cell RNA sequencing reveals expression heterogeneity in the tumor microenvironment. Sci Adv. 2021;7(17).(132)De Jonge MM, De Kroon CD, Jenner DJ, et al. Endometrial Cancer Risk in Women With Germline BRCA1 or BRCA2 Mutations: Multicenter Cohort Study.(133)Tlemsani C, Takahashi N, Pongor L, et al. Whole-exome sequencing reveals germline-mutated small cell lung cancer subtype with favorable response to DNA repair-targeted therapies. Sci Transl Med. 2021;13(578). (see sidebar on Unraveling the Complexities of Cancer Genomics).
Not all mutations acquired by a cell lead to cancer. In fact, the genes that are mutated, and the order and speed at which a cell acquires mutations, determine whether a cancer will develop and, if a cancer does develop, the length of time it will take to happen. The progressive nature of cancer provides distinct time points for medical intervention to prevent, detect, and/or intercept cancer early, and to treat progressive disease (149)Fantini MC, Guadagni I. From inflammation to colitis-associated colorectal cancer in inflammatory bowel disease: Pathogenesis and impact of current therapies. Dig Liver Dis. 2021;53(5).. In general, the further a cancer has progressed, the harder it is to stop the chain of events that leads to the emergence of metastatic disease, which is the cause of most deaths from solid tumors. Therefore, understanding the cellular and molecular events that contribute to the transition from healthy to precancerous cells to progressive disease is an area of immense scientific focus (150)Jiang Q, Isquith J, Ladel L, et al. Inflammation-driven deaminase deregulation fuels human pre-leukemia stem cell evolution. Cell Rep. 2021;34(4).(151)Rozenblatt-Rosen O, Regev A, Oberdoerffer P, et al. The Human Tumor Atlas Network: Charting Tumor Transitions across Space and Time at Single-Cell Resolution. Cell. 2020;181(2).(152)Tilston-Lunel A, Mazzilli S, Kingston NM, et al. Aberrant epithelial polarity cues drive the development of precancerous airway lesions. Proc Natl Acad Sci U S A. 2021;118(18)..
In addition to genetic mutations, changes in the physical structure of DNA caused by chemical modifications of the DNA and/or the proteins associated with it, termed epigenetic modifications, can lead to cancer development (see sidebar on Genetic and Epigenetic Control of Cell Function). Epigenetic modifications regulate how and when our genes are turned “on” or “off,” and they are made by specialized proteins that “add” or “erase” unique chemical modifications of DNA and/or histones (153)MA D. The cancer epigenome: Concepts, challenges, and therapeutic opportunities. Science. 2017;355(6330):1147-1152.. In contrast to genetic mutations, epigenetic changes are often reversible, providing an opportunity for therapeutic intervention. Our understanding of the role of epigenetics in cancer is continuously evolving, and ongoing research is uncovering the enormous potential of targeting epigenetic pathways in cancer treatment (154)Chung C, Sweha SR, Pratt D, et al. Integrated Metabolic and Epigenomic Reprograming by H3K27M Mutations in Diffuse Intrinsic Pontine Gliomas. Cancer Cell. 2020;38(3).(155)Sroka MW, Vakoc CR. An epigenetic tipping point in cancer comes under the microscope. Nature. 2021;590(7846)..
Research aimed at the identification of genetic and epigenetic alterations that drive cancer development has led to the development of a new class of treatments—molecularly targeted therapeutics—which aim to rectify the cellular changes that arise due to such alterations. While these advances have revolutionized clinical cancer care for some patients, they have also brought attention to the fact that racial or ethnic minorities are grossly underrepresented in genetic databases and in most clinical research investigations, including those in cancer science and medicine (156)Landry LG, Ali N, Williams DR, Rehm HL, Bonham VL. Lack Of Diversity In Genomic Databases Is A Barrier To Translating Precision Medicine Research Into Practice. https://doi.org/101377/hlthaff20171595. 2018;37(5):780-785.(157)Sirugo G, Williams SM, Tishkoff SA. The Missing Diversity in Human Genetic Studies. Cell. 2019;177(1):26-31.. The lack of diversity in cancer genomic studies limits our understanding of cancer biology, including inherited cancer predisposition, in underrepresented populations. Rectifying this issue is an area of active research investigation, as reported in the AACR Cancer Disparities Progress Report 2020 (91)Sengupta R, Honey K. AACR Cancer Disparities Progress Report 2020 : Achieving the Bold Vision of Health Equity for Racial and Ethnic Minorities and Other Underserved Populations . Cancer Epidemiol Biomarkers Prev. Published online 2020..
