Envisioning the Future of Cancer Science and Medicine
In this section, you will learn:
- Innovative technologies are enabling a deeper understanding of cancer at a single cell and single molecule level.
- Artificial intelligence is poised to revolutionize cancer research and patient care.
- Wearable technologies have immense potential to improve health outcomes for patients with cancer.
- In-depth understanding of cancer biology is fueling progress in treating intractable cancers, such as glioblastoma multiforme and pancreatic cancer.
- Modulating the human microbiome is the next frontier in cancer therapeutics.
Unprecedented breakthroughs in cancer science and medicine, some of which are covered in this report, have accelerated the pace of progress against cancer. Thanks to decades of research-fueled fundamental discoveries and clinical advances, cancer mortality rates have decreased by 33 percent in the past three decades, and more cancer survivors are living fuller and longer lives.
Cancer researchers, including 2023-2024 AACR president Philip D. Greenberg, MD, FAACR, are confident that this progress will continue to advance the frontiers of cancer science and medicine for the benefit of patients with cancer. Researchers across the nation’s cancer centers are also extremely hopeful that the newly established AACR Cancer Centers Alliance will serve as a catalyst to fostering innovative and synergistic collaborations that address the many hurdles currently facing the nation’s cancer centers and accelerate transformative lifesaving scientific discoveries for patients with cancer.
Revolutionizing Cancer Science and Medicine
These are exciting times as technological innovations continue to advance the frontiers of cancer science and medicine and accelerate the pace of progress against cancer. Within just two decades of the publication of the human genome, researchers have devised revolutionary new ways to study cancer in real time at single cell and single molecule levels. Knowledge gleaned from these in-depth observations is providing exciting new opportunities to detect, treat, and manage cancer. This section highlights some of the breakthrough technological advances that are moving the field of cancer science and medicine into a new era of progress against cancer.
New Frontiers in Cancer Research
Discovery science has played a pivotal role in progress against cancer, as documented in this and the prior 12 editions of the annual AACR Cancer Progress Report. Researchers are continually working to devise new ways to study unresolved mysteries of cancer, some of which are described below.
Spatial transcriptomics, or the study of transcriptomes (see RNA Variations) at the single cell level in intact tissue, allows researchers to examine how cancer cells change during the course of cancer progression (663)Williams CG, et al. (2022) Genome Med, 14: 68. [LINK NOT AVAILABLE]. While studying the transcriptome of a single cell is not a new approach (664)Tang F, et al. (2009) Nat Methods, 6: 377. [LINK NOT AVAILABLE], spatial transcriptomics takes this approach to the next level because it helps examine cells in the tumor or normal tissue where they are naturally located, thus minimizing any molecular changes that may occur during the process of isolating cells from a tumor or normal tissue. In recent years, researchers have employed spatial transcriptomics to answer some of the most elusive questions in cancer research, such as characterizing treatment responses (665)Fan L, et al. (2022) Cancer Res, 82: 2034. [LINK NOT AVAILABLE], tracking tumor evolution (666)Tian L, et al. (2022) Nat Biotechnol, 41: 773. [LINK NOT AVAILABLE], mapping and targeting the tumor microenvironment (667)Peng Z, et al. (2022) J Transl Med, 20: 302. [LINK NOT AVAILABLE], and delineating tumor heterogeneity (668)Zhang Q, et al. (2022) Nat Commun, 13: 5983. [LINK NOT AVAILABLE].
Extrachromosomal DNA (ecDNA) is large, circular, highly amplified pieces of DNA that have untethered themselves from chromosomes. ecDNAs are not found in normal tissues, and they are commonly detected in many of the most aggressive forms of cancer among children and adults (669)Kim H, et al. (2020) Nat Genet, 52: 891. [LINK NOT AVAILABLE], including during the transition from precancerous condition to cancer (670)Luebeck J, et al. (2023) Nature, 616: 798. [LINK NOT AVAILABLE]. Extrachromosomal DNAs are enriched for cancer-promoting oncogenes that drive tumor formation and growth, as well as gene regulatory elements that control their expression (671)Hung KL, et al. (2021) Nature, 600: 731. [LINK NOT AVAILABLE]. Extrachromosomal DNAs can also contain genes, the products of which may help tumors escape immunotherapies (670)Luebeck J, et al. (2023) Nature, 616: 798. [LINK NOT AVAILABLE].
