Looking to the Future of Cancer Science and Medicine
In this section, you will learn:
- Better technologies and lower costs have led to the ability of sequencing individual cancer cells allowing researchers to unravel the complexities of cancer.
- Researchers are developing tools to detect pre-cancers and intercept cancers before they develop.
- Use of the immune system to fight cancer has become one of the most promising areas of cancer research. Current efforts are focused on developing immunotherapies that work in various ways for more types of cancers and increasing the robustness of these therapies.
- Artificial intelligence (AI) is utilizing the power of computation to help clinicians and pathologists better diagnose and treat cancer. AI is also assisting researchers in decoding cancer’s complexities and answering some of the most elusive problems such as tumor heterogeneity.
Advances in cancer science and medicine over the past decade have contributed to more people living through and beyond their cancer, which brings much excitement and anticipation for what will come in the next decade. AACR President, 2022-2023, Lisa M Coussens, PhD, FAACR, is thrilled about what the future holds and is confident that the steady progress toward reducing the burden from all cancers will continue for years to come. Scientific progress against cancer involves efforts from multiple scientific disciplines through national and international collaborations. The new wave of scientific and technological innovations (see sidebar on Technologies Driving Progress Against Cancer) driven by cross-disciplinary team science will have a transformative impact on patient care. The following sections highlight what the future of cancer care may look like based on the new technologies that are being developed right now.
Looking at Individual Cells
By some estimates, a tumor the size of a pea contains about one billion cells which include cancer cells with a range of genetic and epigenetic alterations as well as other cell types such as immune cells and cells that make up blood vessels called endothelial cells (518)Del Monte U. Does the cell number 10(9) still really fit one gram of tumor tissue? Cell Cycle 2009;8:505-6. [LINK NOT AVAILABLE]. This cellular diversity, also called heterogeneity (see Tumor Heterogeneity), leads to genetic diversity within tumors and can contribute to treatment resistance and disease recurrence. Currently, standard practice for genetically profiling a patient’s tumor is to take a biopsy of tissue and sequence the DNA of all the cells together. We now know that this broad stroke approach overlooks the complex changes that happen within the individual cells of the tumor, each with unique DNA mutations.
Overcoming this heterogeneity presents an immense challenge (see Tumor Heterogeneity). Promisingly, scientific research has led to extraordinary progress in the development of less expensive, faster, and higher quality technologies that researchers can use to isolate and profile individual cells from a tumor. These technologies have opened new frontiers for decoding cancer’s complexities, e.g., understanding how individual cells or small subgroups of cells contribute to cancer initiation, progression, and metastasis.
Single Cell Profiling
Single cell sequencing is a powerful technology that uses a machine to separate thousands to millions of cells and sequence the genetic material of each cell independently; this increases the resolution of the cellular differences within a diverse population of cells, such as in a tumor. As one example, in a recent study using single cell sequencing of 300,000 lung cancer cells, researchers evaluated the functional impact of a range of alterations in common cancer-causing genes such as TP53 and KRAS. By analyzing the resulting changes in the RNA levels, the researchers aim to better understand how different mutations in the two genes contribute to cancer development (527)Ursu O, et al. Massively parallel phenotyping of coding variants in cancer with Perturb-seq. Nat Biotechnol 2022;40:896-905. [LINK NOT AVAILABLE]. Other groups have sequenced RNA instead of DNA, which gives an accurate perspective on what a cell is doing functionally. These data can compare how RNA molecules are expressed in a tumor differently when compared to a healthy cell and this knowledge can help clinicians make decisions on treatment strategies (528)Seryakov A, et al. RNA sequencing for personalized treatment of metastatic leiomyosarcoma: Case report. Front Oncol 2021;11:666001. [LINK NOT AVAILABLE](529)Newman S, et al. Genomes for kids: The scope of pathogenic mutations in pediatric cancer revealed by comprehensive DNA and RNA sequencing. Cancer Discov 2021;11:3008-27. [LINK NOT AVAILABLE]. In one study of HBV-associated hepatocellular carcinoma, researchers sequenced the RNA of both the tumor cells and the immune cells to understand how spatial interactions between these cell populations changed, which can lead to evasion of cancer cells from the immune system (530)Ho DW, et al. Single-cell RNA sequencing shows the immunosuppressive landscape and tumor heterogeneity of HBV-associated hepatocellular carcinoma. Nat Commun 2021;12:3684. [LINK NOT AVAILABLE].
