Decoding Cancer Complexity. Integrating Science. Transforming Patient Outcomes.
In this section, you will learn:
- Researchers are harnessing the knowledge gleaned from the molecular underpinnings of cancer initiation and progression to develop safer and more effective treatments.
- Advances in novel and innovative approaches to surgery, radiotherapy, chemotherapy, molecularly targeted therapy, and immunotherapy—the five pillars of cancer treatment—are saving and improving lives.
- From August 1, 2021, to July 31, 2022, FDA has approved eight new anticancer therapeutics and two new imaging agents, and has expanded the use of 10 previously approved anticancer therapeutics to treat additional cancer types.
- Included in the FDA approvals are a first combination of a radiodiagnostic agent to visualize cancer and a radiotherapeutic agent to kill cancer; several new and improved molecularly targeted therapeutics to treat rare cancers and cancers of the blood system; a new immune checkpoint inhibitor against a novel target; and a new immunotherapeutic to treat a rare and deadly form of eye cancer for which there was no standard treatment available.
- While these exciting new advances have the potential to transform patient care, much work is needed to ensure equitable access to these treatments for all populations.
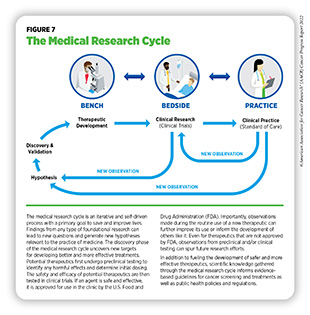
Recent decades have seen tremendous progress against cancer, as evidenced by a 26 percent decline in cancer-related deaths in the U.S. between 2000 and 2019, the most recent data year (5)National Cancer Institute. Surveillance, Epidemiology, and End Results program explorer. Accessed: June 30, 2022.[cited 2020 Jul 15]. (see Cancer in 2022). The rapid pace of progress against cancer is in part driven by the availability of new and effective ways to treat cancers. Furthermore, advances in other aspects of cancer care have significantly improved the quality of life for cancer survivors (see Supporting Cancer Patients and Survivors). Breakthroughs in cancer science and medicine are catalyzed by years-long cross disciplinary collaborations among stakeholders working throughout the medical research cycle (see Figure 7).
Medical Research
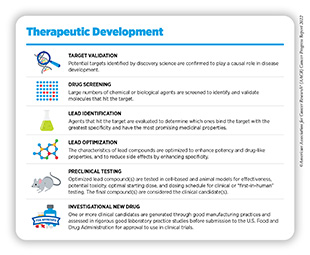
Medical research is a continuous process with the goal to refine and improve clinical care and overall health for all individuals (see Figure 7). During this process, researchers address the hypothesis of whether a new discovery or an observation—made at any step of the medical research cycle—has any biological and/or clinical significance. This is achieved through experiments in a wide range of models that mimic healthy and diseased conditions. Findings from these experiments can identify new targets for drug development; inform approaches for preventive intervention; determine new strategies for early detection; or uncover predictive or prognostic biomarkers, each of which has the potential to improve public health. Once a potential therapeutic target is identified, several candidate therapeutics against the target are carefully tested to determine the appropriate dosage, dosing schedules, and potential toxicities (see sidebar on Therapeutic Development). These preclinical studies help determine the most promising candidates, which are then tested in clinical trials.
Clinical Studies
Once FDA approves a clinical candidate for testing in humans (see sidebar on Therapeutic Development), researchers conduct studies to evaluate safety and efficacy of the clinical candidate in humans. These clinical studies are an integral part of medical research and, together with basic research, make up the backbone of cancer science and medicine. Evidence gathered from clinical studies is reviewed by FDA to determine whether a therapeutic should be approved for use in the clinic.
There are various types of clinical studies, each developed to address specific research questions—although many can also provide answers to additional research questions (292)Li A, et al. Clinical trial design: Past, present, and future in the context of big data and precision medicine. Cancer 2020;126:4838-46. [LINK NOT AVAILABLE] (see sidebar on Types of Clinical Studies).
Clinical studies, also called clinical trials, are carefully designed by clinicians and researchers involved in the study, and the conduct of clinical trials is meticulously reviewed and approved by institutional review boards. Clinical studies in which participants are randomly assigned to receive experimental treatment or standard of care treatment are called randomized clinical trials and are considered the most rigorous. Although an inactive agent, also called placebo, is rarely used in cancer clinical trials, researchers sometimes include it in the clinical trial design when there is no standard of care treatment available.
The design of randomized clinical trials prevents biases and increases the accuracy of the outcomes (293)Hariton E, et al. Randomised controlled trials – the gold standard for effectiveness research: Study design: randomised controlled trials. BJOG 2018;125:1716. [LINK NOT AVAILABLE]. The rigor in design and conduct of clinical trials is essential because these studies involve the use of experimental therapeutics and aim to extrapolate conclusions for the entire population based on data from a group of participants. For this reason, clinical trials are also closely monitored throughout the duration of the study to ensure safety of the participants.
The design of a treatment clinical trial depends on the type of anticancer agent and whether it is being tested alone or in combination with other anticancer treatments; the type of cancer; size and demographics of the clinical trial participants; and the infrastructure in place to support successful completion of the clinical trial, among other considerations. Furthermore, rapidly evolving understanding of the molecular underpinnings of cancer development means that there is a marked increase in the number of drugs that act against specific molecular targets. This knowledge has spurred the growth of precision medicine and has necessitated changes to the design and conduct of clinical trials (see sidebar on Conduct of Clinical Trials). Furthermore, clinical studies can take years to complete. In recent years, FDA—the federal agency that oversees clinical trials and drug approvals—has made important procedural changes to expedite the conduct and review of clinical trials (see Diversifying and Decentralizing Clinical Trials).
Despite improvements in both the clinical trial design and drug approval regulatory processes, there are other opportunities to further minimize the time it takes for a lifesaving anticancer therapeutic to reach patients who will benefit from it the most. The COVID-19 pandemic, despite its adverse effects on nearly all aspects of cancer science and medicine (8)American Association for Cancer Research. AACR Report on the Impact of COVID-19 on Cancer Research and Patient Care. Accessed: June 30, 2022.[cited 2020 Jul 15]., including on clinical trials, has offered a blueprint of success to further revise and reform clinical trials and the drug approval process for the benefit of cancer patients.
Researchers are hopeful that the modifications to clinical trials as a result of the knowledge gleaned from the COVID-19 pandemic may also address the serious lack of representation from racial and ethnic minorities and other underserved populations which remains the most pressing challenge in clinical research. Recruitment of clinical trial participants who are representative of the U.S. population groups who experience the greatest inequities in the burden of cancer (see sidebar on Which U.S. Population Groups Experience Cancer Health Disparities?) is essential to accurately extrapolate the safety and efficacy of new treatments to all population groups. Diversity among participants is even more critical to effectively evaluate cutting-edge precision medicine drugs, such as molecularly targeted therapeutics or immunotherapeutics, because these treatments are closely linked to the unique characteristics of an individual’s cancer, immune system, and lifestyle, among other factors.
As detailed in the recently released AACR Cancer Disparities Progress Report 2022, barriers to participation in clinical trials, especially among racial and ethnic minorities, are numerous, including historical, such as mistrust of the health care system; systemic, such as implicit biases among health care providers; financial, such as cost of the cancer treatment; structural, such as transportation to the study site; and psychological, such as fear of using an experimental drug (13)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2022. Accessed: June 30, 2022.[cited 2020 Jul 15].. Overcoming these barriers for all U.S. population groups and addressing the root causes of low participation in clinical trials require concerted and coordinated efforts from stakeholders in the cancer research and care community (see sidebar on The Medical Research Community: Driving Progress Together).
Progress Across the Spectrum of Cancer Treatment
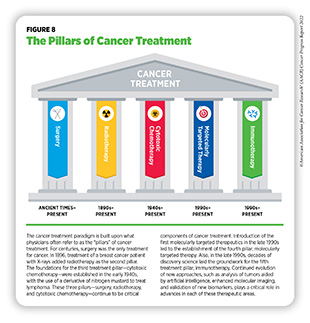
Research drives our understanding of how cancer develops mechanistically; how its risk can be reduced proactively; how it can be detected early; how it can be treated effectively; and how it can impact those affected by it differently. This knowledge, in part, leads to FDA approval of new anticancer drugs and medical devices that not only help save more lives, but also accelerate the pace of medical research through new real-world observations (see Figure 7). The overall goal is to improve outcomes for patients with cancer using—alone or in various combinations—surgery,
chemotherapy, radiotherapy, molecularly targeted therapy, and immunotherapy, the five pillars that constitute the current paradigm for cancer treatment (296)Falzone L, et al. Evolution of cancer pharmacological treatments at the turn of the third millennium. Front Pharmacol 2018;9:1300. [LINK NOT AVAILABLE] (see Figure 8).
This section discusses eight new anticancer therapeutics and two new imaging agents approved by FDA during the 12-month period—August 1, 2021, to July 31, 2022—covered by this report. Also highlighted are the 10 previously approved anticancer drugs that were approved by FDA for treating additional types of cancer during the same time (see Table 4, and Supplemental Table 2). Not discussed are the previously approved anticancer drugs for which FDA approved either a supplementary dosing schedule or additional uses during treatment of the same cancer type for which they were originally approved, for example, an expansion for treatment at a less advanced stage of the disease.
As we make strides toward effectively treating cancers, it is important to note that not all patients receive and/or benefit from the care recommended for the type and stage of cancer with which they have been diagnosed. Gaps in equitable and affordable access to cancer treatment can result in adverse survival rates that disproportionately affect certain population groups (sidebar on Disparities in Cancer Treatment,). It is essential for stakeholders across the cancer research continuum to address these disparities urgently and collectively if the vision of cancer health equity is to be realized.