Because ecDNAs do not follow the normal rules of chromosomal inheritance and are randomly inherited by daughter cells during cell division, much like bacteria, ecDNA enables tumor cells to change quickly, potently contributing to tumor heterogeneity, high oncogene copy number, and rapid treatment resistance (672)Lange JT, et al. (2022) Nat Genet, 54: 1527. [LINK NOT AVAILABLE], and resulting in shorter survival for patients (669)Kim H, et al. (2020) Nat Genet, 52: 891. [LINK NOT AVAILABLE].
Extrachromosomal DNA was first discovered in the 1960s, but was thought to be rare and of unclear importance. The application of powerful basic science technologies, DNA sequencing, and computational tools has revealed that ecDNA is very common among many of the most aggressive forms of cancer, contributing to poor outcomes for patients (669)Kim H, et al. (2020) Nat Genet, 52: 891. [LINK NOT AVAILABLE](673)Hung KL, et al. (2022) Nat Genet, 54: 1746. [LINK NOT AVAILABLE](674)Wu S, et al. (2019) Nature, 575: 699. [LINK NOT AVAILABLE](675)Zhu Y, et al. (2021) Cancer Cell, 39: 694. [LINK NOT AVAILABLE](676)Gu X, et al. (2020) J Exp Clin Cancer Res, 39: 215. [LINK NOT AVAILABLE](677)Zeng X, et al. (2020) Signal Transduct Target Ther, 5: 277. [LINK NOT AVAILABLE]. The first ecDNA-directed anticancer therapy is now in clinical trials (678)ClinicalTrials.gov. Study of the CHK1 Inhibitor BBI-355, an ecDNA-directed Therapy, in Subjects With Tumors With Oncogene Amplifications (POTENTIATE). Accessed: July 31, 2023. .
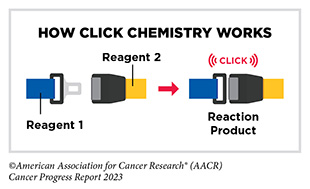
The 2022 Nobel Prize in Chemistry was awarded to K. Barry Sharpless, PhD, Morten Meldal, PhD, and Carolyn Bertozzi, PhD, for their pioneering roles in the discovery of click chemistry. Click chemistry refers to a class of simple chemical reactions that permit the joining of two molecules together. In medical research, click chemistry allows researchers to attach a chemical, such as a fluorescent probe that can be visualized by imaging, to a molecule or a protein present on or inside cells (679)Dubash SR, et al. (2016) J Nucl Med, 57: 1207. [LINK NOT AVAILABLE]. Click chemistry is poised to revolutionize drug discovery and treatment of diseases, including cancer (680)Bauer D, et al. (2023) Nat Protoc, 18: 1659. [LINK NOT AVAILABLE]. As one example, researchers used click chemistry to specifically remove a type of sugar molecule commonly present on the surface of cancer cells, which resulted in enhanced antitumor immune response and improved effectiveness of immunotherapy (681)Stanczak MA, et al. (2022) Sci Transl Med, 14: eabj1270. [LINK NOT AVAILABLE].
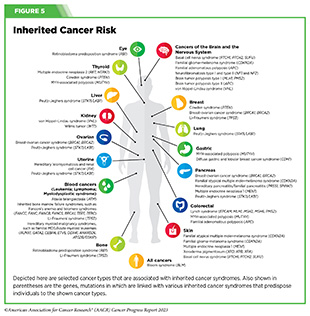
The approaches highlighted here are fueling our ever-expanding knowledge of cancer initiation and progression and are providing new and better ways to understand and treat cancer The success of mRNA-based vaccines in controlling the COVID-19 pandemic has resulted in renewed interest in developing therapeutic (see A New Era of mRNA-based Cancer Vaccines) and preventive vaccines for use in clinical cancer care. For example, researchers are investigating the potential of vaccines in preventing cancers in individuals who are diagnosed with inherited cancer syndromes, such as Lynch syndrome (see Figure 5).
