Understanding How Cancer Develops
In this section, you will learn:
- Research provides our understanding of the biology of cancer, which is not one disease, but a collection of diseases characterized by the uncontrolled growth of cells.
- Genetic mutations underpin cancer initiation and development in most cases; mutations are inherited in about 10 percent of patients.
- Cancer initiation and progression are strongly influenced by interactions of cancer cells with cellular and molecular factors in their environment, referred to as the tumor microenvironment.
- Identification of genetic, epigenetic, protein, and cellular alterations that drive cancer are an important part of the cancer care decision-making process and form the foundation of precision medicine.
The term cancer describes a collection of diseases characterized by the inability of a cell to respond to normal biological cues that regulate processes including cell division, growth, and life span. When cells acquire these traits, they begin to divide uncontrollably and accumulate in organs and tissues forming a mass called a tumor, whereas in the blood or bone marrow they crowd out normal cells. Cancer cells use the blood and lymphatic systems to leave the organ of origin and move to distant sites. Growth of cancer cells in another organ distant from its original site is called metastasis and is the primary cause of death in most cancers. Understanding the biological mechanisms underlying tumor initiation and metastasis and identifying ways to prevent these processes are key focus areas of ongoing research (40)Nguyen B, et al. Genomic characterization of metastatic patterns from prospective clinical sequencing of 25,000 patients. Cell 2022;185:563-75 e11. [LINK NOT AVAILABLE] (41)Yang D, et al. Lineage tracing reveals the phylodynamics, plasticity, and paths of tumor evolution. Cell 2022;185:1905-23 e25. [LINK NOT AVAILABLE]. Our understanding of the hallmarks that define cancer development has increased tremendously in the past two decades, thanks to major advances in basic research resulting from generous federal investments (42)Hanahan D. Hallmarks of cancer: New dimensions. Cancer Discov 2022;12:31-46. [LINK NOT AVAILABLE] (see sidebar on What Is Basic Research and How Does It Drive Progress Against Cancer?).
Cancer Development: Influences Inside the Cell
Cells of the human body rely on instructions in their genetic material, known as deoxyribonucleic acid (DNA), to function. DNA is a complex molecule made up of four types of building blocks, called bases, each of which are unique molecules, designated A, T, C, and G (see sidebar on Genetic and Epigenetic Control of Cell Function). Anywhere from 50 to 250 million of these bases are linked together to form individual DNA strands. Two strands of the same length are then paired together to form a helical structure, which is packaged with proteins, known as histones, into structures called chromosomes that are located inside the nucleus of a cell.
Each chromosome contains hundreds to thousands of genes, which are segments of DNA that contain the code for a protein, the functional unit of the cell. To make a protein, a cell copies the “message” embedded in a gene from DNA to make another type of molecule called ribonucleic acid (RNA) in a process called transcription. The cell can make many copies of this messenger RNA (mRNA) from a single sequence of DNA, amplifying the amount of the message in the cell. The cell then “translates” the information from mRNA into proteins. It is important to note that cellular functions are controlled by many additional layers of complexity. For example, not all parts of DNA make mRNA and not all RNA molecules make proteins, but both DNA and RNA have important roles in determining overall cell function (44)Lin CP, et al. Noncoding RNAs in cancer development. Annu Rev Canc Biol 2017;1:163-84. [LINK NOT AVAILABLE] (45)Segal E, et al. From DNA sequence to transcriptional behaviour: a quantitative approach. Nat Rev Genet 2009;10:443-56. [LINK NOT AVAILABLE].
Cancers arise when there are alterations in the DNA sequence, referred to as mutations (see sidebar on Alterations That Lead to Cancer). The mutations change the way the cell reads DNA, which can alter the sequence or amount of mRNA and the resulting protein that is produced. Mutations can develop differently for each individual and are attributed to a range of genetic as well as environmental factors. Therefore, a patient’s cancer can have one or more unique combinations of genetic mutations. Furthermore, as cancer cells divide, new mutations can arise. Several research efforts are aimed at building databases that catalogue different types of mutations contributing to cancer initiation and progression (46)Drews RM, et al. A pan-cancer compendium of chromosomal instability. Nature 2022;606:976-83. [LINK NOT AVAILABLE]. The following section describes the types of mutations that occur in the DNA and how these changes alter the normal functions of the cell, which leads to cancer.