Cancer Development: Influences Outside the Cell
Cancer arises due to the disruption of normal cellular functions through genetic and epigenetic changes in a cell. Once cancer is initiated, however, complex interactions between cancer cells and their surrounding environment—known as the tumor microenvironment—contribute to disease progression.
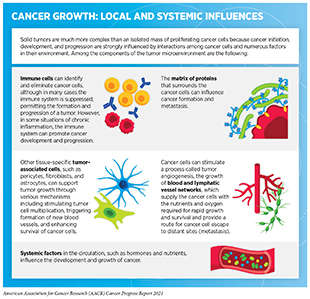
The tumor microenvironment is a specialized niche surrounding the cancer cells in a tumor and consists of immune cells—components of one’s natural defense mechanism—as well as other cellular and molecular elements (see sidebar on Cancer Growth: Local and Global Influences). Bidirectional communications between cancer cells and their microenvironment affect tumor progression and metastasis (158)Zewdu A, Casadei L, Pollock RE, Braggio D. Adipose Tumor Microenvironment. In: Advances in Experimental Medicine and Biology. Vol 1226. ; 2020.(159)Hawkins ED, Duarte D, Akinduro O, et al. T-cell acute leukaemia exhibits dynamic interactions with bone marrow microenvironments. Nature. Published online 2016.(160)Mcgranahan N, Swanton C. Review Clonal Heterogeneity and Tumor Evolution : Past , Present , and the Future. Cell. 2017;168(4):613-628.. The tumor microenvironment can also shelter cancer cells from the effects of radiation, chemotherapy, and immunotherapy, thereby rendering them resistant to treatment (161)Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18(16).(162)Khalaf K, Hana D, Chou JTT, Singh C, Mackiewicz A, Kaczmarek M. Aspects of the Tumor Microenvironment Involved in Immune Resistance and Drug Resistance. Front Immunol. 2021;12..
Ongoing research is likely to uncover additional mechanisms by which the tumor microenvironment interacts with cancer cells which may, in turn, lead to the development of new and improved treatments against cancer. For instance, two new studies unraveled key cellular and molecular features of the tumor microenvironment that are critical for keeping tumors in check and may be utilized for future therapeutic targeting of aggressive cancers such as pancreatic cancer and melanoma (163)Chen Y, Kim J, Yang S, et al. Type I collagen deletion in αSMA+ myofibroblasts augments immune suppression and accelerates progression of pancreatic cancer. Cancer Cell. 2021;39(4).(164)Lim SA, Wei J, Nguyen TLM, et al. Lipid signalling enforces functional specialization of Treg cells in tumours. Nature. 2021;591(7849).. Yet another series of recent articles characterized lung cancer cells and their microenvironment (165)Swanton C. Take lessons from cancer evolution to the clinic. Nature. 2020;581(7809).(166)Casanova-Acebes M, Dalla E, Leader AM, et al. Tissue-resident macrophages provide a pro-tumorigenic niche to early NSCLC cells. Nature. Published online 2021.. The data provide deep insights into the interactions between the immune microenvironment and cancer cells in different regions within a tumor and across different tumors in a patient (167)K J, MR de M, M I, et al. Spatial heterogeneity of the T cell receptor repertoire reflects the mutational landscape in lung cancer. Nat Med. 2019;25(10):1549-1559.(168)K A, SEA R, R R, et al. Geospatial immune variability illuminates differential evolution of lung adenocarcinoma. Nat Med. 2020;26(7):1054-1062.. The studies further characterize the wide range of alterations, including mutations, within immune and cancer cells that enable lung cancers to evade attack and elimination by the immune system (169)S L, EL L, S H, et al. Interplay between whole-genome doubling and the accumulation of deleterious alterations in cancer evolution. Nat Genet. 2020;52(3):283-293.(170)R R, EL C, R S, et al. Neoantigen-directed immune escape in lung cancer evolution. Nature. 2019;567(7749):479-485.(171)E G, JL R, JY H, et al. The T cell differentiation landscape is shaped by tumour mutations in lung cancer. Nat cancer. 2020;1(5):546-561.. Collectively, these discoveries have critical implications in understanding cancer progression and relapse, as well as a tumor’s response to state-of-the-art treatments such as immunotherapies.
