- Investments in Research Fuel a Healthier Future
- A Diverse Cancer Research and Care Workforce Drives Innovation
- Ensuring Safe and Effective Cancer Therapies Through Regulatory Science
- Diversifying and Decentralizing Trials
- Rapidly Delivering Safe and Effective Therapies to Patients
- Addressing Cancer Drug Shortages
- FDA-NCI Collaborations to Promote Innovative Clinical Research
- Advancing Policy to Strengthen Cancer Prevention and Screening Programs
- Accelerating Progress Against Pediatric Cancer
- Building Health Equity by Addressing Cancer Disparities
Advancing the Future of Cancer Research and Patient Care Through the Adoption of Evidence-based Policies
In this section, you will learn:
- Continued investment in medical research through NIH and NCI is essential to making progress against all aspects of cancer, including prevention, early detection, treatment, and survivorship care.
- FDA’s leadership and many innovative initiatives are ensuring that patients are receiving safe and effective treatments in an expedited time frame.
- Expanded insurance coverage for cancer screening and newly proposed standards to reduce tobacco-related illness are important steps to preventing cancer burden.
- Legislation that reauthorizes essential research and data collection programs is important to improve outcomes for children and adolescents diagnosed with cancer.
The National Institutes of Health (NIH) is the largest source for medical research funding in the United States and the world (744)National Institutes of Health. Impact of NIH Research. Accessed: July 5, 2023. . Its goals are to promote innovative research, develop scientific resources, expand the knowledge base in science and medicine, and foster the highest levels of scientific integrity (745)National Institutes of Health. Missions and Goals. Accessed: July 5, 2023. . Within NIH, approximately 1,200 Principal Investigators perform medical research including behavioral research with the goal of changing lives and advancing medical research. Additionally, NIH awarded 58,368 grants to outside organizations in fiscal year (FY) 2022 for projects that have the potential to transform medical research and deliver lifesaving medical breakthroughs (746)National Institutes of Health. FY 2022 By the Numbers: Extramural Grant Investments in Research – NIH Extramural Nexus. Accessed: 2023July 5. .
The National Cancer Institute (NCI) plays a pivotal role in driving progress against cancer. NCI conducts and supports research, training, and other activities related to the prevention, diagnosis, and treatment of cancer, while furthering basic science to advance our knowledge on cancer-causing factors like cell growth and differentiation. Furthermore, NCI-designated cancer centers across the United States serve as a model for advancing research from the laboratory to the clinic and introducing cutting-edge discoveries to patients and the greater community.
Both NIH and NCI rely on robust, sustained, and predictable funding for medical research from Congress to carry out their missions. Support from key members, most specifically the Chair and Ranking member on the Appropriations Subcommittee on Labor, Health and Human Services, Education, and Related Agencies in the House [Representatives Robert Aderholt (R-Alabama) and Rosa DeLauro (D-Connecticut), respectively] and Senate [Senators Tammy Baldwin (D-Wisconsin) and Shelley Moore Capito (R-West Virginia)], respectively,] is vital to ensuring predictable funding increases for medical research.
Another federal entity that shares NIH’s commitment to advancing cancer research is the Advanced Research Projects Agency for Health (ARPA-H). Officially formed in March 2022, ARPA-H is the nation’s newest medical research agency that functions as an independent entity within the organizational structure of NIH. Unlike other federal agencies involved with medical research, ARPA-H focuses on advancing high-potential, high-impact research that cannot be achieved through traditional research or commercial pathways. Cancer is one of three diseases the new research agency will initially prioritize, and in July 2023, ARPA-H launched a first-of-its-kind program to develop new technologies to help surgeons to remove tumors with greater precision and accuracy (747)ARPA-H. New ARPA-H program to develop novel technologies for more precise cancer tumor removal. Accessed: August 2, 2023. . While the future of ARPA-H holds potential, we are united in asking that its funding supplement, rather than supplant the core investments that are provided to NIH and NCI.