DNA Mutations
Most cancer-causing mutations are acquired over an individual’s lifetime due to errors arising during normal cell multiplication or because of environmental exposures, lifestyle factors, or health conditions that fuel chronic inflammation. These acquired mutations are referred to as somatic mutations.
In about 10 percent of cancer cases the mutations are inherited (see Table 2). When multiple individuals in a family carry a mutation in a gene that is associated with cancer-causing processes, and there is strong evidence that the mutation significantly increases risk of cancer, these types of inherited mutations are called “pathogenic.” Decades of research have led to the identification of numerous genes that are associated with cancers as well as specific inherited mutations in those genes that are pathogenic (see Table 2) (47)Huang KL, et al. Pathogenic germline variants in 10,389 adult cancers. Cell 2018;173:355-70 e14. [LINK NOT AVAILABLE]. Pathogenic mutations continue to be identified by genetically profiling cells from different types of cancer and decoding these cancer-causing mutations. For example, a recent study of 214,000 patients identified seven new pathogenic mutations that were associated with increased risk of developing leukemia, or stomach, pancreatic, kidney, and bladder cancers, and intrahepatic bile duct tumors, expanding our understanding of what types of pathogenic mutations lead to cancer (48)Zeng C, et al. Association of pathogenic variants in hereditary cancer genes with multiple diseases. JAMA Oncol 2022;8:835-44. [LINK NOT AVAILABLE].
One example of inherited mutations is those in BRCA1/2 genes, which are important for repairing damaged DNA in cells. Mutations in either BRCA1 or BRCA2 can increase the risk of breast, ovarian, pancreatic, and prostate cancer. New data demonstrate that mutations in BRCA1/2 genes can also increase the risk of biliary tract cancer, esophageal, and/or gastric cancer, expanding our understanding of the role of pathogenic mutations (49)Momozawa Y, et al. Expansion of cancer risk profile for BRCA1 and BRCA2 pathogenic variants. JAMA Oncol 2022;8:871-8. [LINK NOT AVAILABLE].
These discoveries demonstrate the far-reaching contributions of cancer research that help expand our understanding of how cancer develops.
RNA Alterations
As described earlier, when a cell “reads” a gene, it copies the information from the DNA into an RNA molecule called messenger RNA or mRNA. By changing the number of times DNA is copied into mRNA, the cell can increase or decrease the amount of the mRNA, which helps control the amount of protein a cell makes; the cellular levels of a protein can drastically affect cell function. Furthermore, a cell is usually making copies of mRNA from hundreds to thousands of different genes at once, producing many different types of proteins, each of which performs different functions and influences the cell’s biology. Advances in nucleic acid sequencing technology have allowed researchers to determine the sequences of genes, as well as the types and levels of mRNA produced, to create molecular profiles that often match the function of the cell. Profiling cancer cells from a tumor and comparing the profile to that of cells from the normal tissue helps researchers identify specific characteristics that contribute to cancer development.
Examining mRNA in cancer cells has some advantages over exclusively studying DNA. A key reason for this is that mRNA is what makes protein, and therefore changes that occur in mRNA are more consequential. Researchers in one study looked at the ability of mRNA sequencing to detect the presence of a type of mutation called a gene fusion, which occurs in certain cancers (see sidebar on Alterations That Lead to Cancer). The researchers were not only able to identify the same gene fusions detected by DNA testing using mRNA sequencing, but also to identify additional fusions that were not originally picked up during DNA analysis. Furthermore, by analyzing the mRNA sequence, researchers were able to identify which genes were fused, and for a subset of patients, this led to the use of targeted treatments against the mutation which was previously unknown (51)Hehir-Kwa JY, et al. Improved gene fusion detection in childhood cancer diagnostics using RNA sequencing. JCO Precis Oncol 2022;6:e2000504. [LINK NOT AVAILABLE].