Subcellular Profiling
Some of the most cutting-edge technologies go beyond looking at individual cells and instead examine individual parts of a single cell. One such technology uses an automated laser technique to precisely remove the nucleus of a cell and measure different levels of proteins present (531)Mund A, et al. Deep visual proteomics defines single-cell identity and heterogeneity. Nat Biotechnol 2022. [LINK NOT AVAILABLE]. This type of research allows scientists to understand not only how each cell of a tumor influences cancer, but also how different compartments within an individual cell can influence a cell’s function.
Detecting the Earliest Changes During Cancer Development
Catching cancer early is the best way to prevent it from developing into a more aggressive, harder to treat disease. This often happens during routine screenings, where a precancer, which can be a cell or cluster of cells that could develop into cancer but has not yet, is found and tested. Several research groups are sequencing the genomes of precancerous lesions to identify what mutations are present and if these mutations lead to cancer. For instance, Barrett’s esophagus is a disorder characterized by inflammation of the esophagus caused by acid reflux and can often lead to esophageal adenocarcinoma. Researchers identified 80 patients with Barrett’s esophagus and sequenced areas of precancer. Of the 80 patients originally identified, 40 developed esophageal adenocarcinoma. When researchers sequenced all 80 patients again, they found that those that developed esophageal adenocarcinoma had changes in the gene TP53. Interestingly, the type of mutation found in this gene could be detected up to six years prior to cancer diagnosis (532)Paulson TG, et al. Somatic whole genome dynamics of precancer in Barrett’s esophagus reveals features associated with disease progression. Nat Commun 2022;13:2300. [LINK NOT AVAILABLE].
Based on tests like these, recommendations can be made to start or abstain from treatment and opt for surveillance instead. The road from a precancer to cancer is not very well understood, so understanding the path a precancerous cell takes to becoming a cancerous cell, such as looking at TP53 in Barrett’s esophagus, would help inform clinicians about whether to move forward with treatment or not.
Larger research efforts, such as with the Precancer Atlas, are compiling the genetic profiles of precancers into large databases. By studying the different types of mutations that occur in precancerous cells and how those lead to the development of cancer, researchers are understanding more about why this transition occurs and the contribution of both the cancer cell and its surrounding environment.
Artificial Intelligence
Artificial intelligence (AI) is the ability of a machine, often a computer, to do tasks that are normally done by a human and is being used increasingly in cancer science and medicine (see sidebar on Artificial Intelligence in Early Detection). Previous chapters have highlighted the utilization of AI in cancer screening and diagnosis as well as in increasing the precision of radiation therapy. That is because using AI in the fight against cancer comes from its ability to analyze vast amounts of data that continue to accumulate from around the world, with cancer researchers and clinicians adding new data. Therefore, the future of cancer research, screening, diagnosis, and treatment will benefit greatly from the application of AI.
AI in Basic Research
To understand how a healthy cell becomes a cancer cell and eventually a tumor, researchers are using AI to look at each individual cell of the tumor to identify the genes that are turned on or off. In a recent study, scientists designed a type of AI program that looked at 9,800 patients with 33 different types of cancer to identify the most common ways the genome becomes mutated. The algorithm analyzed the “preference” a particular cancer had for a certain type of mutation and used this information to develop a favorites list of common mutations within that type of cancer. This type of algorithm could be applied to new cases of cancer to understand the likelihood that a particular mutation could develop during the cancer’s evolution (539)Steele CD, et al. Signatures of copy number alterations in human cancer. Nature 2022;606:984-91. [LINK NOT AVAILABLE].
The vast amount of information gathered from an experiment, thanks to rapid advances in single cell technologies, can make analysis by a person extremely time-consuming and difficult. One study utilized AI to analyze data generated from single cell sequencing to help differentiate breast cancer cells from healthy cells. It went on to group cancer cells based on common characteristics. After AI learned how to do this, researchers were able to apply this more broadly to other cancers, which could aid other researchers in answering questions about cancer evolution (540)Dohmen J, et al. Identifying tumor cells at the single-cell level using machine learning. Genome Biol 2022;23:123. [LINK NOT AVAILABLE]. With increased use of single cell sequencing technology outlined above, AI approaches will be indispensable to analyze and integrate the vast amounts of data generated.