As genetic testing becomes more common and more people get tested for hereditary cancer syndromes, researchers estimate that one in every 288 people may be diagnosed with Lynch syndrome. These findings underscore the need to develop vaccines that can prevent cancer initiation and progression in individuals with hereditary cancer syndromes. Thanks to research, a new clinical trial is investigating a vaccine that will offer an effective, safe, and easy method of preventing Lynch syndrome-related cancers (682)ClinicalTrials.gov. Cancer Preventive Vaccine Nous-209 for Lynch Syndrome Patients – Full Text View – ClinicalTrials.gov. Accessed: July 5, 2023. . Success of this study will lead to a new frontier in cancer prevention where preventive vaccines will play a pivotal role in reducing cancer burden.
Artificial Intelligence
Artificial intelligence (AI) is the ability of a computer to perform tasks commonly associated with human intelligence, such as how to act, reason, and learn. The use of AI in aiding health care professionals for early detection of cancer has shown tremendous potential, and several AI-assisted software and medical devices have already been approved by FDA for use in the clinic (see Realizing the Potential of Artificial Intelligence for Early Detection of Cancers). Ongoing research, some of which is highlighted below, is exploring the potential of AI in other aspects of cancer research and patient care (683)Shreve JT, et al. (2022) Am Soc Clin Oncol Educ Book, 42: 1. [LINK NOT AVAILABLE].
One exciting use of AI-assisted software is to extract existing knowledge from different sources of information—genomic data, test results, health care professionals’ notes during clinic visits, patient reported outcomes, data from wearables, and scientific publications related to a patient’s cancer—and present a complete view of a patient’s health to clinicians. As one example, researchers used available clinical information from 1,348 patients with early-stage lung cancer to develop an AI-assisted model which accurately identified patients who were at low or high risk of cancer recurrence (684)Torrente M, et al. (2022) Cancers (Basel), 14. [LINK NOT AVAILABLE]. Another machine learning model analyzed images and genomic datasets from 14 different types of cancer and discovered features that could accurately predict poor or favorable health outcomes (685)Chen RJ, et al. (2022) Cancer Cell, 40: 865. [LINK NOT AVAILABLE].
Another way AI is helping to accelerate the pace of progress against cancer is by uncovering previously unknown aspects of cancer cells. For example, a deep learning model revealed that mitochondria—the powerhouses of cells—are organized differently in lung tumors with high metabolism (more aggressive), compared to those with low metabolism (less aggressive) (686)Han M, et al. (2023) Nature, 615: 712. [LINK NOT AVAILABLE]. Researchers can use this new information in a number of ways For example, a new imaging technique that examines the location of mitochondria in lung cancer can be developed to distinguish highly aggressive tumors from less aggressive tumors.
An emerging strategy in early cancer detection is to screen for multiple cancers simultaneously (see Moving Toward Minimally Invasive Cancer Screening). However, such tests generate a wealth of information, and a limitation so far has been the accurate and timely analysis of results from these tests. AI-based approaches have immense potential of overcoming this limitation. Artificial intelligence is also contributing to other aspects of cancer science and medicine. Researchers are already leveraging AI-based analyses of large genomic datasets in classifying tumors at a molecular level (”687″) determining tumor heterogeneity (688)Wang K, et al. (2018) PLoS One, 13: e0205548. [LINK NOT AVAILABLE], diagnosing primary and metastatic cancers (689)Grewal JK, et al. (2019) JAMA Netw Open, 2: e192597. [LINK NOT AVAILABLE], identifying biomarkers for treatment selection (690)Kather JN, et al. (2019) Nat Med, 25: 1054. [LINK NOT AVAILABLE], and predicting overall survival (691)Weiss J, et al. (2023) Nat Commun, 14: 2797. [LINK NOT AVAILABLE], among other applications across the cancer care continuum (692)Tran KA, et al. (2021) Genome Med, 13: 152. [LINK NOT AVAILABLE].