Advances in Cancer Treatment with Surgery
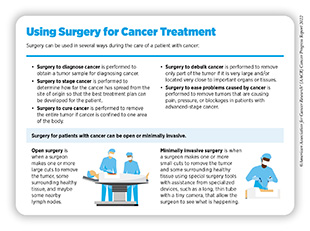
Surgery can be used in several ways during the care of a patient with cancer (see sidebar on Using Surgery for Cancer Treatment). For centuries, surgery was the only available cancer treatment and remains an important treatment option for many patients (see Figure 7).
According to a recent study, more than nine million patients globally needed surgery for treatment of their cancer in 2018—researchers estimate this number to increase by five million by 2040 (307)Perera SK, et al. Global demand for cancer surgery and an estimate of the optimal surgical and anaesthesia workforce between 2018 and 2040: a population-based modelling study. Lancet Oncol 2021;22:182-9. [LINK NOT AVAILABLE].
Despite the rapid pace of development and approvals of molecularly targeted therapies and immunotherapies in recent decades, the use of surgery in cancer care, especially alongside one or more classes of therapies available for the diagnosed cancer type, remains common. The goal of combining surgery with other classes of therapies is to eliminate any cancer cells that surgery might not remove. For many cancer patients, a multidisciplinary team of medical professionals including those specialized in performing surgery, administering radiation, and treating the diagnosed cancer, as well as other individuals as appropriate (e.g., nurses, social workers), reviews treatment options and makes an expert recommendation for treatment with the goal to maximize the benefit and minimize harms from surgery.
Sometimes, additional therapy is given before, after, or around the time of surgery based on specifics of the situation (see sidebar on Commonly Used Terms and Benchmarks in Clinical Studies). Researchers have found that this approach not only improves the surgeon’s ability to remove the tumor (for example by shrinking the tumor when given before the surgery), but also increases the patient’s overall survival and/or quality of life (308)Burotto M, et al. Adjuvant and neoadjuvant cancer therapies: A historical review and a rational approach to understand outcomes. Semin Oncol 2019;46:83-99. [LINK NOT AVAILABLE].
Visualizing Ovarian Tumors Precisely During Surgery
Ovarian cancer is the number five cause of cancer deaths among women, accounting for more deaths than any other cancer of the female reproductive system. According to recent estimates, nearly 20,000 women will be diagnosed with ovarian cancer and more than 12,000 will die because of ovarian cancer in the U.S. 2022 (1)Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [LINK NOT AVAILABLE].
Research has revealed that a complete surgical removal of ovarian cancer following standard-of-care chemotherapy increases overall survival by nearly 14 months (309)Harter P, et al. Randomized trial of cytoreductive surgery for relapsed ovarian cancer. N Engl J Med 2021;385:2123-31. [LINK NOT AVAILABLE]. Surgeons have thus far relied on either imaging tumors before surgery, visually inspecting tumors under normal light during surgery, or examining tumors by touch to identify cancerous tissue, all of which are imprecise methods. In November 2021, FDA approved pafolacianine (Cytalux)—the first tumor-targeted imaging agent for ovarian cancer—to assist surgeons in visualizing hard to detect lesions in adult patients with ovarian cancer during the surgery.
Pafolacianine is given via an intravenous injection between one and nine hours before surgery. The fluorescent agent binds to the folate receptor, a protein which is present in abundance on the surface of ovarian cancer cells (310)Vergote IB, et al. Role of the folate receptor in ovarian cancer treatment: evidence, mechanism, and clinical implications. Cancer Metastasis Rev 2015;34:41-52. [LINK NOT AVAILABLE]. Ovarian cancer cells, with the imaging agent bound to folate receptor on their surface, are then visualized during surgery using a Near-Infrared fluorescence imaging system, which was simultaneously approved by FDA specifically for use with pafolacianine.
FDA approval of pafolacianine was based on a randomized clinical trial that evaluated the safety and efficacy of the imaging agent in women who were diagnosed with ovarian cancer or had high likelihood of having ovarian cancer and were scheduled to undergo surgery (311)Tanyi JL, et al. Phase 3, randomized, single-dose, open-label study to investigate the safety and efficacy of pafolacianine sodium injection (OTL38) for intraoperative imaging of folate receptor positive ovarian cancer. Journal of Clinical Oncology 2021;39:5503-. [LINK NOT AVAILABLE]. A dose of the imaging agent was given to 134 women (ages 33 to 81). When evaluated under both normal and fluorescent light during surgery, pafolacianine detected in 27 percent of women at least one cancerous lesion that was not observed by standard visual inspection. Approval of pafolacianine is expected to substantially improve ovarian cancer surgery by enhancing a surgeon’s ability to find otherwise undetectable ovarian lesions.
Improvements in Radiation-based Approaches to Cancer Care
Radiotherapy is the use of high-energy rays (e.g., gamma rays and X-rays) or particles (e.g., electrons, protons, and carbon nuclei) to control or eradicate cancer. Discovery of X-rays in 1895 allowed visualization of internal organs at low doses and the effective use of X-rays at high doses to treat a breast cancer patient a year later established radiotherapy as the second pillar of cancer treatment (see Figure 8). Radiotherapy plays a central role in the management of cancer and works primarily by damaging DNA, leading to cancer cell death. The use of radiotherapy in treatment and management of cancer continues to increase, as indicated by a 16.4 percent increase in radiation facilities across the U.S. between 2005 and 2020 (312)Maroongroge S, et al. Geographic access to radiation therapy facilities in the United States. Int J Radiat Oncol Biol Phys 2022;112:600-10. [LINK NOT AVAILABLE].
There are many types and uses of radiotherapy (see sidebar on Using Radiation in Cancer Treatment). However, it is important to note that radiotherapy may also have harmful side effects, partly because of the radiation-induced damage to cells in healthy organs surrounding the tumor tissue (313)Wang K, et al. Radiation therapy-associated toxicity: Etiology, management, and prevention. CA Cancer J Clin 2021;71:437-54. [LINK NOT AVAILABLE]. Researchers are continuously working on making radiotherapy safer and more effective and designing novel radiotherapeutics (alone or in combination) to target more cancer types and benefit more patients. Recent technological advances, such as the development of sophisticated computer analytic programs assisted by AI, are helping optimize the delivery of the radiation to the tumor while minimizing exposure to normal tissues (314)Santoro M, et al. Recent applications of artificial intelligence in radiotherapy: Where we are and beyond. Applied Sciences-Basel 2022;12:3223. [LINK NOT AVAILABLE].
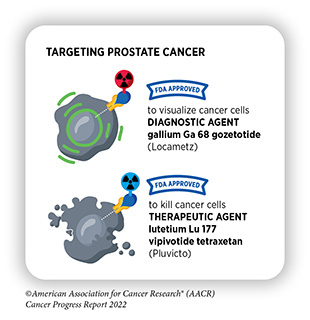
Delivering Radiation Precisely to Metastatic Prostate Cancer
One of the most exciting areas of research in radiotherapy is theragnostics (315)Duan H, et al. Radiotheranostics – Precision medicine in nuclear medicine and molecular imaging. Nanotheranostics 2022;6:103-17. [LINK NOT AVAILABLE]. Theragnostics, also called theranostics, is a treatment approach in which cancer is visualized by positron emission tomography (PET) or computer tomography (CT) imaging using molecules that are linked to weak radionuclides and bind to specific proteins on the surface of cancer cells. Once the presence of cancer is confirmed, the same targeting agents—labeled with more potent radioactive compounds—are then used to kill cancer cells. Breakthrough advances in this area are reflected by the recent FDA approvals, highlighted in the AACR Cancer Progress Reports 2018 and 2021 (21)American Association for Cancer Research. AACR Cancer Progress Report 2021. Accessed: Dec 19, 2021.[cited 2020 Jul 15].(316)American Association for Cancer Research. AACR Cancer Progress Report 2018. Accessed: June 30, 2022.[cited 2020 Jul 15]. and discussed below, of a radiodiagnostic agent and a radiotherapeutic agent for the visualization and treatment of metastatic castrate-resistant prostate cancer (mCRPC).
Prostate cancer is the most diagnosed cancer, after non-melanoma skin cancer, and is the second leading cause of cancer deaths after lung cancer in U.S. men (1)Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [LINK NOT AVAILABLE]. Early-stage prostate cancer cells rely on normal levels of testosterone to proliferate. Although not routinely used, one of the treatment strategies for early-stage prostate cancer is to inhibit androgen receptor activity, which helps lower testosterone to very low levels and thus slows cancer growth. However, some advanced-stage prostate cancer cells treated with anti-androgen therapy adapt to become independent of testosterone and continue to proliferate, and often spread to other organs even when the testosterone levels in the body are very low. This type of prostate cancer is called metastatic castrate-resistant prostate cancer (mCRPC) and can be very difficult to treat.
In March 2022, FDA approved lutetium Lu 177 vipivotide tetraxetan (Pluvicto), referred as 177Lu-PSMA-617, for the treatment of adult mCRPC patients who have been treated with a combination of androgen receptor inhibition and chemotherapy, and whose cancer cells have a protein called prostate-specific membrane antigen (PSMA) on their surface. Concurrently, FDA approved gallium Ga 68 gozetotide (Locametz), also called 68Ga-PSMA-11, which is the first radioactive diagnostic agent approved for the selection of patients for radiotherapy.
The FDA approval of both agents was based on a clinical trial in which patients with previously treated mCRPC first received radiodiagnostic agent 68Ga-PSMA-11, a weak radionuclide that binds to PSMA protein on the surface of prostate cancer cells (317)Sartor O, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med 2021;385:1091-103. [LINK NOT AVAILABLE]. Patients in which PET imaging detected at least one tumor lesion with 68Ga-PSMA-11 were selected for treatment with the radiotherapeutic agent 177Lu-PSMA-617, which also binds to PSMA but is a much stronger radionuclide. Of 831 PSMA-positive mCRPC patients who fulfilled the requirements of the study, 551 patients received both the best standard of care (BSoC) and 177Lu-PSMA-617 every six weeks for up to a total of six doses; the remaining 280 patients received BSoC alone. Findings of the study showed an increase of 5.3 months in progression-free survival and an increase of 4.0 months in overall survival among patients who received the radiotherapeutic and BSoC compared to those who only received BSoC (317)Sartor O, et al. Lutetium-177-PSMA-617 for metastatic castration-resistant prostate cancer. N Engl J Med 2021;385:1091-103. [LINK NOT AVAILABLE] (see sidebar on Commonly Used Terms and Benchmarks in Clinical Studies).