AI in Diagnosing Cancers
Diagnosing a cancer typically involves taking a biopsy of tissue and then examining it under a microscope by a pathologist who is trained to find signs of cancer using specialized training and judgment. As this method of diagnosis can be laborious and time-consuming, and can sometimes miss signs of cancer, using AI-based approaches offers a promising way to diagnose cancer.
Currently, studies are exploring the ability of AI to diagnose cancer by comparing results from AI to manual detection done by a pathologist. So far, AI appears to have a high degree of accuracy, even outperforming human pathologists in diagnosing certain types of cancer (541)Mahmood H, et al. Artificial Intelligence-based methods in head and neck cancer diagnosis: an overview. Br J Cancer 2021;124:1934-40. [LINK NOT AVAILABLE](542)Nassif AB, et al. Breast cancer detection using artificial intelligence techniques: A systematic literature review. Artif Intell Med 2022;127:102276. [LINK NOT AVAILABLE](543)Bera K, et al. Predicting cancer outcomes with radiomics and artificial intelligence in radiology. Nat Rev Clin Oncol 2022;19:132-46. [LINK NOT AVAILABLE]. For example, AI was used to detect precancerous colonic polyps, which can develop into colorectal cancer. The researchers found that when AI was used in conjunction with a traditional colonoscopy, this led to a two-fold reduction in missed identification of precancerous lesions compared to diagnoses by a pathologist alone (544)Wallace MB, et al. Impact of artificial intelligence on miss rate of colorectal neoplasia. Gastroenterology 2022;163:295-304 e5. [LINK NOT AVAILABLE]. Recently, FDA has approved AI for cancer early detection and diagnosis, demonstrating the effectiveness of this approach (see Recent Advances in Cancer Screening and Early Detection).
Other approaches that use AI focus on being able to predict the likelihood of developing metastasis. In one study that looked at bone metastasis in patients with breast cancer, an AI algorithm was able to correctly predict the likelihood of bone metastasis 88 percent of the time based on 311,408 different cases (545)Liu WC, et al. Using machine learning methods to predict bone metastases in breast infiltrating ductal carcinoma patients. Front Public Health 2022;10:922510. [LINK NOT AVAILABLE].
To have the most accurate and equitable AI-based screening approaches, these technologies must be applied to a broad range of groups, including racial and ethnic minorities, especially because there has been a demonstrated bias in the use of AI-based screening approaches in the past. (546)Guo LN, et al. Bias in, bias out: Underreporting and underrepresentation of diverse skin types in machine learning research for skin cancer detection-A scoping review. J Am Acad Dermatol 2022;87:157-9. [LINK NOT AVAILABLE](547)Obermeyer Z, et al. Dissecting racial bias in an algorithm used to manage the health of populations. Science 2019;366:447-53. [LINK NOT AVAILABLE](548)Uche-Anya E, et al. Artificial intelligence in gastroenterology and hepatology: how to advance clinical practice while ensuring health equity. Gut 2022:gutjnl-2021-326271. [LINK NOT AVAILABLE](549)Yala A, et al. Multi-institutional validation of a mammography-based breast cancer risk model. J Clin Oncol 2022;40:1732-40. [LINK NOT AVAILABLE]. For instance, in a meta-analysis of AI programs that were developed to detect melanoma from images of skin lesions, only six out of 136 studies disclosed skin type and only 12 disclosed race and ethnicity. Without inclusion of data on darker skin colors (which are often underrepresented) and reporting of race and ethnicity, AI cannot develop inclusive algorithms, which leads to biased technologies that can have a diagnosis that is inconclusive or false.
The bias often stems from a lack of representation of samples from these groups in the datasets from which the program learns. For instance, The Cancer Genome Atlas is made up of samples predominantly from majority European ancestry, which leads to underrepresentation of prognostic, diagnostic, and therapeutic genetic signatures across races. In contrast, AI algorithms that use data collected from global populations can be applied to broad populations with a high degree of accuracy. For instance, one algorithm called Mirai, was used to predict the development of breast cancer at five years from 128,793 mammograms from 62,185 patients across five countries including the United States, Israel, Sweden, Taiwan, and Brazil. The researchers found that their AI had a high degree of accuracy in predicting breast cancer development, regardless of the country being studied, because of the inclusiveness of the algorithm (549)Yala A, et al. Multi-institutional validation of a mammography-based breast cancer risk model. J Clin Oncol 2022;40:1732-40. [LINK NOT AVAILABLE].