Another exciting application of AI in cancer medicine is its potential to create models that can predict how patients will respond to a treatment. In a recent study, researchers used clinical and genomic data from 700 patients with cancer, who were treated with ICIs, to develop a prediction model based on machine learning—a type of AI that is programmed to learn over time from the data provided to make predictions or decisions (693)Kong J, et al. (2022) Nat Commun, 13: 3703. [LINK NOT AVAILABLE]. The model accurately predicted how patients with melanoma, gastric cancer, and bladder cancer would have responded to the ICI treatment. Furthermore, the model’s predictions were more accurate compared to those based on biomarkers currently being used in the clinic to predict a patient’s response to the ICI treatment (693)Kong J, et al. (2022) Nat Commun, 13: 3703. [LINK NOT AVAILABLE].
Cancer science and medicine are on the verge of an AI-driven revolution. However, it is important to be cognizant of the fact that AI-assisted approaches can introduce unintended biases in analyses and can further widen inequities in the burden of cancer experienced by medically underserved populations (694)Michel A, et al. (2023) Breast Cancer Res Treat, 200: 237. [LINK NOT AVAILABLE] (see Sidebar 24). It is vital that the datasets used to train AI-based models accurately and proportionally represent population groups affected by the type of cancer being studied.
Wearable Technologies
Wearable technology, also called wearables, is a category of electronic devices that can be worn as accessories, embedded in clothing, implanted in the user’s body, or affixed on the skin. Wearables are hands-free microcomputers with the ability to send and receive data via the Internet as well as to perform a variety of functions, such as counting steps or monitoring heart rate.
An exciting area of cancer research is the use of wearables that can be implanted on or inside the patient’s body for delivering drugs effectively and automatically (695)Kar A, et al. (2022) Biomaterials, 283: 121435. [LINK NOT AVAILABLE](696)Beg S, et al. (2022) Drug Discov Today, 27: 103314. [LINK NOT AVAILABLE]. Patients with cancer, especially elderly patients, often have to take many medications. According to one study of patients with cancer ages 70 years or older, 61 percent of the study’s participants were taking five or more medications a day (697)Mohamed MR, et al. (2023) Cancer, 129: 1096. [LINK NOT AVAILABLE]. Many of the anticancer therapeutics are pills taken orally, which can cause distress and inconvenience for patients, resulting in patients not taking their medication on time or at all (698)Partridge AH, et al. (2002) J Natl Cancer Inst, 94: 652. [LINK NOT AVAILABLE], thus increasing the risk of adverse health outcomes (697)Mohamed MR, et al. (2023) Cancer, 129: 1096. [LINK NOT AVAILABLE].
Researchers recently reported the development of a wearable that can be attached to skin for delivering anticancer drugs (699)Ma X, et al. (2023) Adv Sci (Weinh), 10: e2205343. [LINK NOT AVAILABLE]. The study showed that the wearable successfully delivered anticancer drugs in a preclinical mouse model of melanoma and prevented recurrence of cancer. Importantly, there was no noticeable effect of the delivered drugs on other organs in the body (699)Ma X, et al. (2023) Adv Sci (Weinh), 10: e2205343. [LINK NOT AVAILABLE].
Another focus of ongoing research is examining the potential of wearables to diagnose cancer. Recently, researchers reported the development of a flexible patch containing a miniaturized ultrasound scanner. The patch can be attached to a bra, which when worn scans the breast tissue. The ability to attach the patch in six different positions on the bra allows the entire breast to be imaged (700)Du W, et al. (2023) Science Advances, 9: eadh5325. [LINK NOT AVAILABLE]. Although the patch needs to be tested in a large number of individuals, such innovative applications of wearables are opening exciting new frontiers in cancer science and medicine.
Smart watches and health and fitness (activity) trackers are among the most commonly used wearables. According to a 2020 report, one in five U.S. adults regularly wears a smart watch or a fitness tracker, and more than half of those who do support sharing of data from these devices with their health care providers and with researchers (701)Pew Research Center. About one-in-five Americans use a smart watch or fitness tracker. Accessed: July 5, 2023. .