These improvements mark a significant progress against an advanced-stage, difficult-to-treat cancer and provide hope for mCRPC patients, who otherwise have limited treatment options. Researchers are also actively working on developing additional theragnostic agents for the treatment of metastatic prostate cancer (318)Farolfi A, et al. Theragnostics in prostate cancer. Q J Nucl Med Mol Imaging 2021;65:333-41. [LINK NOT AVAILABLE].
Advances in Treatment with Cytotoxic Chemotherapy
Cytotoxic chemotherapy—use of chemicals to kill cancer cells— was first introduced as a pillar of cancer treatment in the early to mid-20th century (299)DeVita VT, Jr., et al. A history of cancer chemotherapy. Cancer Res 2008;68:8643-53. [LINK NOT AVAILABLE]. Chemotherapy remains a backbone of cancer treatment and its use is continually evolving to minimize potential harms to cancer patients, while maximizing its benefits.
As with surgery, chemotherapy is more commonly being used to treat cancer in combination with one or more types of therapies. Furthermore, FDA continues to grant approvals to newer and more effective chemotherapeutics. FDA routinely expands the use of previously approved chemotherapeutics to treat patients with subtypes of the same cancer for which the chemotherapeutic was previously approved (for example, the approval in May 2022 of azacitidine [Vidaza] to treat pediatric patients with juvenile myelomonocytic leukemia). Many FDA approvals of chemotherapeutics are in combination with other previously approved targeted therapies (for example, the approval in December 2021 of chemotherapy to be used in combination with a molecularly targeted therapeutic rituximab [Rituxan] to treat certain types of childhood blood cancer). In addition, FDA approves new formulations of previously approved chemotherapeutics (for example, the approval in September 2020 of a new formulation of azacitidine [Onureg] that could be taken orally allowing patients with acute myeloid leukemia to continue their medication in the midst of the COVID-19 pandemic without needing to visit a clinic).
Advances in Treatment with Molecularly Targeted Therapy
Discovery science has played a key role in unraveling the complexities of cancer and in providing a deeper understanding of the numerous genetic mutations that drive cancer development. As a result, recent decades have seen major advances in precision medicine. Cancer patients now have many treatment options that are specific to the genetic changes driving their cancer, or based on the characteristics of their cancer type. These anticancer agents—called molecularly targeted therapeutics—target cancer cells within a tumor more precisely, making the treatment more effective and less toxic than cytotoxic chemotherapy. Thus, molecularly targeted therapeutics are not only saving the lives of cancer patients, but are also leading to a higher quality of life for cancer survivors after treatment.
Adding Precision to the Treatment of Rare Cancers
According to NCI, a cancer is considered rare if it affects fewer than 15 per 100,000 people every year (319)National cancer Institute. About rare cancers. Accessed: June 30, 2022.[cited 2020 Jul 15].. Of all the various cancer types, 27 percent are considered rare, including all childhood cancers. Because of the rarity of these cancers, it is harder for the patient to find a provider who is an expert in treating the rare cancer with which the patient is diagnosed, and it is harder for researchers to obtain biospecimens to learn about the cancer or find enough participants for clinical trials to test new treatments.
Despite the challenges, there have been significant advances in recent decades toward treating rare cancers, as highlighted by the FDA approvals covered in this report. These approvals include new or improved treatment options for cancers that are caused by a rare inherited cancer syndrome; for a family of rare soft tissue tumors; and for bile duct cancer.
The von Hippel-Lindau (VHL) syndrome is a rare, inherited disorder that is caused by a mutation in the VHL gene. It is estimated that between 3,000 and 30,000 people in U.S. have VHL syndrome (1)Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [LINK NOT AVAILABLE]. Patients with VHL disease have an increased risk of developing certain types of cancer, especially kidney cancer and pancreatic cancer, and thus far, the treatment options for patients with VHL syndrome have been limited to surgery and/or radiotherapy.
In August 2021, FDA approved belzutifan (Welireg) for adult patients who have several tumors associated with VHL disease that do not require immediate surgery. Specifically, the drug is approved to treat VHL-associated renal cell carcinomas (a type of kidney cancer), central nervous system hemangioblastomas (tumors that arise from the linings of blood vessels), or pancreatic neuroendocrine tumors (tumors of specialized cells called neuroendocrine cells that have the properties of both nerve- and hormone-producing cells, and regulate important body functions, such as blood flow).
Belzutifan is an inhibitor of hypoxia-inducible factor, a protein that functions as an oxygen sensor and helps cancer cells grow in low oxygen conditions that are a characteristic of the tumor microenvironment (see The Blood System) (320)Kim LC, et al. Hypoxia-inducible factors in cancer. Cancer Res 2022;82:195-6. [LINK NOT AVAILABLE]. The FDA approval of belzutifan was based on results from a small clinical trial that tested the drug in patients with VHL-associated renal cell carcinoma; all of the patients also had other VHL-associated primary tumors (321)Jonasch E, et al. Belzutifan for renal cell carcinoma in von Hippel-Lindau disease. N Engl J Med 2021;385:2036-46. [LINK NOT AVAILABLE]. After 18 months, kidney tumors in 49 percent of the patients shrank at least 30 percent, and tumors in a majority of the patients were still responding after one year. Belzutifan also shrank VHL-associated brain, pancreatic, and eye tumors in 30 percent, 77 percent, and 100 percent of patients, respectively. Belzutifan is the first FDA-approved drug for the treatment of patients with VHL-associated tumors such as Alexandra Vitale (321)Jonasch E, et al. Belzutifan for renal cell carcinoma in von Hippel-Lindau disease. N Engl J Med 2021;385:2036-46. [LINK NOT AVAILABLE].
Perivascular epithelioid cell tumors, also called PEComas, are a family of very rare tumors that form in the soft tissues of the stomach, intestines, lungs, female reproductive organs, and genitourinary organs. The tumors occur in about one case per million people, often in children with an inherited condition called tuberous sclerosis. It is estimated that there are about 100 to 300 new patients per year in the U.S. Most perivascular epithelioid cell tumors are benign (not cancerous), however patients with malignant PEComas have a very poor outcome, with median survival of only 16 months after treatment with chemotherapy (322)National Organization for Rare Disorders. Perivascular epithelioid cell neoplasm. Accessed: June 30, 2022.[cited 2020 Jul 15]..
In November 2021, FDA approved sirolimus protein-bound particles as an injectable suspension (Fyarro) for adult patients with malignant PEComa that cannot be removed surgically or has spread to other organs. Sirolimus is a chemical compound that inhibits activity of the mechanistic target of rapamycin (mTOR), a protein important for cell division and survival (323)Zou Z, et al. mTOR signaling pathway and mTOR inhibitors in cancer: progress and challenges. Cell Biosci 2020;10:31. [LINK NOT AVAILABLE]. In the formulation newly approved by FDA, sirolimus is bound to albumin, which is a common protein present in blood and soluble in water. Efficacy of the approved drug was evaluated in 31 patients with malignant PEComa that had spread to other organs or could not be removed by surgery. Thirty-nine percent of patients responded to the treatment, including two patients who had complete remission. Among the patients who responded, 67 percent and 58 percent had a response that lasted more than 12 months and 24 months, respectively (324)Wagner AJ, et al. nab-Sirolimus for patients with malignant perivascular epithelioid cell tumors. J Clin Oncol 2021;39:3660-70. [LINK NOT AVAILABLE].
Cells use glucose as a primary source of energy to divide and perform other essential functions. Cells convert glucose into fuel through a series of steps, each of which is controlled by essential proteins, or enzymes. Isocitrate dehydrogenase (IDH) is one such protein, and the cells have two closely related forms of IDH—IDH1 and IDH2 (325)Tommasini-Ghelfi S, et al. Cancer-associated mutation and beyond: The emerging biology of isocitrate dehydrogenases in human disease. Sci Adv 2019;5:eaaw4543. [LINK NOT AVAILABLE].
Research has revealed that IDH1/2 proteins are modified in certain cancers, such as those of the blood, brain, and bile duct (326)Dang L, et al. IDH mutations in cancer and progress toward development of targeted therapeutics. Ann Oncol 2016;27:599-608. [LINK NOT AVAILABLE]. Modifications in IDH1/2 proteins cause cancer cells to acquire changes in their epigenome (see Epigenetic Changes), that help them divide faster than normal cells (327)Du X, et al. The Roles of 2-Hydroxyglutarate. Front Cell Dev Biol 2021;9:651317. [LINK NOT AVAILABLE]. Development of molecularly targeted therapeutics against the modified forms of IDH1/2 is an exciting area of research (328)Jusakul A, et al. Whole-genome and epigenomic landscapes of etiologically distinct subtypes of cholangiocarcinoma. Cancer Discov 2017;7:1116-35. [LINK NOT AVAILABLE].
Cholangiocarcinoma is a group of cancers that begin in the bile ducts, which are the tubes that connect liver and gallbladder to small intestine. Cholangiocarcinomas, or bile duct cancers, are rare and affect about 8,000 Americans every year (1)Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [LINK NOT AVAILABLE]. Bile duct cancers are associated with poor outcomes for patients both at early and advanced stages of the disease. About 10 percent of bile duct cancers carry mutations in IDH1/2 genes (326)Dang L, et al. IDH mutations in cancer and progress toward development of targeted therapeutics. Ann Oncol 2016;27:599-608. [LINK NOT AVAILABLE].
In August 2021, FDA approved ivosidenib (Tibsovo) for adult patients with cholangiocarcinoma who were previously treated, had locally advanced or metastatic disease and an IDH1 mutation. FDA also approved the Oncomine Dx Target Test as a companion diagnostic to detect IDH1 mutation for selecting patients for treatment with ivosidenib.