Additionally, the U.S. Food and Drug Administration (FDA) is a key federal agency in our nation’s efforts against cancer. For example, the FDA Oncology Center for Excellence leverages the skills of regulatory scientists to support the approval of safe and effective oncologic medical products. Additionally, as tobacco products, including electronic cigarettes, remain the leading preventable cause of cancer, recent FDA efforts through its Center for Tobacco Products seek to reduce the use of these products.
The Centers for Disease Control and Prevention (CDC) is another federal agency that plays an important role in driving progress against cancer. The agency’s Division of Cancer Prevention and Control (DCPC) works with state, local, territorial, and tribal health entities to develop and implement effective cancer prevention and screening practices. Key CDC cancer initiatives include programs to support early detection of breast, cervical, and colorectal cancer, as well as support for central cancer registries that are vital to data collection on cancer.
The Biden administration remains committed to “ending cancer as we know it.” In February 2022, the administration relaunched the Cancer Moonshot, a whole-of-government initiative that aims to reduce the cancer death rate in the U.S. by 50 percent by 2047 (748)The White House. Fact Sheet: President Biden Reignites Cancer Moonshot to End Cancer as We Know It. Accessed: July 5, 2023. . To help achieve the goals of the Cancer Moonshot, the National Cancer Plan lays out eight goals to accelerate progress against cancer (309)National Cancer Institute. National Cancer Plan. Accessed: July 5, 2023. .
Funding support for medical research and the many cancer programs at NIH, NCI, FDA, and CDC remains critical, but the June 2023 agreement to raise the debt ceiling is making the prospect of increased appropriations for these agencies in FY 2024 very challenging. To ensure that federal agencies can advance progress against cancer, the medical research community must continue to advocate before Congress the importance of supporting robust, sustained, and predictable investments, most especially for the medical research supported by NIH.
Investments in Research Fuel a Healthier Future
Remarkable advances in medical research have led to significant improvements in cancer prevention and reductions in cancer mortality. Investments in cancer research have resulted in a 33 percent decrease in cancer deaths since 1991, preventing an estimated 3.8 million deaths. This progress is a result of NIH and NCI investments in research that developed state-of-the-art anticancer therapies and more effective screening tools to detect cancers at the earliest possible stage, as well as initiatives through FDA and CDC to raise public awareness of the importance of cancer prevention and cancer screenings. As a result of these efforts, there are now more than 18 million cancer survivors living in the United States (see Supporting Cancer Patients and Survivors).
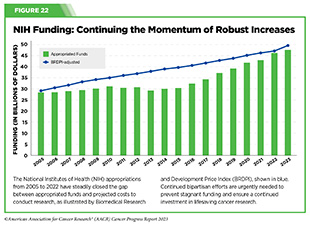
Continued federal investments are needed to further the progress against cancer. Beginning in FY 2005, a decade of stalled funding at NIH caused budgets to be eroded by inflation (see Figure 22). As a result, NIH’s purchasing power—the amount that each dollar invested can buy—was reduced by nearly 25 percent compared to the previous decade. This had a devastating impact on the ability of NIH to adequately fund research and meet the needs of the medical research community. The current political climate surrounding appropriations and NIH funding has once more created an uncertain environment for this necessary research.
The FY 2024 Presidential Budget Request includes $48.6 billion in discretionary and mandatory resources for NIH, an overall increase of $811 million from FY 2023 (749)The ASCO Post. President Biden Prioritizes Cancer Research, Access to Care in FY 2024 Budget Proposal. Accessed: July 5, 2023. . Within this budget, $7.8 billion (an increase of $503 million) was proposed for NCI (750)National Cancer Institute. Congressional Justification FY 2024. Accessed: July 5, 2023. ; $2.5 billion was requested for ARPA-H (an increase of $1 billion); and $716 million for the reauthorization of the Cancer Moonshot through FY 2026.