Researchers are testing the potential use of smart watches and activity trackers in clinical cancer care in several ways (702)Low CA (2020) NPJ Digit Med, 3: 140. [LINK NOT AVAILABLE]. Findings from a number of studies have shown that use of wearables among cancer patients and survivors helps encourage physical activity (703)Teo NR, et al. (2023) Semin Oncol Nurs, 39: 151403. [LINK NOT AVAILABLE]; predict whether patients receiving active cancer treatment are at an increased risk of hospitalization (704)Low CA, et al. (2018) Ann Behav Med, 52: 88. [LINK NOT AVAILABLE]; identify patients who are fit for certain types of cancer treatments (705)Ohri N, et al. (2019) Int J Radiat Oncol Biol Phys, 105: 745. [LINK NOT AVAILABLE]; examine association of sleep patterns with overall survival (706)Kos M, et al. (2023) Crit Rev Oncol Hematol, 185: 103979. [LINK NOT AVAILABLE]; and determine quality of life (707)Torrente M, et al. (2022) Clin Med (Lond), 22: 36. [LINK NOT AVAILABLE]. Future studies with large numbers of patients will further strengthen the utility of wearables in cancer science and medicine as an exciting new frontier that can measure biometric parameters in patients and deliver care remotely (708)Thanarajasingam G, et al. (2023) medRxiv. [LINK NOT AVAILABLE], thus harnessing technological advances to further expand and improve telemedicine.
Tackling Difficult-to-Treat Cancers
The consistently declining rates of U.S. cancer deaths in recent years underscore the unprecedented progress against cancer (see Cancer in 2023). However, treatment options for certain types of cancer, such as cancers of pancreas, and those that originate in the nervous system, remain limited. Thanks to rapid advances in our understanding of how cancer develops (see Understanding the Path to Cancer Development), researchers are taking innovative approaches to tackling difficult-to-treat cancers. Here we present an overview of some of the most promising breakthroughs in treating two types of cancer— glioblastoma and pancreatic cancer—as examples of progress against currently intractable cancers.
Glioblastoma
Glioblastoma—the most common and deadly type of brain cancer—is highly resistant to chemotherapy, and surgery and/or radiotherapy are ineffective. No new drugs have been approved for glioblastoma over the past decade, and only 6.9 percent of patients survive five years after being diagnosed with glioblastoma (29)Ostrom QT, et al. (2022) Neuro Oncol, 24: v1. [LINK NOT AVAILABLE], underscoring an urgent need to find better treatments for the disease.
An active area of scientific discovery is understanding the relationship between resistance to treatment and how the tumor utilizes nutrients. Two recent studies found that certain types of gliomas, a larger category of cancers of the nervous system that includes glioblastoma, produce large quantities of chemicals, called pyrimidine nucleotides, which are the building blocks of DNA. Findings of the studies show that gliomas become dependent on these chemicals for survival, and when the production of pyrimidine nucleotides is blocked with a drug, tumors shrink in animal models of glioma (709)Shi DD, et al. (2022) Cancer Cell, 40: 939. [LINK NOT AVAILABLE](710)Pal S, et al. (2022) Cancer Cell, 40: 957. [LINK NOT AVAILABLE]. Researchers are now planning clinical trials to test the drug in people with gliomas.
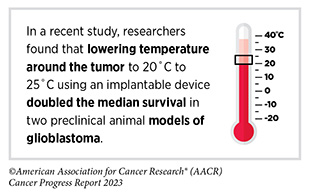
One of the challenges in effectively treating glioblastoma (and other cancers that originate in the brain) is delivering drugs to the brain, which is naturally protected from pathogens and other toxins by a thin layer of tissue and blood vessels, called the blood–brain barrier. Even the most potent chemotherapy drugs cannot cross this barrier. Researchers are testing novel approaches to increase the concentration of drugs in the brains of patients with glioblastoma. In a phase I clinical trial, an ultrasound device was implanted in the brains of 17 patients who had recurrent glioblastoma (711)Sonabend AM, et al. (2023) Lancet Oncol, 24: 509. [LINK NOT AVAILABLE]. When activated, the device repeatedly, but temporarily, opened the blood–brain barrier, increasing the concentration of a chemotherapy drug by sixfold in patients’ brains without causing any serious side effects (711)Sonabend AM, et al. (2023) Lancet Oncol, 24: 509. [LINK NOT AVAILABLE]. The study is now in phase II of clinical development.