Ivosidenib, an inhibitor of the mutated form of IDH1, was approved based on findings of a phase III clinical trial that evaluated the efficacy of the drug in 187 patients (329)Zhu AX, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: The phase 3 randomized clinical ClarIDHy trial. JAMA Oncol 2021;7:1669-77. [LINK NOT AVAILABLE]. Patients were randomly assigned to receive ivosidenib or placebo (see sidebar on Commonly Used Terms and Benchmarks in Clinical Studies, p. 72). Initial findings from the study reported that ivosidenib treatment nearly doubled progression-free survival (2.7 months in the ivosidenib group versus 1.4 months in the placebo group) (330)Abou-Alfa GK, et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol 2020;21:796-807. [LINK NOT AVAILABLE]. Subsequent findings from the clinical trial revealed that the overall survival doubled in patients treated with ivosidenib (10.3 months in the ivosidenib group versus 5.1 months in the placebo group) (329)Zhu AX, et al. Final overall survival efficacy results of ivosidenib for patients with advanced cholangiocarcinoma with IDH1 mutation: The phase 3 randomized clinical ClarIDHy trial. JAMA Oncol 2021;7:1669-77. [LINK NOT AVAILABLE]. The expanded use of ivosidenib—previously approved by FDA in 2018 to treat acute myeloid leukemia patients with IDH1 mutation—brings hope for patients with an aggressive form of cancer, for which there are limited treatment options.
In July 2022, FDA approved crizotinib (Xalkori) to treat adult and pediatric patients (1 year of age and older) who have inflammatory myofibroblastic tumors (IMT) that have returned or have become resistant to other cancer treatments and cannot be surgically removed. The approval is specifically to treat patients whose IMT have alterations in the gene for anaplastic lymphoma kinase (ALK); these alterations increase the activity of the enzyme, which helps cancer cells divide faster. Crizotinib was first approved to treat lung cancer with alterations in the ALK gene in 2011.
IMT is a very rare and usually benign (noncancerous) tumor. An estimated 150 to 200 people are diagnosed with IMT in the U.S. annually. IMT forms in tissues called mucosal surfaces and mesentery, and primarily develops in children and young adults. Mucosal surfaces are found in eyes, nose, mouth, digestive tract, lungs, and genital and urinary tracts.
Mesentery connects the organs in the abdomen. IMT contains a lot of immune cells, making the tumor look “inflamed” like an infection. IMT can cause many issues for patients as it can grow in the way of important organs such as the lung or stomach and, in very rare cases, can spread to distant organs. Recent studies have shown that about 50 percent of IMT tumors carry alterations in the ALK gene (331)Lovly CM, et al. Inflammatory myofibroblastic tumors harbor multiple potentially actionable kinase fusions. Cancer Discov 2014;4:889-95. [LINK NOT AVAILABLE](332)Antonescu CR, et al. Molecular characterization of inflammatory myofibroblastic tumors with frequent ALK and ROS1 gene fusions and rare novel RET rearrangement. Am J Surg Pathol 2015;39:957-67. [LINK NOT AVAILABLE].
FDA approval of crizotinib, which inhibits the activity of ALK protein, to treat patients with IMT was based on findings of two small clinical studies, one with 14 pediatric participants with IMT and the second with seven adult participants with IMT. A total of 12 of the pediatric patients and five of the seven adult patients partially or completely responded to the treatment (333)U.S. Food and Drug Administration. FDA approves crizotinib for ALK-positive inflammatory myofibroblastic tumor. Accessed: July 28, 2022.[cited 2020 Jul 15].. The approval expands the treatment options of this rare cancer type.
According to NCI, a quarter of all cancer deaths in the U.S. each year are due to rare cancers (319)National cancer Institute. About rare cancers. Accessed: June 30, 2022.[cited 2020 Jul 15]., making rare cancers a major public health challenge and a key priority for NCI (see sidebar on National Cancer Institute Initiatives to Understand and Treat Rare Cancers). FDA approvals of the drugs discussed in this section, as well as the new immunotherapeutics to treat certain forms of rare cancers (see Advances in Cancer Immunotherapy), address a key unmet need in cancer care and signal major advances toward treating rare cancers.
Expanding Treatment Options for Patients with Blood Cancers
Leukemia is a type of blood cancer that begins in the bone marrow and moves into blood as cancer cells proliferate and overcrowd the cavity inside the bone where the bone marrow resides.
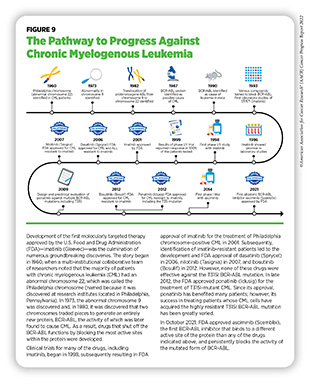
Chronic myeloid leukemia (CML) is a slow-growing cancer of myeloid cells. CML is characterized by the presence of a structural genetic variation in which pieces of chromosomes 9 and 22 break off and trade places. As a result, the ABL gene from chromosome 9 joins the BCR gene on chromosome 22 to form an entirely new and abnormal gene called BCR-ABL. The structurally altered chromosome 22 with the BCR-ABL gene is called the Philadelphia chromosome and is present in more than 90 percent of all CML (also called Philadelphia positive or Ph+ CML) patients (see Figure 9). The BCR-ABL gene makes the BCR-ABL protein, which makes the cancer cells proliferate faster than normal myeloid cells (348)U.S. Food and Drug Administration. FDA grants accelerated approval to dabrafenib in combination with trametinib for unresectable or metastatic solid tumors with BRAF V600E mutation. Accessed: June 30, 2022.[cited 2020 Jul 15]..
It is estimated that 8,860 new cases of CML will be diagnosed in the U.S. in 2022, and 1,220 people will die from it (1)Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [LINK NOT AVAILABLE]. Patients with Ph+ CML have several treatment options available, and typically respond well to therapies that are targeted against the BCR-ABL protein (see Figure 9). However, in some cancer cells, the BCR-ABL gene acquires additional mutations or doubles in number, thus conferring resistance against the existing therapies and posing a challenge to successful treatment of the disease (see sidebar on The Challenge of Treatment Resistance).
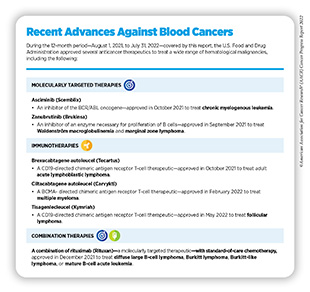
In October 2021, FDA granted accelerated approval to asciminib (Scemblix) for Ph+ CML patients whose cancer is in a chronic phase, i.e., when cancer cells account for less than 10 percent of the total number of cells in bone marrow or blood samples, and who have been previously treated with two or more inhibitors of tyrosine kinases. Tyrosine kinases, such as ABL, belong to a large family of specialized proteins, called enzymes, and play critical roles in cell proliferation, differentiation, and identity (335)K KB, et al. Protein tyrosine kinases: Their roles and their targeting in leukemia. Cancers (Basel) 2021;13. [LINK NOT AVAILABLE]. FDA also approved asciminib for adult patients with Ph+ CML, whose cancer is in a chronic phase and who have the T315I mutation in the BCR-ABL protein (see Figure 10).
FDA approval was based on findings from two clinical trials; in one study, 233 patients were randomly assigned to receive either asciminib or bosutinib—the BCR-ABL inhibitor that was approved by FDA in 2012 to treat Ph+ CML (336)Rea D, et al. A phase 3, open-label, randomized study of asciminib, a STAMP inhibitor, vs bosutinib in CML after 2 or more prior TKIs. Blood 2021;138:2031-41. [LINK NOT AVAILABLE]. Patients were evaluated at 24 weeks for major molecular response, which means that the number of Ph+ CML cells in blood or bone marrow—as assessed by a molecular test—is 1/1000th or less of what is expected in a CML patient who has not been treated. Major molecular response was 25 percent in patients treated with asciminib compared to 13 percent in patients who received bosutinib.
The second clinical trial evaluated the efficacy of asciminib in patients with Ph+ CML who also had the T351I mutation
(337)Hughes TP, et al. Asciminib in chronic myeloid leukemia after ABL kinase inhibitor failure. N Engl J Med 2019;381:2315-26. [LINK NOT AVAILABLE]. Major molecular response was achieved in 42 percent of the patients by 24 weeks, and in 49 percent of the patients by 96 weeks. Importantly, the response lasted for more than two years, indicating that asciminib is highly effective in treating Ph+ CML patients who have developed resistance to other available molecularly targeted therapies.
The approval of asciminib is a major advance toward successfully treating Ph+ CML patients who develop resistance to currently available treatments and face significantly worse outcomes.
B cells are a type of white blood cells in the immune system that make antibodies to help the body defend against foreign substances, such as toxins, and pathogens, such as viruses. Antibody production by B cells is, in part, regulated by a tyrosine kinase, called Bruton’s tyrosine kinase (BTK) (338)Weber ANR, et al. Bruton’s tyrosine kinase: An emerging key player in innate immunity. Front Immunol 2017;8:1454. [LINK NOT AVAILABLE]. As mentioned above, tyrosine kinases are specialized proteins that catalyze many important chemical reactions in the cell and regulate critical cellular functions, such as cell proliferation, differentiation, and identity (339)Lemmon MA, et al. Cell signaling by receptor tyrosine kinases. Cell 2010;141:1117-34. [LINK NOT AVAILABLE]. In normal B cells, BTK is only active when the body needs additional B cells and/or antibodies. However, in cancers of B cells, mutations in the BTK gene result in chronic activation of the BTK protein. The mutated BTK helps cancer cells to divide and the tumors to grow faster than normal cells, and has become an attractive target for development of molecularly targeted therapeutics to treat cancers of B cells (340)Hendriks RW, et al. Targeting Bruton’s tyrosine kinase in B cell malignancies. Nat Rev Cancer 2014;14:219-32. [LINK NOT AVAILABLE].
In August 2021, FDA expanded the use of zanubrutinib (Brukinsa)—an inhibitor of BTK originally approved in 2019 to treat another rare form of blood cancer called mantle cell lymphoma—to treat adult patients with Waldenström macroglobulinemia (WM).