Every effort must be made to reduce biases in technologies, which can be done by incorporating a health equity lens early on in development (i.e., incorporating health equity into the AI program); increasing recruitment and representation of a diverse population in AI clinical trials; and implementing reporting standards and auditing (548)Uche-Anya E, et al. Artificial intelligence in gastroenterology and hepatology: how to advance clinical practice while ensuring health equity. Gut 2022:gutjnl-2021-326271. [LINK NOT AVAILABLE].
Using AI to Predict Treatment Success
In an age of precision medicine, there are multiple factors including tumor-associated and inherited genetic alterations, lifestyle, environmental exposures, general health, and medical history, many of which evolve over time, that health care providers must consider before selecting the most appropriate therapy (550)Derbal Y. Can artificial intelligence improve cancer treatments? Health Informatics J 2022;28:14604582221102314. [LINK NOT AVAILABLE].
This approach helps to tailor treatment plans to individual patients. In one study that used AI to generate a radiotherapy regimen for prostate cancer, among the 100 patients studied, 89 percent of the radiotherapy treatment plans generated were deemed clinically acceptable, with 72 percent deemed superior to those devised by human experts (551)McIntosh C, et al. Clinical integration of machine learning for curative-intent radiation treatment of patients with prostate cancer. Nat Med 2021;27:999-1005. [LINK NOT AVAILABLE]. Another study, which used AI to identify patients with head and neck cancers who would benefit from a reduction in the intensity of their radiotherapy or chemotherapy, showed that AI correctly predicted which patients would benefit from treatment de-escalation (552)Corredor G, et al. An imaging biomarker of tumor-infiltrating lymphocytes to risk-stratify patients with HPV-associated oropharyngeal cancer. J Natl Cancer Inst 2022;114:609-17. [LINK NOT AVAILABLE].
Using the Immune System to Fight Cancer
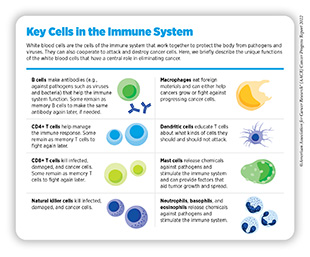
The immune system can identify and eliminate cancer cells the way it does disease-causing pathogens such as bacteria, viruses, and toxins. The immune system, which is made up of many different types of cells (see sidebar on Key Cells in the Immune System), detects foreign objects by using protein sensors on the cell surface. While the immune system is extremely effective in eliminating threats, cancer cells often develop mechanisms to hide from immune cells, escape death, and grow into a tumor. A new class of cancer treatments called immunotherapies utilizes what we know about the immune system to fight cancer. While there has been tremendous progress in this area, immunotherapies do not work for all patients nor are all cancers approved for treatment with specific immunotherapies. In addition, cell-based immunotherapy production is not robust, with long waiting lists for treatment access. This necessitates the discovery of new targets and improved manufacturing technologies. This section details selected examples of what is to come in the research area of immunotherapies and the technologies being developed to expand this type of lifesaving therapy.
The Future of Immune Checkpoint Inhibitors
Cancer cells can have proteins on their surface that can turn off certain immune cells when there is contact, thus evading elimination. In the past decade, cancer researchers have developed therapies called immune checkpoint inhibitors that inactivate these proteins and allow the immune system to recognize and eliminate the cancer cell. Unfortunately, only a fraction of patients responds to immune checkpoint inhibitors (ICIs), and many who do respond initially develop resistance over time.
Continued efforts in this area focus on identifying new proteins specific to different cancers and designing drugs to target them, such as the recent FDA approval of nivolumab and relatimab-rmbw (Opdualag), which targets the protein LAG-3 on the surface of T cells, to treat melanoma.