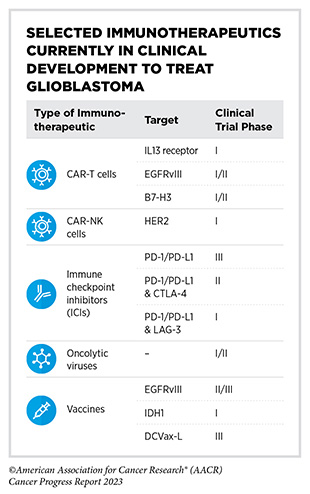
Clinical advances and breakthroughs in immunotherapy for other tumors (see Immunotherapy: Pushing the Frontier of Cancer Medicine) have inspired treatment of glioblastoma with immunotherapeutics. Different classes
of immunotherapeutics are at various stages of clinical development to treat glioblastoma (713)Yuan B, et al. (2022) Hum Vaccin Immunother, 18: 2055417. [LINK NOT AVAILABLE]. There are several ongoing CAR T-cell candidates for glioblastoma treatment, including CARs directed against proteins, such as EGFRvIII, IL13Rα2, and B7-H3, that are abundantly present on the surface of glioblastoma cells (714)Yu MW, et al. (2021) Front Immunol, 12: 676301. [LINK NOT AVAILABLE]. In addition, CAR-NK cells are being evaluated in clinical studies of glioblastoma against different targets, such as HER2 (715)Xiong Q, et al. (2023) Front Oncol, 13: 1192128. [LINK NOT AVAILABLE].
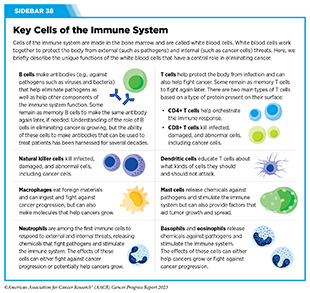
Therapeutic vaccines have also shown promise in the treatment of glioblastoma. In a phase III clinical trial, researchers added a vaccine based on dendritic cells (see Sidebar 38) to the standard of care treatment (716)Liau LM, et al. (2023) JAMA Oncol, 9: 112. [LINK NOT AVAILABLE]. Addition of vaccine more than doubled the overall survival in patients who were newly diagnosed with glioblastoma or who had recurrence of the disease at 60 and 30 months, respectively (716)Liau LM, et al. (2023) JAMA Oncol, 9: 112. [LINK NOT AVAILABLE]. These results demonstrate a remarkable advance and underscore the promise of immunotherapy in treating currently intractable cancers.
Pancreatic Cancer
According to U.S. estimates for the year 2023, 64,050 new cases of pancreatic cancer will be diagnosed and 50,550 people will die from the disease (28)American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . Pancreatic cancer is a highly aggressive disease, with a 12 percent 5-year survival rate, and treatment options primarily limited to surgery, radiation therapy, and chemotherapy.
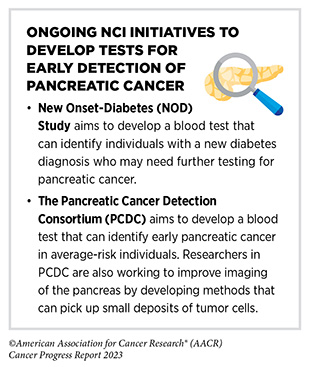
Researchers are actively working to uncover novel ways to improve health outcomes for patients with pancreatic cancer. One of the ways to tackle this disease is to identify risk factors that can contribute to the development of pancreatic cancer. For example, a new diagnosis of diabetes, sometimes also called new-onset diabetes, is a known risk factor for developing pancreatic cancer, although the reasons are not clear (717)Mellenthin C, et al. (2022) Cancers (Basel), 14. [LINK NOT AVAILABLE]. Another active area of research is to develop safe and reliable methods of detecting pancreatic cancer early. Currently, USPSTF does not recommend screening for pancreatic cancer in individuals who do not have any symptoms of the disease (718)Owens DK, et al. (2019) JAMA, 322: 438. [LINK NOT AVAILABLE]. Ongoing studies are focused on developing prediction models and genetic tests to detect pancreatic cancer in individuals who are at high risk of developing the disease (719)Yang J, et al. (2021) Cancer Commun (Lond), 41: 1257. [LINK NOT AVAILABLE].