Cancer Development: Integrating Our Knowledge
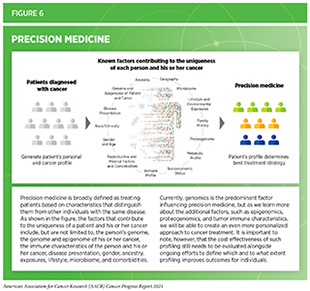
The remarkable progress in discovery science during the past five decades has transformed our understanding of the complex group of diseases we call cancer. We have learned that cancer development is influenced by many factors, including a patient’s biological characteristics, social and environmental exposures, and lifestyle. As each person’s experience is unique, so is his or her cancer. As a result, we are beginning to see a major shift from a “one size fits all” paradigm to cancer prevention, screening, and treatment to a more personalized approach called precision medicine (see Figure 6).
Notably, we have also learned that even among patients with the same type of cancer based on traditional classification, each patient’s response to treatment is unique. For example, a recent analysis of the cellular and molecular characteristics of tumors derived from patients with metastatic cancers showed that the response to the same treatment can vary widely among patients. In fact, the researchers were able to identify a subset of patients who responded exceptionally well to therapeutics that are usually only effective in fewer than 10 percent of individuals with similar cancer types (173)Wheeler DA, Takebe N, Hinoue T, et al. Molecular Features of Cancers Exhibiting Exceptional Responses to Treatment. Cancer Cell. 2021;39(1).. The researchers further identified patterns within tumor cells and the microenvironment that could potentially explain the exceptional responses to treatment. Knowledge gained from studies such as these, and other ongoing and future investigations that characterize the genetic and epigenetic alterations within tumors and the microenvironment will help us move closer to the promise of delivering precision medicine for all patients with cancer.
Precision medicine aims to use genetic and other information about a patient’s tumor, as well as other factors, to help diagnose, plan treatment, determine how well treatment is working, or make a prognosis, with the overarching goal of improving clinical outcomes and minimizing unnecessary diagnostic and therapeutic interventions. Currently, tumor genetics is the predominant factor guiding precision medicine in cancer, and there are emerging data that the adoption of this approach provides substantial survival benefits for patients (174)Howlader N, Forjaz G, Mooradian MJ, et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med. 2020;383(7).(175)MJ P, EM B, JR B, et al. Overall survival in patients with pancreatic cancer receiving matched therapies following molecular profiling: a retrospective analysis of the Know Your Tumor registry trial. Lancet Oncol. 2020;21(4):508-518.(176)JK S, S K, R O, et al. Molecular profiling of cancer patients enables personalized combination therapy: the I-PREDICT study. Nat Med. 2019;25(5):744-750.. Ongoing efforts are focused on identifying innovative approaches, such as new tools that harness genetic information to predict patients’ responses to cancer treatments, to maximize the number of patients who can benefit from precision medicine (177)Lee JS, Nair NU, Dinstag G, et al. Synthetic lethality-mediated precision oncology via the tumor transcriptome. Cell. 2021;184(9).. Researchers are also pursuing novel avenues beyond tumor genetics, e.g., protein composition of tumor cells, microbial composition in the gut, and restrictive or altered diets, among others, to boost the power of precision medicine for cancer patients (178)Davar D, Dzutsev AK, McCulloch JA, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science (80- ). 2021;371(6529).(179)Kaiser J. Special diets might boost the power of drugs to vanquish cancers. Science (80- ). Published online 2021.. Integration of data collected through such multipronged approaches will provide further insights into cancer diagnosis and treatment, opening new opportunities in precision oncology. Yet another area of active focus is the accumulation of relevant data from racial and ethnic minorities, the lack of which substantially minimizes the current implementation of precision medicine for these populations (91)Sengupta R, Honey K. AACR Cancer Disparities Progress Report 2020 : Achieving the Bold Vision of Health Equity for Racial and Ethnic Minorities and Other Underserved Populations . Cancer Epidemiol Biomarkers Prev. Published online 2020.. Going forward, concerted efforts from all stakeholders in medical research and public health will be critical to ensure that every cancer patient in the United States benefits from the promise of precision medicine.