Normal cells copy the message from DNA in pieces of mRNA that are assembled in a process called splicing to complete the message. In cancer cells, this process can be altered to generate abnormal proteins, which can fuel uncontrolled cell proliferation and growth (see sidebar on Alterations That Lead to Cancer). For instance, in non-small cell lung cancer, the gene MET can become alternately spliced, generating an abnormal protein called METex14. If genetic tests reveal the presence of this alternately spliced mRNA, MET-targeted therapeutics can be used for the treatment of such patients (52)Lee JK, et al. Characterization of non-small-cell lung cancers with MET exon 14 skipping alterations detected in tissue or liquid: Clinicogenomics and real-world treatment patterns. JCO Precis Oncol 2021;5:1354-76. [LINK NOT AVAILABLE] (53)Kim EK, et al. Molecular diagnostic assays and clinicopathologic implications of MET exon 14 skipping mutation in non-small-cell lung cancer. Clin Lung Cancer 2019;20:e123-e32. [LINK NOT AVAILABLE].
Protein Modifications
Cells use networks of proteins, often referred to as signaling networks, to sense important information regarding internal conditions such as cellular energy levels as well as external conditions like temperature, integrating this information to mount a cellular response; for example, a cell can increase the level of energy production to grow and divide based on sensing external stimuli permitting it to do so; conversely, an unhealthy cell, upon sensing factors released by the immune system, can initiate a programmed sequence of events that leads to its elimination before it can become cancerous. Importantly, cancer cells can often alter the signaling networks, through mutations, upregulation, or modification of proteins, to suit their development and progression. By doing this, they no longer respond to normal cues, leading to uncontrolled cell division or evasion of cell death.
Analyses of DNA or mRNA cannot always reliably predict changes in the level or function of the corresponding proteins and how those may affect signaling networks. Proteomics—the comprehensive analysis of all the proteins inside a cell—is extremely important in cancer research and has proven to be a powerful tool to gain novel insights into a patient’s tumor that cannot be realized by genomics alone.
Researchers strongly believe that when used together, cancer proteomics and genomics can truly open new opportunities in the diagnosis, prognosis, and treatment of cancers and transform the landscape of patient care. According to NCI, proteogenomics is defined as the study of how information about the DNA in a cell or organism relates to the proteins made by that cell or organism.
Although this is an emerging area of investigation, several major NCI programs including Clinical Proteomics Tumor Analysis Consortium (CPTAC) are studying how proteins and the signaling networks associated with them are different between cancers. Furthermore, several research groups are utilizing information about signaling changes to identify how different cancer types will respond to different types of cancer therapy combinations (54)Clark DJ, et al. Integrated proteogenomic characterization of clear cell renal cell carcinoma. Cell 2019;179:964-83 e31. [LINK NOT AVAILABLE] (55)Rodriguez H, et al. The next horizon in precision oncology: Proteogenomics to inform cancer diagnosis and treatment. Cell. Volume 184: Elsevier Inc.; 2021. p 1661-70. [LINK NOT AVAILABLE].
Epigenetic Changes
In addition to genetic mutations, changes caused by chemical modifications of DNA and/or the proteins associated with it, termed epigenetic modifications, can lead to cancer development (see sidebar on Alterations That Lead to Cancer). Epigenetic modifications regulate how and when our genes are turned on or off. Specialized proteins add or erase unique epigenetic modifications to and from DNA and histones (56)Lu Y, et al. Epigenetic regulation in human cancer: the potential role of epi-drug in cancer therapy. Mol Cancer 2020;19:79. [LINK NOT AVAILABLE]. In contrast to genetic mutations, epigenetic changes are often reversible, providing an opportunity for therapeutic intervention.
Our understanding of the epigenetic contributions to cancer development is constantly evolving. As one example, one recent study found that when mutations occur in areas of the DNA that do not code for a gene, this leads to epigenetic
modifications many bases away from the gene (57)Grishin D, et al. Allelic imbalance of chromatin accessibility in cancer identifies candidate causal risk variants and their mechanisms. Nat Genet 2022;54:837-49. [LINK NOT AVAILABLE]. This means that even when a mutation does not occur within a gene, it can still have far-reaching consequences for the development of cancer through epigenetic effects.
Emerging evidence shows that environmental influences such as diet, stress, smoking tobacco products, and exposure to pollutants can result in epigenetic changes. Understanding these influences is especially important for groups who continue to be disproportionately and negatively affected by environmental influences, such as racial and ethnic minorities and other underserved populations, as discussed in detail in the recently released AACR Cancer Disparities Progress Report 2022 (13)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2022. Accessed: June 30, 2022.[cited 2020 Jul 15]..