WM begins in B cells and is a rare type of non-Hodgkin lymphoma. Each year, an estimated 1,000 to 1,500 U.S. adults are diagnosed with WM (1)Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [LINK NOT AVAILABLE]. Cancer cells in patients with WM make a large amount of a certain type of antibody called macroglobulin. The buildup of this protein in the body can lead to excess bleeding, problems with vision, and problems with the nervous system (341)Yun S, et al. Waldenstrom macroglobulinemia: Review of pathogenesis and management. Clin Lymphoma Myeloma Leuk 2017;17:252-62. [LINK NOT AVAILABLE].
WM typically develops in adults older than 70 years and grows slowly in most patients. Although several molecularly targeted therapeutics have received FDA approval in recent years for treatment of WM, patients often develop resistance to the existing therapies and the disease can still progress to more aggressive lymphoma, underscoring the continued need to develop newer and better therapeutics against the disease.
The FDA approval of zanubrutinib to treat adult patients with WM was based on a phase III clinical trial that compared the efficacy of zanubrutinib with that of ibrutinib (Imbruvica), another FDA-approved BTK inhibitor for the treatment of patients with WM (342)Tam CS, et al. A head-to-head Phase III study comparing zanubrutinib versus ibrutinib in patients with Waldenstrom macroglobulinemia. Future Oncol 2018;14:2229-37. [LINK NOT AVAILABLE]. Patients were divided into two groups: one group received either zanubrutinib or ibrutinib, while the other group—in which patients either had normal MYD88 gene, which contributes to the survival of WM cells, or had a certain mutation in the MYD88 gene—received only zanubrutinib. Approval was based on response rate and duration of response to zanubrutinib. The response rate in the first group was 77.5 percent, and 94.4 percent of the respondents had no signs of cancer 12 months after starting the treatment. The response was 50 percent in the second group. The study is ongoing and, although the initial findings are encouraging, additional data will further inform the efficacy and safety of zanubrutinib (342)Tam CS, et al. A head-to-head Phase III study comparing zanubrutinib versus ibrutinib in patients with Waldenstrom macroglobulinemia. Future Oncol 2018;14:2229-37. [LINK NOT AVAILABLE].
In September 2021, FDA also approved zanubrutinib for adult patients with relapsed or refractory marginal zone lymphoma (MZL)—another rare type of B-cell cancer— and who have received at least one anti-CD20-based treatment, a common treatment option for patients with cancers of B cells.
MZL is the most common type of slow-growing lymphoma and accounts for about 5-10 percent of all lymphomas (1)Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [LINK NOT AVAILABLE]. MZL remains largely understudied, making it challenging to define a single treatment approach for MZL patients. FDA approval of zanubrutinib for the treatment of adult MZL patients was based on findings from two clinical trials. One study included 66 MZL patients who had received at least one prior anti-CD20-based treatment, while the other study included 20 patients who had been previously treated for MZL. In the first trial, the overall response rate was 56 percent, with 20 percent of respondents achieving complete remission (343)Opat S, et al. The MAGNOLIA Trial: Zanubrutinib, a next-generation Bruton tyrosine kinase inhibitor, demonstrates safety and efficacy in relapsed/refractory marginal zone lymphoma. Clin Cancer Res 2021;27:6323-32. [LINK NOT AVAILABLE]. In the second trial, the overall response rate was 80 percent, with 20 percent of respondents achieving complete remission. At the time of the approval, the duration of response at the one-year mark was estimated to be in 85 percent and 72 percent of respondents, respectively (344)Phillips T, et al. Zanubrutinib monotherapy in relapsed/refractory indolent non-Hodgkin lymphoma. Blood Adv 2022;6:3472-9. [LINK NOT AVAILABLE].
The approval of zanubrutinib for the treatment of adult MZL patients provides clinicians with additional treatment options, especially for patients in which the cancer has returned.
In December 2021, FDA expanded the use of rituximab (Rituxan) in combination with chemotherapy for pediatric patients who are between 6 months and 18 years of age; have not been previously treated; and are at an advanced stage of one of the following rare forms of B-cell cancers—diffuse large B-cell lymphoma (DLBCL); Burkitt lymphoma (BL); Burkitt-like lymphoma (BLL); or mature B-cell acute leukemia (B-AL)—that have the CD20 protein on their surface.
Rituximab was first approved by FDA in 1997 to treat non-Hodgkin lymphoma (NHL) and has become a main treatment option for a broad variety of B-cell cancers (344)Phillips T, et al. Zanubrutinib monotherapy in relapsed/refractory indolent non-Hodgkin lymphoma. Blood Adv 2022;6:3472-9. [LINK NOT AVAILABLE]. Rituximab is an antibody that binds to the CD20 protein, found in abundance on the surface of cancerous B cells, and directs other immune cells to the tumor where they kill the target cancer cells, a process called antibody-dependent cell-mediated toxicity. The clinical study evaluating the efficacy and safety of the combination therapy is ongoing (346)Minard-Colin V, et al. Rituximab for high-risk, mature B-cell non-Hodgkin’s lymphoma in children. N Engl J Med 2020;382:2207-19. [LINK NOT AVAILABLE]. An interim analysis of the findings was performed using data from 328 patients who were followed up for nearly 40 months. Patients receiving the combination therapy did not have detectable cancer after three years, and had an 11.6 percentage points higher survival rate (93.9 percent with combination therapy versus 82.3 percent with chemotherapy alone) (346)Minard-Colin V, et al. Rituximab for high-risk, mature B-cell non-Hodgkin’s lymphoma in children. N Engl J Med 2020;382:2207-19. [LINK NOT AVAILABLE].
Together, these approvals fulfill the unmet needs in treatment of many rare but aggressive forms of blood cancers and highlight the rapid advances spurred by the field of precision medicine (see sidebar on Recent Advances Against Blood Cancers).
Combining Therapeutics to Improve Outcomes
BRAF is an enzyme with a critical role in controlling cell growth. The BRAF gene is changed in approximately six percent of all human cancers, including melanoma and colorectal cancer (347)Dankner M, et al. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene 2018;37:3183-99. [LINK NOT AVAILABLE]. Most cancer-related changes in the BRAF gene cause the protein to continuously stay active, thus helping cancer cells grow faster than normal cells.
One of the most common cancer-related changes in the BRAF gene is called BRAF V600E mutation. The BRAF V600E mutation is found in about 50 percent of melanoma patients, 10 percent of colorectal cancer patients, and 2-5 percent of non– small cell lung cancer (NSCLC) patients. Presence of BRAF V600E mutation is associated with poor outcomes for patients with certain types of cancer (347)Dankner M, et al. Classifying BRAF alterations in cancer: new rational therapeutic strategies for actionable mutations. Oncogene 2018;37:3183-99. [LINK NOT AVAILABLE].
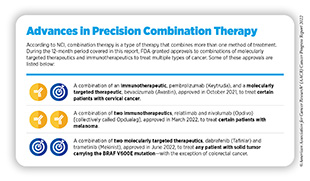
In June 2022, FDA approved a combination of two molecularly targeted therapeutics that inhibit activity of the BRAF protein— dabrafenib (Tafinlar) and trametinib (Mekinist)—for the treatment of adult and pediatric patients (age 6 and older) who have a solid tumor harboring the BRAF V600E mutation. The approval is specifically for patients whose cancer has either spread in the body following prior treatment or cannot be surgically removed, and who do not have any satisfactory alternative treatment options. It is important to note that the combination is not approved for patients with colorectal cancer even if the cancer carries the BRAF V600E mutation because colorectal cancer is resistant to treatment with therapeutics that inhibit the activity of BRAF.
FDA approval was based on findings from three clinical trials that evaluated efficacy of the combination in adult and pediatric patients (age six and older). Patients enrolled in the studies had one of 24 different types of tumors, including cancers of skin, lung, bile duct, thyroid, brain, and gastrointestinal tract (348)U.S. Food and Drug Administration. FDA grants accelerated approval to dabrafenib in combination with trametinib for unresectable or metastatic solid tumors with BRAF V600E mutation. Accessed: June 30, 2022.[cited 2020 Jul 15].. A total of 41 percent of patients experienced an objective response to the treatment. The overall response rate was 46 percent for bile duct cancer, 33 to 50 percent for brain cancer depending upon how advanced the cancer was. Among pediatric patients, 25 percent experienced an overall response rate that lasted for about six months in 78 percent of patients and for about two years in 44 percent of patients (348)U.S. Food and Drug Administration. FDA grants accelerated approval to dabrafenib in combination with trametinib for unresectable or metastatic solid tumors with BRAF V600E mutation. Accessed: June 30, 2022.[cited 2020 Jul 15]..
The approval of the dabrafenib and trametinib combination to treat any solid tumor—with the exception of colorectal cancer—that carries the BRAF V600E mutation offers a new way to treat a wide range of patients, such as Tyler Richards, who do not have other suitable treatment options. The approval also underscores the power of combining two or more targeted therapies to treat different types of cancer that share unique genetic features (see sidebar on Advances in Precision Combination Therapy).
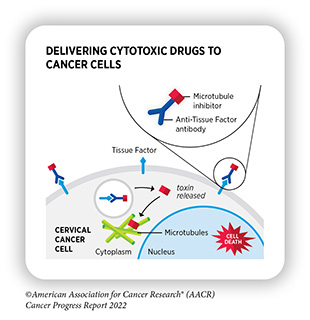
Delivering a Cytotoxic Drug Precisely to Cervical Cancer Cells
Researchers are continuously developing precise strategies that selectively target cancer cells for eradication without harming the normal tissue. Antibody-drug conjugates (ADCs), which use antibodies to deliver an attached toxin specifically to cancer cells, constitute one such strategy. The antibody used in an ADC is usually directed against a protein that is present in abundance on the surface of cancer cells. The choice of cytotoxic agent is informed by the cancer type as well as other pharmacological considerations, such as the effective dose of the toxin needed to kill cancer cells and the stability of the toxin inside the body. Once the antibody binds to its target on the cancer cell surface, the ADC is taken up by the cell where it releases the cytotoxic drug, which ultimately kills the cancer cell (349)Tarantino P, et al. Antibody-drug conjugates: Smart chemotherapy delivery across tumor histologies. CA Cancer J Clin 2022;72:165-82. [LINK NOT AVAILABLE]. This approach minimizes the side effects of the cytotoxic agent compared to a traditional systemic delivery.