Recent studies have also unveiled potential new drug targets for the treatment of pancreatic cancer. For example, one study found that pancreatic cancer meets its nutrient needs by using a metabolic pathway that is predominantly active in infants and is rarely used by normal cells in adults (722)Lee MS, et al. (2023) Nature, 616: 339. [LINK NOT AVAILABLE]. When researchers blocked the pathway in preclinical animal models of pancreatic cancer with a therapeutic that blocks the metabolic pathway, tumor growth was severely inhibited (722)Lee MS, et al. (2023) Nature, 616: 339. [LINK NOT AVAILABLE]. In another preclinical study, researchers found that pancreatic cancer cells use a different type of fuel, called uridine, when they do not have access to sugar, the most common type of fuel used by cells in the human body. Starving pancreatic tumors of uridine stopped tumors from growing (723)Nwosu ZC, et al. (2023) Nature, 618: 151. [LINK NOT AVAILABLE].
A recent scientific discovery revealed that pancreatic cancer cells make an abnormal form of a protein called collagen. Collagen is a protein in the extracellular space which provides structure to tissues and is found almost everywhere in the human body (724)Chen Y, et al. (2022) Cancer Cell, 40: 818. [LINK NOT AVAILABLE]. The abnormal collagen produced by pancreatic cancer cells promotes tumor growth. When its production or function was blocked using a therapeutic in a preclinical animal model, cancer-fighting immune cells started to move into the tumors, and tumors shrank dramatically in response to a commonly used immunotherapy drug, which was otherwise not very effective in the presence of abnormal collagen (724)Chen Y, et al. (2022) Cancer Cell, 40: 818. [LINK NOT AVAILABLE].
Findings of these preclinical studies are encouraging and pave the way to develop novel and effective treatment options for patients with pancreatic cancer in the near future.
An in-depth understanding of the genetic makeup of pancreatic cancer has identified mutations in several genes, such as KRAS, that contribute to pancreatic cancer development (726)Roth MT, et al. (2020) F1000Res, 9. [LINK NOT AVAILABLE]. One KRAS mutation, called G12D, is the most common in pancreatic cancer and is present in about 35 percent of patients with pancreatic cancer.
In recent years, there have been exciting new advances in targeting the KRAS G12D mutation. As one example, a recent study showed that a molecularly targeted therapeutic directed against the mutation shrank tumors or stopped their growth in preclinical animal models of pancreatic cancer (727)Kemp SB, et al. (2023) Cancer Discov, 13: 298. [LINK NOT AVAILABLE]. Notably, the drug also allowed the cancer-fighting immune cells to enter the tumor microenvironment. Researchers are now planning to combine this drug with an immunotherapeutic to evaluate whether the combination will be even more effective against pancreatic cancer.
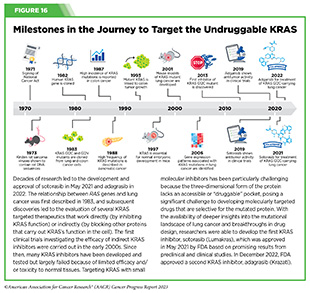
In a phase I/II clinical trial, treatment with another KRAS targeted therapeutic, sotorasib, shrank tumors in about 20 percent of participants (728)Strickler JH, et al. (2023) N Engl J Med, 388: 33. [LINK NOT AVAILABLE]. Sotorasib, which has already been approved to treat patients with lung cancer, is directed against a different KRAS mutation called G12C (see Figure 16), which is present in approximately 1 to 2 percent of patients with pancreatic cancer. Findings from these studies are encouraging and underscore the continued efforts to develop effective treatments against an intractable form of cancer.
Immunotherapy, especially adoptive cell therapy and vaccines, has shown great promise against pancreatic cancer. As of March 2022, multiple CAR T-cell therapies, targeting various proteins such as CD133, EGFR, HER2, and MSLN, were in clinical trials for treatment of pancreatic cancer (729)Yeo D, et al. (2022) Mol Ther Oncolytics, 24: 561. [LINK NOT AVAILABLE]. Together, these research efforts are ushering effective methods for early detection and treatment of pancreatic cancer into a new era, with immense potential of bringing hope to patients with this aggressive form of cancer.