Identifying the patients who are most likely to have durable responses to ICIs is key to guiding treatment decisions and is an area of active investigation. Researchers are trying to understand how the cellular and molecular characteristics of a patient’s tumor as well as the patient’s immune system can predict how well ICIs inhibitors will work (553)Naranbhai V, et al. HLA-A*03 and response to immune checkpoint blockade in cancer: an epidemiological biomarker study. The Lancet Oncology 2022;23:172-84. [LINK NOT AVAILABLE](554)Jaiswal A, et al. An activation to memory differentiation trajectory of tumor-infiltrating lymphocytes informs metastatic melanoma outcomes. Cancer Cell 2022;40:524-44 e5. [LINK NOT AVAILABLE]. As one example, research has revealed that the extracellular matrix surrounding tumors that has high levels of a supportive molecule called collagen could predict how well a patient would respond to ICIs (555)Mariathasan S, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544-8. [LINK NOT AVAILABLE](556)Wang L, et al. EMT- and stroma-related gene expression and resistance to PD-1 blockade in urothelial cancer. Nat Commun 2018;9:3503. [LINK NOT AVAILABLE]. Researchers are now trying to identify unique collagen biomarkers released into the blood to develop blood-based tests, which are less invasive and can be repeated over time to aid researchers and physicians in understanding the influence of extracellular matrix on patient response to cancer therapy and predict survival (557)Wang S, et al. Blood-based extracellular matrix biomarkers as predictors of survival in patients with metastatic pancreatic ductal adenocarcinoma receiving pegvorhyaluronidase alfa. J Transl Med 2021;19:39. [LINK NOT AVAILABLE](558)Jensen C, et al. Serological assessment of collagen fragments and tumor fibrosis may guide immune checkpoint inhibitor therapy. J Exp Clin Cancer Res 2021;40:326. [LINK NOT AVAILABLE]. Currently, researchers can detect fragments of collagen that are released during the production of extracellular matrix structures or during collagen degradation and remodeling (559)Jensen C, et al. Granzyme B degraded type IV Collagen products in serum identify melanoma patients responding to immune checkpoint blockade. Cancers (Basel) 2020;12. [LINK NOT AVAILABLE](560)Karsdal MA, et al. Novel combinations of post-translational modification (PTM) neo-epitopes provide tissue-specific biochemical markers–are they the cause or the consequence of the disease? Clin Biochem 2010;43:793-804. [LINK NOT AVAILABLE](561)Leeming DJ, et al. Post-translational modifications of the extracellular matrix are key events in cancer progression: opportunities for biochemical marker development. Biomarkers 2011;16:193-205. [LINK NOT AVAILABLE].
Cell-Based Immune Therapies
Our increasing knowledge of the immune system and how it interacts with cancer cells is rapidly being harnessed to develop a type of immunotherapy called cell-based immunotherapy, which uses an immune cell as a therapeutic agent to attack cancer cells.
An approach that has already garnered a lot of attention is adoptive T-cell therapy, which has immense potential to boost the killing power of a type of immune cells called T cells (see sidebar on What Is Adoptive T-Cell Therapy?). The goal is to dramatically increase the number of functional cancer-killing T cells in a patient. Six of these new types of immunotherapies, known as CAR T-cell therapies, have been approved by the FDA for treating patients with a range of blood cancers. Notably, some of the patients treated with these therapies during the first clinical trial in 2010 are still living cancer free (562)Penn Today. Decade-long remission after CAR T cell therapy. Accessed: June 30, 2022.[cited 2020 Jul 15].(563)Children’s Hospital of Philadelphia. First child to receive revolutionary CAR T therapy celebrates 10 years cancer free. Accessed: June 30, 2022.[cited 2020 Jul 15]..