Major steps towards a cancer-free future for all can be achieved with these supplementary investments, as well as other FY 2024 allocations. A proposed $394.5 million investment is slated for CDC’s Division of Cancer Prevention and Control (DCPC) programs. The National Comprehensive Cancer Control Program, including the Cancer Genomics program, intends to increase the number of individuals who are appropriately referred to genetic counseling and testing; the National Breast and Cervical Cancer Early Detection Programs to enhance breast and cervical cancer screening and diagnostic services for uninsured and underinsured American women, addressing inequity in cancer prevention efforts; and the Colorectal Cancer Control Program to increase colorectal cancer screening rates among people ages 45 to 75. The Division works with state and local governments, community organizations, and health care providers to promote cancer prevention and early detection. These collaborations include funding for central cancer registries; comprehensive cancer control, which includes state, tribal, local, and territorial organizations; the National Breast and Cervical Cancer Early Detection Program; and initiatives focused on colorectal, skin, prostate, and ovarian cancer, as well as HPV-associated cancers (751)The White House. FACT SHEET: President Biden’s Budget Accelerates Progress Toward the Goal of Ending Cancer as We Know It. Accessed: July 5, 2023. .
While Congress has made decisive commitments to medical research over the last eight fiscal years, a now strained budget is leading to a more uncertain future for medical research. Investments have been proposed to expand opportunities in medical research, cancer prevention, and cancer treatment. But the risk of not passing President Biden’s FY 2024 Budget, which is already below the One Voice Against Cancer (OVAC) budget provided to Congress, threatens the future funding of NIH, NCI, and other institutions. The need for increased funding, as opposed to flat funding, comes directly from the NCI’s budget projections, with their Professional Judgment Budget detailing the need for a $2.7 billion increase.
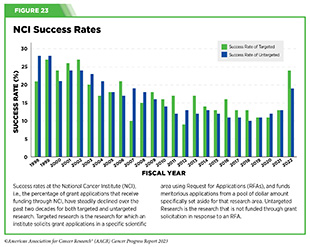
Flat funding can set a precedent for lower funding in the future because future budget requests will have to be built from the FY 2024’s final budget. Additionally, if funding is kept at the same rate as FY 2023, it will not meet or address new costs brought on by inflation. The previous stagnant period for NCI funding prior to FY 2015 prevented the institute from keeping up with the then 3.7-3.8 percent biomedical inflation rate (752)Hitt E (2008) Mol Oncol, 2: 290. [LINK NOT AVAILABLE]; the current biomedical inflation rate is 4.5 percent (753)Office of the Budget NIH. Biomedical Research and Development Price Index: Fiscal Year 2022 Update and Projections for FY 2023–FY 2028. Accessed: July 5, 2023. . Successfully funded applications dropped below 20 percent during this period (see Figure 23), deterring young investigators from entering the field and decreasing America’s competitive stance against other global biomedical research institutions. Keeping these applications funded is essential to combating cancer and spurring future discoveries (see Research: Driving Progress Against Cancer).
With so many scientific opportunities available to make progress against cancer and other diseases, it is imperative that our elected leaders continue to provide robust, sustained, and predictable funding increases for medical research and cancer prevention at NIH, NCI, CDC, and FDA. The Senate Committee on Appropriations Chair Patty Murray (D-WA) and the Vice Chair Susan Collins (R-ME) remain steadfast in their bipartisan commitment to medical research, as do many members of the Senate. However, some House Republicans are demanding that federal budget cuts must go even deeper than what President Biden and Speaker Kevin McCarthy agreed to in the recent bipartisan debt limit compromise that limited overall federal spending for the next two fiscal years. This is creating a contentious budget environment in the House of Representatives that will further complicate efforts to secure a funding increase for NIH and NCI in FY 2024.
A Diverse Cancer Research and Care Workforce Drives Innovation
To realize the full potential of the medical research enterprise, research institutions must be proactive in recruiting, supporting, and retaining a cancer research and care workforce that reflects the diversity of our society. Collaborative and intentional efforts are needed to train and support the current and next generations of cancer researchers throughout their career paths to prevent and cure all cancers. Within the cancer research and care workforce, early-career researchers invigorate progress against cancers as they bring innovative ideas and new questions. NIH and NCI play an important role in fostering the career development of young researchers to become the scientific and clinical leaders of the future.