Targeting the Microbiome in Cancer Treatment
The human microbiome is the collection of all microorganisms (e.g., bacteria and fungi) and viruses that live in the gut, skin, and mouth, among other parts of the body. Most microorganisms that make up the human microbiome are helpful to our health, but a smaller number are potentially harmful. Accruing evidence suggests that the balance between helpful and potentially harmful microorganisms in the microbiome contributes to overall health, and an imbalance can contribute to a number of diseases, including cancer (730)Kho ZY, et al. (2018) Front Microbiol, 9: 1835. [LINK NOT AVAILABLE].
Composition of the human microbiome is affected by genetics as well as lifestyle factors, such as diet, environment, and antibiotic use (731)Doocey CM, et al. (2022) BMC Microbiol, 22: 53. [LINK NOT AVAILABLE]. An imbalanced microbiome can cause inflammation in the intestine and contribute to colorectal cancer (732)Purcell RV, et al. (2017) PLoS One, 12: e0171602. [LINK NOT AVAILABLE](733)Vijay-Kumar M, et al. (2010) Science, 328: 228. [LINK NOT AVAILABLE]. There is mounting evidence that an imbalanced microbiome can also modulate tumor formation, metastasis, and resistance to treatment (734)Weersma RK, et al. (2020) Gut, 69: 1510. [LINK NOT AVAILABLE](735)Ghaddar B, et al. (2022) Cancer Cell, 40: 1240. [LINK NOT AVAILABLE](736)Feng TY, et al. (2022) Cancer Immunol Res, 10: 1309. [LINK NOT AVAILABLE].
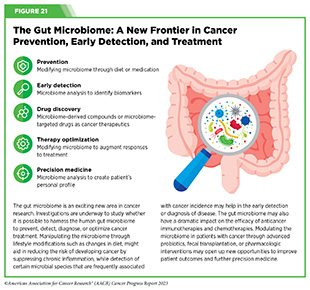
Research has shown that tumors also harbor microorganisms and the type of microorganism present in tumors can predict health outcomes (see Figure 21) (737)Chen Y, et al. (2022) Front Immunol, 13: 935846. [LINK NOT AVAILABLE]. For example, two recent studies, one examining 35 different types of cancer and the other investigating cancers of the lung and gastrointestinal tract, revealed that tumors have unique populations of fungi that can predict cancer-specific outcomes (738)Narunsky-Haziza L, et al. (2022) Cell, 185: 3789. [LINK NOT AVAILABLE](739)Dohlman AB, et al. (2022) Cell, 185: 3807. [LINK NOT AVAILABLE].
Several studies point to the link between microbial composition and response to cancer treatment (740)Liu L, et al. (2022) JAMA Oncol, 8: 1059. [LINK NOT AVAILABLE]. Researchers have found that modulating the microbiome can boost the effectiveness of anticancer treatment. As one example, oral administration of a type of bacteria that is naturally present in the microbiome enhanced the antitumor activity of a PD-1-targeting ICI (741)Si W, et al. (2022) Gut, 71: 521. [LINK NOT AVAILABLE]. Conversely, findings from a retrospective study show that the overall survival of patients who were taking antibiotics that alter the microbiome’s diversity prior to immunotherapy was negatively impacted (742)Eng L, et al. (2023) J Clin Oncol, 41: 3122. [LINK NOT AVAILABLE]. In another study, researchers found that patients with B-cell lymphoma who were not treated
with antibiotics showed better clinical response to CAR T-cell therapy (743)Stein-Thoeringer CK, et al. (2023) Nat Med, 29: 906. [LINK NOT AVAILABLE].
There are many ongoing clinical trials investigating the role of the microbiome in cancer therapy (740)Liu L, et al. (2022) JAMA Oncol, 8: 1059. [LINK NOT AVAILABLE]. Some are modulating the microbiome as a way to reduce or prevent toxic side effects of cancer treatment, while others are combining modulation of the gut microbiome with immunotherapy to enhance the effectiveness of the latter. Findings from these studies have the potential to revolutionize future cancer treatment and care.
Next Section: Advancing the Future of Cancer Care Through Evidence-based Policies Previous Section: Supporting Cancer Patients and Survivors