CAR T-cell therapies involve collecting T cells directly from the blood of a patient with cancer and genetically altering them outside of the patient to target cancer cells. Unfortunately, the process for manufacturing CAR T cells is extremely time-consuming and resource intensive, which has led to long waiting lists for patients desperately seeking treatment (564)Stat News. ‘How do you decide?’: Cancer treatment’s CAR-T crisis has patients dying on a waitlist. Accessed: June 30, 2022.[cited 2020 Jul 15].. CAR T-cell therapy can also lead to several, sometimes life-threatening side effects including a phenomenon known as cytokine storm (565)Sterner RC, et al. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J 2021;11:69. [LINK NOT AVAILABLE]. Promisingly, many manufacturers and research groups have been able to streamline some parts of the manufacturing process and reduce the manufacturing time from the current standard of 16 days to about 48 or even 24 hours (566)Novartis. Novartis announces T-Charge™, next-generation CAR-T platform with first-in-human data at ASH 2021. Accessed: June 30, 2022.[cited 2020 Jul 15].(567)Ghassemi S, et al. Rapid manufacturing of non-activated potent CAR T cells. Nat Biomed Eng 2022;6:118-28. [LINK NOT AVAILABLE]. However, other technologies are being developed to reduce the cost and amount of time it takes to produce these therapies.
A novel approach to CAR T-cell manufacturing that is being evaluated is to deliver the genetic modifications directly to T cells in a patient using nanoparticles (568)Parayath NN, et al. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat Commun 2020;11:6080. [LINK NOT AVAILABLE], eliminating the most time-consuming aspects of the process—mainly, the isolation, shipping back and forth between manufacturing facilities, and reintroduction of cells into the patient, also called the “vein-to-vein” time. These nanoparticles can be available in the clinic on an as needed basis, reducing time to treatment initiation and quality control problems (569)Xin T, et al. In-vivo induced CAR-T cell for the potential breakthrough to overcome the barriers of current car-t cell therapy. Front Oncol 2022;12:809754. [LINK NOT AVAILABLE]. Other upcoming technologies that are being tested include mixing T cells with the tools that genetically modify them to fight cancer into a type of sponge that can be implanted in the patient, which can be done in the clinic, eliminating shipping cells to the manufacturing facility (570)Agarwalla P, et al. Bioinstructive implantable scaffolds for rapid in vivo manufacture and release of CAR-T cells. Nat Biotechnol 2022. [LINK NOT AVAILABLE].
Ongoing clinical trials are also testing immune cells from healthy donors that can be reprogrammed to be used in patients with cancer. In previous studies, using CAR T cells from another person led to rejection by the recipient because the donor cells were viewed by the patient’s immune system as foreign. By using a different type of immune cells called natural killer (NK) cells, researchers have found a way to circumvent this problem (571)Lorenzo-Herrero S, et al. NK cell-based immunotherapy in cancer metastasis. Cancers 2019;11:29. [LINK NOT AVAILABLE](572)Marofi F, et al. CAR-engineered NK cells; a promising therapeutic option for treatment of hematological malignancies. Stem Cell Res Ther 2021;12:374. [LINK NOT AVAILABLE]. Another advantage of these so-called CAR NK cells is that they do not stay in the bloodstream as long as traditional CAR T cells, reducing the potential for long-term adverse effects such as development of autoimmune side effects, a common issue among immunotherapy recipients (573)Lupo KB, et al. Natural killer cells as allogeneic effectors in adoptive cancer immunotherapy. Cancers 2019;11:769. [LINK NOT AVAILABLE]. Current clinical trials in several countries are exploring the use of these cells, which promise to overcome many of the challenges seen with CAR T-cell therapy (368)Zhang L, et al. CAR-NK cells for cancer immunotherapy: from bench to bedside. Biomark Res 2022;10:12. [LINK NOT AVAILABLE].
Apart from CAR T-cell and CAR NK-cell therapies, another new cell-based therapy that is beginning to show promise in clinical trials uses a type of immune cells called tumor-infiltrating lymphocytes (TILs) that are derived from a patient’s tumor. After the TILs are isolated from a tumor biopsy and expanded in numbers outside of the patient, they are reintroduced along with immune-stimulating agents into the patient.
TILs have advantages over other types of adoptive cell therapies because they are isolated directly from a tumor and do not need to be genetically manipulated. Because of this, they recognize multiple characteristics of the patient’s tumor, which contrasts with CAR T cells that only recognize a single cancer-associated marker targeted by the engineered CAR.