In September 2021, FDA approved tisotumab vedotin-tftv (Tivdak) for adult patients with recurrent or metastatic cervical cancer with disease progression on or after chemotherapy. Tisotumab vedotin-tftv is an ADC in which an antibody directed against tissue factor—a protein that is present in abundance on the surface of cervical cancer cells and is an indicator of poor outcome (350)Unruh D, et al. Beyond thrombosis: the impact of tissue factor signaling in cancer. J Hematol Oncol 2020;13:93. [LINK NOT AVAILABLE]—is linked with a potent inhibitor of cell division. Tisotumab vedotin-tftv is the first and the only FDA-approved ADC to treat cervical cancer.
According to the 2022 estimates, more than 14,000 new cases of invasive cervical cancer will be diagnosed, and more than 4,000 women will die from cervical cancer in the U.S. (1)Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [LINK NOT AVAILABLE]. Although both incidence and mortality from cervical cancer have declined by greater than 50 percent since 1975 (5)National Cancer Institute. Surveillance, Epidemiology, and End Results program explorer. Accessed: June 30, 2022.[cited 2020 Jul 15].—thanks to advances in prevention, early detection, and treatment—cervical cancer that returns after initial course of treatment and/or metastasizes to other organs has a very poor outcome for patients and its treatment remains a significant challenge (351)Pang SS, et al. Current management of locally advanced and metastatic cervical cancer in the United States. JCO Oncol Pract 2022;18:417-22. [LINK NOT AVAILABLE].
The FDA-approval of tisotumab vedotin-tftv was based on a phase II clinical trial in which researchers evaluated the efficacy of the drug in 101 patients with recurrent or metastatic cervical cancer (352)Coleman RL, et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2021;22:609-19. [LINK NOT AVAILABLE]. Patients received tisotumab vedotin-tftv every three weeks unless the disease started to progress again, or the researchers considered the drug toxic for the patient. According to the study’s findings, 24 percent of patients responded to the treatment, either partially (17 percent of the respondents) or completely (seven percent of the respondents) and the duration of the response was more than eight months (352)Coleman RL, et al. Efficacy and safety of tisotumab vedotin in previously treated recurrent or metastatic cervical cancer (innovaTV 204/GOG-3023/ENGOT-cx6): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol 2021;22:609-19. [LINK NOT AVAILABLE].
Given the poor prognosis for patients with recurrent or metastatic cervical cancer, and the low efficacy of current therapies in treating the disease, approval of tisotumab vedotin-tftv represents a new and substantial advance for women with recurrent or metastatic cervical cancer such as Jennifer Myers.
Targeting New Ways to Treat Lung Cancer
Lung cancer is the leading cause of cancer deaths—nearly 25 percent of all cancer deaths in the U.S. are due to lung cancer— making it a major public health challenge (1)Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [LINK NOT AVAILABLE].
Epidermal growth factor receptor (EGFR) is an important tyrosine kinase that is present on the cell surface of many normal tissues and helps cells proliferate. Research has revealed that the EGFR gene acquires mutations in certain cancer types, including NSCLC (353)Chen J, et al. Expression and function of the epidermal growth factor receptor in physiology and disease. Physiol Rev 2016;96:1025-69. [LINK NOT AVAILABLE]. About 80-85 percent of lung cancers are NSCLC. Sixty percent of all NSCLCs have very high levels of EGFR on their surface and roughly 20 percent have mutations in the EGFR gene (1)Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [LINK NOT AVAILABLE](354)da Cunha Santos G, et al. EGFR mutations and lung cancer. Annu Rev Pathol 2011;6:49-69. [LINK NOT AVAILABLE]. Because of this knowledge, EGFR has become an important therapeutic target, and FDA has approved numerous EGFR-targeted therapies over the last two decades for treatment of NSCLC (21)American Association for Cancer Research. AACR Cancer Progress Report 2021. Accessed: Dec 19, 2021.[cited 2020 Jul 15].. However, patients eventually develop resistance to these therapies as the EGFR gene acquires additional alterations (see sidebar on The Challenge of Treatment Resistance). For example, patients with NSCLC whose tumors harbor certain alterations, such as insertion mutations in a part of the EGFR gene called exon 20, do not respond well to EGFR-targeted therapeutics, such as osimertinib, and generally have poor prognosis (355)Remon J, et al. EGFR exon 20 insertions in advanced non-small cell lung cancer: A new history begins. Cancer Treat Rev 2020;90:102105. [LINK NOT AVAILABLE].
In September 2021, FDA approved mobocertinib (Exkivity) for adult patients who have locally advanced or metastatic NSCLC with EGFR exon 20 insertion mutations, and whose disease has progressed during or after chemotherapy. FDA also approved the Oncomine Dx Target Test as a companion diagnostic to select patients with EGFR exon 20 insertion mutations. Efficacy of the drug was evaluated in 114 patients who received mobocertinib daily. Overall, 28 percent of patients responded to the treatment and the response lasted more than 17 months (356)Zhou C, et al. Treatment outcomes and safety of mobocertinib in platinum-pretreated patients with EGFR exon 20 insertion-positive metastatic non-small cell lung cancer: A phase 1/2 open-label nonrandomized clinical trial. JAMA Oncol 2021;7:e214761. [LINK NOT AVAILABLE]. Progression-free survival was more than seven months, while the overall survival was two years (see sidebar on Commonly Used Terms and Benchmarks in Clinical Studies) (356)Zhou C, et al. Treatment outcomes and safety of mobocertinib in platinum-pretreated patients with EGFR exon 20 insertion-positive metastatic non-small cell lung cancer: A phase 1/2 open-label nonrandomized clinical trial. JAMA Oncol 2021;7:e214761. [LINK NOT AVAILABLE].
Mobocertinib is the first molecularly targeted drug that is directed only against EGFR for the treatment of patients with NSCLC who have EGFR exon 20 insertion mutations, and that can be taken orally.
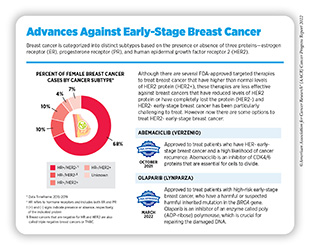
As indicated earlier in the chapter, the AACR Cancer Progress Reports do not include detailed discussions of FDA approvals of anticancer therapeutics that were previously approved for a different stage of the same type of cancer. For example, during the period covered in this report, FDA approved two previously approved molecularly targeted therapeutics for treatment of early-stage breast cancer (see sidebar on Advances Against Early-Stage Breast Cancer).
Advances in Cancer Immunotherapy
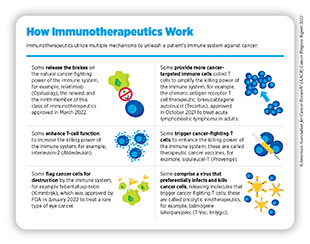
Decades of research have identified ways by which the immune system detects and destroys unwanted organisms, molecules, and toxins in the human body. More recent discoveries in this area of research have allowed researchers to weaponize a patient’s immune system against cancer (362)Dobosz P, et al. The intriguing history of cancer immunotherapy. Front Immunol 2019;10:2965. [LINK NOT AVAILABLE], leading to the establishment of cancer immunotherapy as the most recent addition to the pillars of cancer treatment (see Figure 8). Cancer immunotherapy leverages the natural ability of a patient’s immune system to fight cancer using a class of drugs known as immunotherapeutics (see sidebar on How Immunotherapeutics Work) (363)Waldman AD, et al. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol 2020;20:651-68. [LINK NOT AVAILABLE].
Rapid advances in the development of new and improved immunotherapeutics have revolutionized cancer care of many patients. The remarkable success of these treatments in the clinic is, in part, because of the durable response in some patients with metastatic cancer. As one example, a recent study reported that the immune response in patients who had metastatic skin cancer (melanoma) and responded exceptionally well to immunotherapy lasted up to nine years following treatment (364)Han J, et al. Resident and circulating memory T cells persist for years in melanoma patients with durable responses to immunotherapy. Nat Cancer 2021;2:300-11. [LINK NOT AVAILABLE]. However, research has also found that not all patients who receive immunotherapy experience such an incredible response. Reasons for the suboptimal response to immunotherapy are many, and include the type of cancer a patient has; the stage at which the cancer is being treated; how quickly the cancer is acquiring new alterations; and how cancer cells have modified the environment around them to evade immunotherapeutics, among others (365)Galluzzi L, et al. The hallmarks of successful anticancer immunotherapy. Sci Transl Med 2018;10. [LINK NOT AVAILABLE]. Furthermore, immunotherapeutics currently approved by FDA treat only a subset of cancer types (366)Cancer Research Institute. Approval timelines of active immunotherapies. Accessed: June 30, 2022.[cited 2020 Jul 15]..
Researchers are continuously investigating and developing new and improved strategies to fully realize the potential of immunotherapeutics for treating all cancers. Numerous ongoing clinical trials are evaluating a range of immunotherapeutics against new targets on cancer cells and testing the use of those we already have against additional types of cancer, alone or in combination with other types of cancer treatments (367)Kubli SP, et al. Beyond immune checkpoint blockade: emerging immunological strategies. Nat Rev Drug Discov 2021;20:899-919. [LINK NOT AVAILABLE]. One major breakthrough in cancer immunotherapy is the FDA approval in March 2022 of a revolutionary immunotherapeutic, called relatlimab, in combination with nivolumab (Opdualag). Relatlimab is the first immune checkpoint inhibitor against a new target in eight years since the approval of pembrolizumab (see Releasing the Brakes on the Immune System).