Advanced age has been shown to induce epigenetic changes. To understand this phenomenon, researchers have developed ‘epigenetic clocks’ that estimate an individual’s epigenetic age based on modifications present on a person’s DNA. Previous studies have indicated how advanced epigenetic age can increase the risk of cancer development (58)Morales Berstein F, et al. Assessing the causal role of epigenetic clocks in the development of multiple cancers: a Mendelian randomization study. Elife 2022;11. [LINK NOT AVAILABLE].
Accelerated epigenetic aging has also been shown in patients treated with certain types of cancer therapies regardless of their biological age, which may explain why cancer survivors can develop side effects, such as “excess heart age.” In one study that looked at epigenetic changes in whole blood cells in early-stage breast cancer patients undergoing surgery and radiotherapy, it was found that there was significant epigenetic age acceleration, which affected certain immune cells (59)Sehl ME, et al. The acute effects of adjuvant radiation and chemotherapy on peripheral blood epigenetic age in early stage breast cancer patients. NPJ Breast Cancer 2020;6:23. [LINK NOT AVAILABLE].
Tumor Heterogeneity
The changes in DNA, RNA, and protein can vary among subsets of cells within a single tumor. The phenomenon used to describe cells with different mutations in a single tumor is called tumor heterogeneity. Tumor heterogeneity fuels the cancer’s ability to grow faster and metastasize, resist therapy, and evade destruction by the immune system. Fortunately, the adoption of cutting-edge technologies such as genetic sequencing and single cell profiling has allowed researchers to identify the different mutations within subsets of cells in a tumor with a high resolution (60)Megyesfalvi Z, et al. Expression patterns and prognostic relevance of subtype-specific transcription factors in surgically resected small-cell lung cancer: an international multicenter study. J Pathol 2022;257:674-86. [LINK NOT AVAILABLE]. Additionally, current research is using predictive mathematical models to understand how different groups of cells within a tumor become resistant to therapy and how these unique groups may respond to different types of therapies so they can be treated more effectively (61)Strobl MAR, et al. Spatial structure impacts adaptive therapy by shaping intra-tumoral competition. Commun Med (Lond) 2022;2:46. [LINK NOT AVAILABLE].
Cancer Development: Influences Outside the Cell
Cancer arises due to the disruption of normal cellular functions through genetic and epigenetic changes in a cell. Once cancer is initiated, however, complex interactions between cancer cells and their surrounding environment—known as the tumor microenvironment—contribute to disease progression (see sidebar on Cancer Growth: Local and Systemic Influences). For instance, cancer cells can release molecules that shape their surrounding environment to provide them with nutrients, oxygen, and a supportive structure. This reorganization also aids in the process of metastasis of cancer cells through blood and lymphatic systems.
The Blood System
Because cancer cells grow and divide rapidly, they require high levels of oxygen and nutrients to fuel their growth. To keep up with this demand, tumors release factors into the surrounding environment that increase the amount of blood vessels, a process called angiogenesis. Blood vessels carry nutrients and oxygen to the tumor cells while simultaneously removing waste and carbon dioxide. By increasing blood vessel formation around tumors, cancer cells increase access to these components. Therapeutics that block angiogenesis, therefore, can limit access to these factors, restricting a tumor’s ability to grow, divide, and metastasize.
Advances in cancer therapeutics over the past two decades have led to the development of inhibitors of angiogenesis, such as those that target the protein VEGF, which is a central regulator of angiogenesis; targeting of this protein inhibits the tumor blood supply. The first anti-angiogenic therapy to be approved by FDA, bevacizumab (Avastin) in 2004 was followed by several other drugs including the most recent approval of tivozanib (Fotivda) in 2021.