Targeting Immunotherapies Directly to Tumors
Several immunotherapies that treat cancer work by helping the body’s immune system attack cancer cells; however, their use can have off-target effects that can damage healthy, noncancer cells, leading to debilitating side effects. New technologies are being tested that better target immunotherapies directly to the tumor, reducing the possibility of side effects. Researchers are experimenting with a device that is implanted near the tumor and releases molecules to recruit some immune cell types locally (574)Nash AM, et al. Clinically translatable cytokine delivery platform for eradication of intraperitoneal tumors. Sci Adv 2022;8:eabm1032. [LINK NOT AVAILABLE]. This technology has been used successfully in animal models. Positive data from studies such as this provide rationale for movement into clinical testing. If this technology can be approved, this type of delivery method could help with other types of immunotherapies such as the previously described TILs by helping these cells more efficiently target the tumor from which they were originally isolated.
Controlling CAR T cells once they are in the body can increase their effectiveness by allowing them to home in on a tumor, rather than wasting time moving throughout the body or targeting other, non-cancerous cells and leading to side effects. To regulate CAR T cells, researchers are creating tools that activate these cells at the right time and place using either blue light or ultrasound radiation, which are focused more precisely on the tumor (575)Wu Y, et al. Control of the activity of CAR-T cells within tumours via focused ultrasound. Nat Biomed Eng 2021;5:1336-47. [LINK NOT AVAILABLE]. Another research team is engineering these cells to be smarter by equipping them with a biological computer. These cells have cellular “circuits” that more specifically find cancer cells using multiple identification signals, drastically reducing off-target effects and toxicities (576)Angelici B, et al. An AAV gene therapy computes over multiple cellular inputs to enable precise targeting of multifocal hepatocellular carcinoma in mice. Sci Transl Med 2021;13:eabh4456. [LINK NOT AVAILABLE].
Targeting the Tumor Immune Microenvironment
To make the immune system more effective in eradicating cancer cells, manipulation of the tumor microenvironment is often necessary. This is because cellular and molecular components of the tumor microenvironment can lead to suppression of the antitumor immune system, preventing immunotherapies from working. One group of researchers utilized a nanoparticle delivery system to overcome this issue.
The nanoparticles, which carry an inhibitor that promotes antitumor immune response, only release their cargo when they encounter the inhibitory molecules released by the tumor microenvironment. Once released, the inhibitor neutralizes factors that prevent the immune system from working and increases the response of tumors to immunotherapy (577)Mao C, et al. Delivery of an ectonucleotidase inhibitor with ROS-responsive nanoparticles overcomes adenosine-mediated cancer immunosuppression. Sci Transl Med 2022;14:eabh1261. [LINK NOT AVAILABLE]. Overcoming the immune suppressive effects of the tumor microenvironment by using supplemental therapies such as these are essential to unleash the full power of immunotherapies.
mRNA Cancer Vaccines
The highly successful and rapid development and use of mRNA vaccines against the SARS-CoV-2 virus during the COVID-19 pandemic have reinvigorated interest in using mRNA vaccine platforms in the fight against cancers. In the same way that mRNA vaccines expose the immune system to parts of the virus so that it will subsequently recognize the virus during infection, cancer vaccines expose the immune system to part of a patient’s tumor so the immune system can identify the tumor in the patient and eliminate it quickly and precisely. mRNA vaccines have already been used as a potential cancer therapeutic in several clinical trials over the past decade (578)American Association for Cancer Research. Decades of cancer vaccine research enabled rapid development of COVID-19 vaccines. Accessed: June 30, 2022.[cited 2020 Jul 15]..
The technology developed for the COVID-19 vaccines, which includes using lipid nanoparticles to encase the mRNA or modifying the mRNA molecule itself to be more stable and evade the immune system, was originally developed for cancer vaccines. Now, researchers are using this technology in new clinical trials to improve cancer vaccines (579)ClinicalTrials.Gov. Safety, tolerability, and immunogenicity of mRNA-4157 alone in participants with resected solid tumors and in combination with pembrolizumab in participants with unresectable solid tumors (KEYNOTE-603). Accessed: June 30, 2022.[cited 2020 Jul 15].. To maximize the efficacy of the mRNA vaccine, current studies combine it with immunotherapies, helping to stimulate the immune system. The results are promising, with research groups already seeing success in patients (580)National Cancer Institute. How mRNA vaccines might help treat cancer. Accessed: June 30, 2022.[cited 2020 Jul 15]..