Another area of active research is the use of natural killer (NK) cells to develop a new class of immunotherapeutics (368)Zhang L, et al. CAR-NK cells for cancer immunotherapy: from bench to bedside. Biomark Res 2022;10:12. [LINK NOT AVAILABLE](369)Daher M, et al. Outlook for new CAR-based therapies with a focus on CAR NK cells: What lies beyond CAR-engineered T cells in the race against cancer. Cancer Discov 2021;11:45-58. [LINK NOT AVAILABLE]. NK cells are a type of immune cells that rapidly kill abnormal cells by releasing cytotoxic chemicals. In several clinical studies, NK cells have proven to be safer that T cell-based immunotherapeutics for use in humans, and more effective in killing cancer cells (369)Daher M, et al. Outlook for new CAR-based therapies with a focus on CAR NK cells: What lies beyond CAR-engineered T cells in the race against cancer. Cancer Discov 2021;11:45-58. [LINK NOT AVAILABLE]. Researchers are also developing genetically modified NK cells to further enhance their ability to specifically kill cancer cells (370)Xie G, et al. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine 2020;59:102975. [LINK NOT AVAILABLE]. Ongoing research is also focused on harnessing the ability of certain types of immune cells isolated from tumors to develop anticancer immunotherapeutics (371)Wang S, et al. Perspectives of tumor-infiltrating lymphocyte treatment in solid tumors. BMC Med 2021;19:140. [LINK NOT AVAILABLE] (see Looking to the Future of Cancer Science and Medicine).
Here, we focus on the FDA approvals of new immunotherapeutics and the expansions of the previously approved immunotherapeutics for use against additional types of cancer between August 1, 2021, and July 31, 2022 (see Table 4).
Releasing the Brakes on the Immune System
Breakthrough discoveries over the past few decades have revealed that T cells, a type of immune cell, are naturally capable of destroying cancer cells. Research has also revealed that some tumor cells “learn” to avoid destruction by T cells. One of the ways by which tumor cells do so is by increasing levels of certain proteins on their surface that attach to and activate “brakes” on T cells, thus stopping them from attacking cancers. These brakes are proteins on the surface of T cells and are called immune checkpoint (IC) proteins.
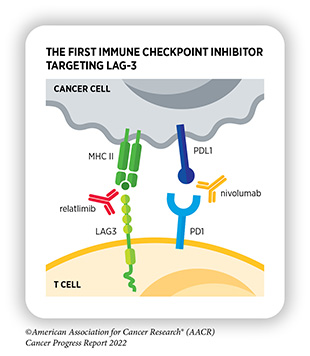
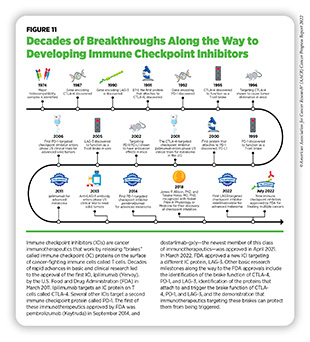
Researchers have identified many IC proteins and their binding partners on tumor cells, four of which—CTLA-4, PD-1, and PD-L1, and most recently, LAG-3—have proven to be effective targets for therapeutic intervention (see Figure 11). This knowledge has led to the development of a new class of therapeutics—called immune checkpoint inhibitors (ICIs)— that can release the brakes on T cells and trigger T cells to destroy cancer cells (372)Marin-Acevedo JA, et al. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol 2021;14:45. [LINK NOT AVAILABLE].
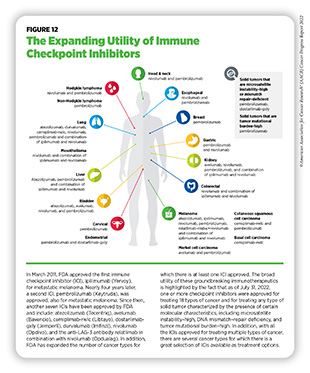
One of the major advantages of ICIs is their effectiveness against different types of cancer, as highlighted by FDA approvals of ICIs to treat multiple types of cancer (see Figure 12). During the 12-month period covered in this report, the FDA approved relatlimab (Opdualag), which binds to and releases a brake called LAG-3 on T cells, in combination with an already approved ICI, nivolumab (Opdivo), to treat metastatic melanoma. As of July 31, 2022, one or more ICIs have been approved for treating 18 types of cancer and for treating any types of solid tumors that are characterized by certain molecular features, such as microsatellite instability (MSI)–high, DNA mismatch–repair deficiency (dMMR), and tumor mutational burden (TMB)–high.
In March 2022, FDA approved a combination of two ICIs, nivolumab and relatlimab. The combination is approved to treat adult and pediatric (≥12 years old) patients with previously untreated melanoma whose cancer has either spread to other organs or cannot be removed by surgery.
Deaths related to invasive melanoma have declined sharply at a rate of four percent every year from 2014 to 2019—the most recent data year—thanks to breakthroughs in treatment of the cancer (1)Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [LINK NOT AVAILABLE](21)American Association for Cancer Research. AACR Cancer Progress Report 2021. Accessed: Dec 19, 2021.[cited 2020 Jul 15].. However, treatment of melanoma that has spread to other parts of the body or cannot be surgically removed remains a significant challenge. The approval of relatlimab is a major advance as it is the first FDA-approved drug to block the activity of LAG-3, a protein on the surface of T cells that also functions as a brake similar to CTLA-4 and PD-1. It is also the first FDA-approved ICI against a new immune checkpoint in more than eight years. Importantly, the approval brings hope for patients, such as Johnny Borgstrom, who had limited options for treating an otherwise difficult-to-treat type of skin cancer.
FDA approval was based on a phase II/III clinical study that compared efficacy of the relatlimab and nivolumab combination with nivolumab alone—which is the standard-of-care—in patients with previously untreated melanoma whose cancer had spread within the body or could not be removed surgically (373)Tawbi HA, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med 2022;386:24-34. [LINK NOT AVAILABLE]. A total of 714 patients were randomly selected to receive the combination of nivolumab and relatlimab or nivolumab alone. Compared to patients who received nivolumab alone, patients who received the drug combination had longer progression-free survival (4.6 months versus 10.1 months, respectively) (373)Tawbi HA, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med 2022;386:24-34. [LINK NOT AVAILABLE].
It is important to note that the relatlimab and nivolumab combination appears to be associated with fewer side effects than another combination of ICIs, also approved for the treatment of melanoma—nivolumab and ipilimumab. Less than 20 percent of patients who received the relatlimab and nivolumab combination reported serious side effects compared to nearly 60 percent of patients who received the nivolumab and ipilimumab combination (374)Frampton AE, et al. A new combination immunotherapy in advanced melanoma. N Engl J Med 2022;386:91-2. [LINK NOT AVAILABLE].
Although the study is ongoing, approval of relatlimab marks a significant advance in the field of ICIs, and provides more treatment options for patients who have advanced, and particularly harder to treat, cancer.
In August 2021, FDA approved dostarlimab-gxly (Jemperli) for adult patients who have no satisfactory alternative treatment options for their advanced stage or returning solid tumors that are dMMR, a specific genetic feature of many types of cancer. FDA also approved the VENTANA MMR RxDx Panel as a companion diagnostic to select patients with dMMR solid tumors for treatment with dostarlimab-gxly.
FDA approval of dostarlimab-gxly, which was first approved in April 2021 to treat advanced endometrial cancer with dMMR (21)American Association for Cancer Research. AACR Cancer Progress Report 2021. Accessed: Dec 19, 2021.[cited 2020 Jul 15]., to treat any solid tumor with dMMR was based on clinical trials that evaluated the efficacy of the immunotherapeutic. Overall, 41.6 percent of patients responded to the treatment. The median duration of the response was nearly three years, with more than 95 percent of the respondents responding for at least six months (376)U.S. Food and Drug Administration. FDA grants accelerated approval to dostarlimab-gxly for dMMR advanced solid tumors. Accessed: June 30, 2022.[cited 2020 Jul 15].. The approval adds dostarlimab-gxly to a growing list of targeted therapeutics being used in the clinic to treat different types of cancer that share similar genetic feature(s).
In addition to the remarkable benefit of ICIs in saving and improving lives of patients across a broad spectrum of cancer types, studies are highlighting the potential utility of ICIs beyond cancer treatment. For example, findings of a recent study indicate that pembrolizumab can increase viral production from dormant HIV-infected cells, which are usually undetected by the host immune system and are unresponsive to ART. By increasing HIV viral production in these latently infected cells, pembrolizumab could facilitate immune recognition and reduce the number of HIV-infected cells that persist after ART, potentially aiding in curing HIV in addition to treating cancer (378)Uldrick TS, et al. Pembrolizumab induces HIV latency reversal in people living with HIV and cancer on antiretroviral therapy. Sci Transl Med 2022;14:eabl3836. [LINK NOT AVAILABLE]. Additional studies will help determine whether this observation may translate into cures for patients with HIV and cancer, and whether pembrolizumab and other ICIs have similar additional benefits beyond cancer.
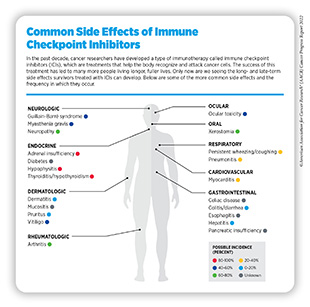
It should be noted that realizing the full potential of ICIs in treating cancers is somewhat restricted by the fact that not all types of tumors respond to these immunotherapeutics and many that do eventually develop resistance to the treatment. Researchers are continuously working to develop innovative and improved strategies to bring the promise of these immunotherapeutics to as many additional cancer patients as possible. It will also be important to evaluate and address the short- and long-term side effects and late effects arising in patients who are treated with these newly approved immunotherapeutics (see sidebar on Common Side Effects of Immune Checkpoint Inhibitor).