The Lymphatic System
Like blood vessels, the lymphatic system branches throughout the body and is essential for the immune system to function. This system is also responsible for maintaining fluid levels, removing waste, detecting pathogens, absorbing fats, and producing immune cells and antibodies in the lymph nodes. Tumor cells can manipulate the lymphatic system to grow new vessels that can transport cancer cells away from the primary tumor during metastasis (62)Secker GA, et al. Regulation of VEGFR signalling in lymphatic vascular development and disease: An update. Int J Mol Sci 2021;22:7760. [LINK NOT AVAILABLE]. Changes in the way the lymph system grows have been observed in many cancers including melanoma, breast cancer, colorectal cancer, and squamous cell carcinoma of the head and neck (63)Dieterich LC, et al. Tumor lymphangiogenesis and new drug development. Adv Drug Deliv Rev 2016;99:148-60. [LINK NOT AVAILABLE]. Ongoing efforts, including several clinical trials, are specifically focused on targeting lymph system growth in cancer (64)Wang C, et al. Advances in drugs targeting lymphangiogenesis for preventing tumor progression and metastasis. Front Oncol 2021;11:783309. [LINK NOT AVAILABLE].
The Immune System
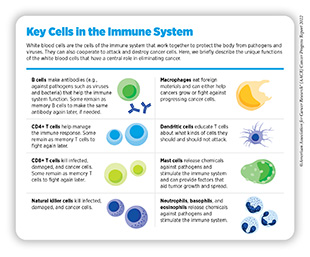
The immune system is composed of a variety of organs, tissues, cells, and molecules that work together to defend the body against external (virus, bacteria) and internal (cancer) threats by recognizing and eliminating them (see sidebars on Cancer Growth: Local and Systemic Influences, and Key Cells in the Immune System). Normally, cells that are precancerous and at risk of turning into cancer cells are eliminated by the immune system. However, certain cancer cells can manipulate the immune system so that they evade elimination. For instance, cancer cells can increase the amount of surface proteins that can put brakes on immune cells, effectively evading detection. Treatments that unleash the brakes on the body’s immune system to fight cancer are called immunotherapies and these agents have drastically improved our ability to fight many types of cancer (see Advances in Cancer Immunotherapy).
Ongoing research is likely to uncover additional mechanisms by which the immune system can be harnessed to eliminate cancer cells which may, in turn, lead to the development of new and improved treatments against cancer.
Cancer Development: Integrating Our Knowledge
The remarkable progress in discovery science during the past five decades has transformed our understanding of cancer. We have learned that cancer development is influenced by many factors, including a patient’s biological characteristics, social and environmental exposures, and lifestyle. As each person’s experience is unique, so is their cancer. As a result, we are now seeing a major shift from a “one size fits all” paradigm of cancer prevention, screening, and treatment to a more personalized approach called precision medicine.
Precision medicine aims to use genetic and other information about a patient’s tumor, as well as other factors, to help diagnose, plan treatment, determine how well treatment is working, or make a prognosis, with the overarching goal of improving clinical outcomes and minimizing unnecessary diagnostic and therapeutic interventions.
Currently, a critical aspect of precision medicine is genetic sequencing of tumors to identify the specific mutations in the cancer cells so that therapies that are designed to target that mutation can be used. The effectiveness of this molecularly targeted strategy is becoming increasingly clear across a broad range of cancer types (see Advances in Treatment with Molecularly Targeted Therapy) (66)Pruis MA, et al. Personalised selection of experimental treatment in patients with advanced solid cancer is feasible using whole-genome sequencing. Br J Cancer 2022. [LINK NOT AVAILABLE] (67)Kornauth C, et al. Functional precision medicine provides clinical benefit in advanced aggressive hematologic cancers and identifies exceptional responders. Cancer Discov 2022;12:372-87. [LINK NOT AVAILABLE]. For instance, it has been shown that comprehensive genetic testing benefited individuals with rare cancers (i.e., diseases that affect fewer that 15 out of 100,000 people each year) by identifying treatment opportunities (68)Hoes LR, et al. Patients with rare cancers in the Drug Rediscovery Protocol (DRUP) benefit from genomics-guided treatment. Clin Cancer Res 2022;28:1402-11. [LINK NOT AVAILABLE] (see Adding Precision to the Treatment of Rare Cancers). Another study retrospectively sequenced the genomes of patients with cancer that were treated with immunotherapies to determine what mutations could be useful in predicting response. Based on common mutations in genes such as KRAS, BRAF, and TP53, researchers developed a model that predicted response to ICIs in treating metastatic cancers (69)Gajic ZZ, et al. Recurrent somatic mutations as predictors of immunotherapy response. Nat Commun 2022;13:3938. [LINK NOT AVAILABLE].