Using Liquid Biopsies to Detect Cancers Earlier
Recent studies have demonstrated that it is possible to use blood or another biofluid sample, or “liquid biopsy,” rather than a traditional tissue/tumor biopsy, to obtain material that can be analyzed to provide valuable information such as the molecular alterations associated with a patient’s cancer (see Moving Toward Minimally Invasive Cancer Testing). Liquid biopsies, therefore, provide a less invasive means to detect or track the status of cancer. There is much excitement in the cancer field that, as opposed to traditional biopsies which only provide a snapshot of the tumor characteristics at one specific time point, liquid biopsy approaches may generate a more complete picture of an individual’s cancer by allowing for the monitoring of disease progression and its response to treatments in real time. While these tests have been approved for over a decade, starting in 2016 for detection of a mutation in the EGFR gene from plasma, they have increased their scope, with the ability to monitor cancer progression, detect genetic mutations, recognize signs of relapse, and even determine if a patient will respond to certain types of therapies. The routine use of liquid biopsies will increase the accuracy of cancer diagnosis and treatment and increase efficiency compared to a traditional biopsy (581)Bai Y, et al. Liquid biopsy in tumors: opportunities and challenges. Ann Transl Med 2018;6:S89. [LINK NOT AVAILABLE].
New evidence from an international clinical study demonstrated that measuring circulating DNA in the blood after surgery of tumors helped guide the future use of chemotherapy. By knowing if a patient was positive for circulating tumor DNA (ctDNA) after surgery, which indicates the possible presence of cancer cells, researchers would be better informed regarding administration of chemotherapy. The study demonstrated that using this type of approach led to reduced use of chemotherapy overall and improved patient outcomes without compromising recurrence-free survival (582)Tie J, et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage ii colon cancer. N Engl J Med 2022;386:2261-72. [LINK NOT AVAILABLE]).
Liquid biopsy approaches are also becoming more precise. By using ctDNA from blood, researchers can not only detect the presence of a tumor but also determine the potential prognosis of that cancer or if it has the potential to progress (583)Madanat-Harjuoja LM, et al. Circulating tumor DNA as a biomarker in patients with stage III and IV wilms tumor: Analysis from a Children’s Oncology Group Trial, AREN0533. J Clin Oncol 2022:JCO2200098. [LINK NOT AVAILABLE](584)Cox A, et al. Chaperonin containing TCP1 as a marker for identification of circulating tumor cells in blood. PLoS One 2022;17:e0264651. [LINK NOT AVAILABLE]. This is done by looking at several different types of ctDNA that are released by a tumor over time since the pattern changes as the tumor evolves. One study looked at ctDNA found in urine and blood of children with either stage III or IV Wilms tumor, one of the most common kidney cancers in children. This study detected the mutations present in the ctDNA and compared them to the mutations found in a biopsy from the tumor itself. The researchers found that the mutations were able to be accurately detected in the ctDNA and that these mutations were useful as prognostic markers for these types of tumors (583)Madanat-Harjuoja LM, et al. Circulating tumor DNA as a biomarker in patients with stage III and IV wilms tumor: Analysis from a Children’s Oncology Group Trial, AREN0533. J Clin Oncol 2022:JCO2200098. [LINK NOT AVAILABLE]. Collectively, these data demonstrate how safer, less invasive biopsies such as using ctDNA from blood can potentially transform clinical cancer care in the future (583)Madanat-Harjuoja LM, et al. Circulating tumor DNA as a biomarker in patients with stage III and IV wilms tumor: Analysis from a Children’s Oncology Group Trial, AREN0533. J Clin Oncol 2022:JCO2200098. [LINK NOT AVAILABLE].
On the other hand, screening healthy individuals for cancers may lead to either over- or underdiagnosis of cancers, which makes some physicians and scientists question the capabilities of liquid biopsy tests. To determine their effectiveness in detecting cancers compared to standard surveillance methods such as mammograms, NCI advisors have endorsed a four-year pilot study that will enroll 24,000 people to assess liquid biopsy tests produced from several commercial companies starting in the year 2023 (585)National Cancer Institute. Cancer screening research network/multi cancer early detection evaluation. Accessed: July 28, 2022.[cited 2020 Jul 15]..
Next Section: Impacting the Future of Cancer Research and Patient Care Through Evidence-Based Policies Previous Section: Supporting Cancer Patients and Survivors