Boosting the Killing Power of the Immune System
T cells are specialized to destroy unwanted microbes, such as infectious pathogens, or cells, such as a cancer cell, in the body. However, T cells only go on the attack after a specialized immune cell has “presented” them with a piece of the microbe or cancer cell that is present in the body (see sidebar on Key Cells in the Immune System). Researchers have leveraged this knowledge to develop a new class of immunotherapeutics, called bispecific antibodies, which bypasses the intermediate step and directly brings the tumor cell and T cell together. Ongoing research is focused on developing safe and effective variations of these immunotherapeutics that can flag cancer cells so that the immune system can destroy them (379)Sedykh SE, et al. Bispecific antibodies: design, therapy, perspectives. Drug Des Devel Ther 2018;12:195-208. [LINK NOT AVAILABLE].
Uveal melanoma, also called ocular melanoma, is a cancer that forms in the middle layer of the wall of the eye. Although rare, this type of cancer is often fatal once it spreads to other parts of the body, which happens in about half of all cases. There was no standard treatment for metastatic uveal melanoma until recently (380)Carvajal RD, et al. Metastatic disease from uveal melanoma: treatment options and future prospects. Br J Ophthalmol 2017;101:38-44. [LINK NOT AVAILABLE].
In January 2022, FDA approved tebentafusp-tebn (Kimmtrak) for treatment of certain patients with metastatic uveal melanoma whose cancer cannot be surgically removed or has spread to other organs in the body. The approval is for adult patients whose cells have a marker on their surface called human leukocyte antigen (HLA)-A02:01, which is a protein present in about half of all White people, the population most affected by the disease. Tebentafusp-tebn is only the second bispecific T-cell engager approved by FDA and is the first and only treatment for patients with metastatic uveal melanoma.
Tebentafusp-tebn is a bispecific antibody, which means that it can recognize two different types of molecules simultaneously. One end of tebentafusp-tebn binds to a protein, called gp100, which is present in abundance on the surface of melanoma cells (381)Martinez-Perez D, et al. gp-100 as a novel therapeutic target in uveal melanoma. Cancers (Basel) 2021;13. [LINK NOT AVAILABLE]. The other end binds to the protein CD3 on T cells, bringing them in close proximity to the melanoma cells, where the immune cells can attack and destroy the cancer cells. For tebentafusp-tebn to recognize and bind to gp100-expressing melanoma cells, these cells must also express HLA-A02:01 (382)Damato BE, et al. Tebentafusp: T cell redirection for the treatment of metastatic uveal melanoma. Cancers (Basel) 2019;11. [LINK NOT AVAILABLE].
The approval was based on a phase III clinical trial, in which 378 patients were randomly assigned to either the tebentafusp-tebn group (252 patients) or the control group (126 patients) (383)Nathan P, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med 2021;385:1196-206. [LINK NOT AVAILABLE]. The control group received one of the three established therapies—pembrolizumab, ipilimumab, or dacarbazine. At the one-year follow-up, the overall survival was 73 percent in the tebentafusp-tebn group and 59 percent in the control group. Treatment with tebentafusp-tebn increased survival without any progression of the disease by 63 percent (31 percent in the tebentafusp-tebn group versus 19 percent in the control group at six months). Furthermore, nine percent of the patients in the tebentafusp-tebn group had their tumors shrink in response to treatment compared to five percent in the control group (383)Nathan P, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med 2021;385:1196-206. [LINK NOT AVAILABLE].
Engineering Immune Cells to Eliminate Cancer
Adoptive cell therapy (ACT), also called cellular immunotherapy, is designed to dramatically increase the number of cancer-killing T cells, thus giving a patient’s immune system a boost to seek and destroy cancer cells (384)Rohaan MW, et al. Adoptive cellular therapies: the current landscape. Virchows Arch 2019;474:449-61. [LINK NOT AVAILABLE] (see sidebar on What Is Adoptive T-Cell Therapy?).
ACT is one of the more recent immunotherapeutic approaches that have revolutionized the treatment of certain types of blood cancer and transformed the lives of many adult and pediatric patients (385)Dana H, et al. CAR-T cells: Early successes in blood cancer and challenges in solid tumors. Acta Pharm Sin B 2021;11:1129-47. [LINK NOT AVAILABLE]. However, treating patients with ACT, such as with one of the FDA-approved CAR T-cell therapies, is a complex procedure that can only be performed at specially certified health care facilities by highly trained medical professionals (see sidebar on What Is Adoptive
T-Cell Therapy?). Furthermore, some of the side effects of CAR T-cell therapies can be potentially life threatening, such as the cytokine release syndrome in which the patient’s immune system overreacts by rapidly releasing certain types of molecules into the blood. CAR T-cell therapies have also proven less successful against solid tumors (386)Morotti M, et al. Promises and challenges of adoptive T-cell therapies for solid tumours. Br J Cancer 2021;124:1759-76. [LINK NOT AVAILABLE]. Developing simpler and safer ways to bring the promise of this class of immunotherapeutics to patients is an area of active research. One exciting approach under investigation is to combine the power of cancer immunotherapy with the potential of stem cells that can be genetically engineered to make “designer” immune cells with enhanced antitumor activity (387)Netsrithong R, et al. Advances in adoptive cell therapy using induced pluripotent stem cell-derived T cells. Front Immunol 2021;12:759558. [LINK NOT AVAILABLE].
The potential of ACT for the effective treatment of several types of blood cancer is underscored by the FDA approvals of the new uses of previously approved CAR T-cell therapies. During the 12 months covered in this report, FDA approved ciltacabtagene autoleucel (Carvykti) to treat multiple myeloma; brexucabtagene autoleucel (Tecartus) to treat acute lymphoblastic leukemia in adults; and tisagenlecleucel (Kymriah) to treat follicular lymphoma.
Multiple myeloma remains one of the most diagnosed blood cancers in the U.S. (1)Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [LINK NOT AVAILABLE]. Despite the advances in recent years against the disease (21)American Association for Cancer Research. AACR Cancer Progress Report 2021. Accessed: Dec 19, 2021.[cited 2020 Jul 15]., many patients eventually develop resistance to treatment over time and then the disease progresses. In February 2022, FDA approved ciltacabtagene autoleucel (also called cilta-cel) for the treatment of adults with myeloma whose cancer has returned or does not respond to treatment after four or more prior lines of lines of treatments.
Cilta-cel is directed against the protein B-cell maturation antigen (BCMA) on cancer cells. Expression of BCMA is largely restricted to plasma cells, which are blood cells that make antibodies to protect against infections (383)Nathan P, et al. Overall survival benefit with tebentafusp in metastatic uveal melanoma. N Engl J Med 2021;385:1196-206. [LINK NOT AVAILABLE], and is much higher in myeloma cells compared to normal plasma cells (389)Yu B, et al. BCMA-targeted immunotherapy for multiple myeloma. J Hematol Oncol 2020;13:125. [LINK NOT AVAILABLE]. Cilta-cel approval was based on results from a phase I/II clinical trial. Study participants received a single infusion of cilta-cel and 98 percent of them responded completely or partially to the treatment. Importantly, there were no signs of the cancer in the bone marrow or blood for 77 percent of participants a year after infusion and the overall survival rate was 89 percent (390)Berdeja JG, et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): a phase 1b/2 open-label study. Lancet 2021;398:314-24. [LINK NOT AVAILABLE]. Approval of cilta-cel marks the second CAR T-cell therapy option for patients with advanced multiple myeloma; in April 2021, FDA approved idecabtagene vicleucel or ide-cel (Abecma), the first ever CAR T-cell therapy to treat multiple myeloma (21)American Association for Cancer Research. AACR Cancer Progress Report 2021. Accessed: Dec 19, 2021.[cited 2020 Jul 15]..
Acute lymphoblastic leukemia (ALL) is a cancer of lymphoblasts, a type of blood cells that eventually develop into the immune system. ALL progresses rapidly and requires immediate treatment. Although most cases of ALL occur in children, about 80 percent of ALL deaths occur in adults (1)Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [LINK NOT AVAILABLE]. Adults with ALL either do not respond at all to the available treatments or those who do respond usually have their cancer return (391)Pulte D, et al. Survival of adults with acute lymphoblastic leukemia in Germany and the United States. PLoS One 2014;9:e85554. [LINK NOT AVAILABLE]. These patients have a renewed hope with the approval of the first CAR T-cell therapy for the treatment of adult ALL.
In October 2021, FDA approved brexucabtagene autoleucel (Tecartus) for adult patients with B-cell precursor acute lymphoblastic leukemia (ALL) that has either returned after initial treatment or does not respond to the treatment. The approval was based on a phase I/II clinical study in which 55 patients received a single infusion of the drug (392)Shah BD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. The Lancet 2021;398:491-502. [LINK NOT AVAILABLE]. Overall, 71 percent of the patients had complete remission with or without their white blood cell count returning to normal at median follow-up of 16 months. The 58 percent of patients who had complete remission had no signs of cancer after more than a year of receiving the treatment, and more than half of all respondents were alive nearly two years after the treatment (392)Shah BD, et al. KTE-X19 for relapsed or refractory adult B-cell acute lymphoblastic leukaemia: phase 2 results of the single-arm, open-label, multicentre ZUMA-3 study. The Lancet 2021;398:491-502. [LINK NOT AVAILABLE]. This is remarkable news for adult patients with ALL who, even when responding to other available immunotherapies, such as blinatumomab (Blincyto), often have their cancer return.
Follicular lymphoma (FL) is a form of B-cell NHL, which is the most common type of lymphoma (1)Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [LINK NOT AVAILABLE]. Although FL responds well to initial treatments, it remains difficult to cure because many patients develop resistance to the treatment (393)Bojarczuk K, et al. Molecular classification of large b-cell non-hodgkin lymphoma. Cancer J 2020;26:357-61. [LINK NOT AVAILABLE]. In May 2022, FDA approved tisagenlecleucel for adult patients with FL whose cancer has returned or become resistant after two or more lines of systemic therapy. Findings of a phase II clinical trial, involving more than 90 patients, led to the FDA approval. Overall, 86 percent of patients responded to the immunotherapeutic, and 69 percent responded completely. Importantly, 75 percent of the respondents were still in response at the nine-month follow-up (394)Fowler NH, et al. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med 2022;28:325-32. [LINK NOT AVAILABLE].
Next Section: Supporting Cancer Patients and Survivors Previous Section: Screening for Early Detection