A clinical trial called the MoleculAr Profiling for Pediatric and Young Adult Cancer Treatment Stratification (MAPPYACTS) performed comprehensive genomic testing of 787 recurrent or refractory malignances in AYA patients with cancer; this included sequencing DNA and RNA. Of this group, 436 patients had at least one genetic mutation identified that would benefit from an existing targeted treatment. Of this group, 30 percent went on to receive the matched therapeutic treatment leading to a response rate in half of those patients (70)Berlanga P, et al. The European MAPPYACTS trial: Precision medicine program in pediatric and adolescent patients with recurrent malignancies. Cancer Discov 2022;12:1266-81. [LINK NOT AVAILABLE]. Many studies are investigating how the use of tumor molecular profiling could identify targeted therapy opportunities for more patients with cancer (71)Church AJ, et al. Molecular profiling identifies targeted therapy opportunities in pediatric solid cancer. Nat Med 2022. [LINK NOT AVAILABLE].
Genetic sequencing of tumors using technologies such as next-generation sequencing is key to guiding precision medicine and has become more widely utilized in a variety of cancers. In 2019, approximately 40 percent of patients with advanced non-small cell lung cancer, metastatic colorectal cancer, metastatic breast cancer, and advanced melanoma had their tumors sequenced using next-generation sequencing technology, which increased from just one percent in 2011 (72)Sheinson DM, et al. Trends in use of next-generation sequencing in patients with solid tumors by race and ethnicity after implementation of the medicare national coverage determination. JAMA Netw Open 2021;4:e2138219. [LINK NOT AVAILABLE]. Unfortunately, tumor sequencing is not utilized by everyone despite the substantial benefits, with decreased likelihood of a patient being referred for a genetic test by a physician as well as lower rates of uptake observed for racial and ethnic minorities as well as those on Medicare (73)Ademuyiwa FO, et al. Genetic counseling and testing in African American patients with breast cancer: A nationwide survey of us breast oncologists. J Clin Oncol 2021;39:4020-8. [LINK NOT AVAILABLE] (74)Kehl KL, et al. Race, poverty, and initial implementation of precision medicine for lung cancer. J Natl Cancer Inst 2019;111:431-4. [LINK NOT AVAILABLE] (75)Palazzo LL, et al. Disparities and trends in genetic testing and erlotinib treatment among metastatic non-small cell lung cancer patients. Cancer Epidemiol Biomarkers Prev 2019;28:926-34. [LINK NOT AVAILABLE] (76)Quinn R, et al. Impact of precision medicine on clinical outcomes: A single-institution retrospective study. Front Oncol 2021;11:659113. [LINK NOT AVAILABLE]. This necessitates increased and equitable access to genetic testing of tumors to maximize treatment potential and lessen unnecessary treatments (72)Sheinson DM, et al. Trends in use of next-generation sequencing in patients with solid tumors by race and ethnicity after implementation of the medicare national coverage determination. JAMA Netw Open 2021;4:e2138219. [LINK NOT AVAILABLE] (77)Brito RA, et al. Total cost of lung cancer care associated with broad panel versus narrow panel sequencing. Journal of Clinical Oncology 2020;38:7077-. [LINK NOT AVAILABLE].
Although genetic sequencing is a powerful tool for understanding changes in cancer cells that can guide treatment decisions, it is becoming increasingly clear that the influences and interactions of multiple additional factors including environmental, genetic, epigenetic, microbiome, and lifestyle factors must be understood for precision medicine to be most effective. More studies that look at these determinants of health from groups of different ancestral, racial, and ethnic backgrounds are necessary to build a better understanding of what leads to cancer. For instance, the All of Us research program launched by NIH in 2018 will enroll 1 million people in the United States to create a diverse database that includes data on biology, lifestyle, and environment and their effects on health. As of March 2022, this project had sequenced the entire genomes of 100,000 people, 50 percent of whom were racially or ethnically diverse. These types of databases will bring new knowledge to understanding the complex interactions that can lead to cancer and other diseases.
Next Section: Preventing Cancer: Identifying Risk Factors Previous Section: A Snapshot of a Year of Progress