More specifically, NCI has taken steps to support early-stage, tenure-track research faculty. For example, NCI has created several programs and policies to help establish independent laboratories including:
- Setting a payline in the 17th percentile for “early-stage investigators” (researchers within 10 years of completing their terminal degree) (754)National Cancer Institute. NCI Full Year Funding Policy for RPG Awards FY 2023. Accessed: July 5, 2023. ;
- Converting the most meritorious NCI R01 applications from early-stage investigators to Method to Extend Research in Time (MERIT) R37 awards (755)National Cancer Institute. MERIT Award (R37). Accessed: July 14, 2022. , which provide longer term support; and
- Supporting the Cancer Moonshot Scholars program, which seeks to diversify the NCI R01 portfolio to increase the number of applications from early-stage investigators from diverse backgrounds, including those that are underrepresented in the STEM workforce (756)National institutes of Health. RFA-CA-22-050: NCI Cancer Moonshot Scholars Diversity Program (CMSDP) (R01 Clinical Trial Optional). Accessed: July 5, 2023. .
The influx of innovative ideas from young scientists with various backgrounds is critical for future breakthroughs against cancer and other diseases.
It is concerning that there has been a decline in the number of postdoctoral researchers in the life sciences, as many have expressed that low compensation, diminishing funding sources, and uncertain career trajectories were key drivers of that dissatisfaction in extramural postdoctoral research training (757)STAT News. Academia’s postdoc system is teetering, imperiling efforts to diversify life sciences. Accessed: July 5, 2023. (758)National Postdoctoral Associations. 2023 Postdoctoral Barriers to Success. Accessed: July 5, 2023. (759)National Science Foundation. Restricted Data Analysis System for the Survey of Earned Doctorates. Accessed: July 5, 2023. . To understand how to address the factors that influence postdoctoral training and retention in academia, NIH released a Request for Information (760)National institutes of Health. NOT-OD-23-084: Request for Information (RFI): Re-envisioning U.S. Postdoctoral Research Training and Career Progression within the Biomedical Research Enterprise. Accessed: July 5, 2023. in February 2023 in order to receive feedback that will inform recommendations to better support the medical research community. As Congress considers both annual appropriations and supplemental funding, it will be vital to invest in additional resources to support early-career researchers. Robust, sustained, and predictable funding increases for NIH and NCI are critical to ensure that these programs continue.
Ensuring Safe and Effective Cancer Therapies Through Regulatory Science
FDA performs a critical role in ensuring that new anticancer therapies are safe and effective for patients. FDA’s complexity and workload have continually increased as new categories of therapies become available and inspections are needed across the globe. Therefore, FDA’s staff, technology, and processes must also be continually enhanced in order to provide effective and efficient oversight of drug development and manufacturing. FDA is funded through user fees paid by the pharmaceutical industry and congressionally appropriated funds, both of which are critical to support staff, technology modernization, and regulatory science programs that improve clinical trials. These crucial funds support FDA’s mission to regulate approximately one fifth of the U.S. economy that includes ensuring medicinal products used by Americans are safe and effective.
FDA’s Oncology Center of Excellence (OCE) was created in 2017 by the 21st Century Cures Act to provide dedicated
staff to improve efficiency in oncology drug development and review. OCE facilitates collaborations between staff members with oncology expertise from other FDA centers, including the Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation (CBER), and Center for Devices and Radiological Health (CDRH).
Diversifying and Decentralizing Trials
Clinical trial participation yields advances in treatment and survival for the types of cancers and groups of patients represented (761)Bleyer A, et al. (2018) Pediatric Blood Cancer, 65: e27074. [LINK NOT AVAILABLE]. Additionally, clinical trial participants frequently experience improved outcomes compared to nonparticipants (762)Koo KC, et al. (2018) BMC Cancer, 18: 468. [LINK NOT AVAILABLE], as they receive a greater level of care during clinical visits (763)Chow CJ, et al. (2013) J Am Coll Surg, 216: 774. [LINK NOT AVAILABLE]. While more than half of adult patients with cancer choose to enroll in trials if asked by their providers (764)Unger JM, et al. (2021) J Natl Cancer Inst, 113: 244. [LINK NOT AVAILABLE], clinical trial participation rates remain very low in the United States. Only 8 percent of adult patients and 19.9 percent of pediatric and adolescent patients with cancer participate in clinical trials(765)Unger JM, et al. (2019) J Natl Cancer Inst, 111: 245. [LINK NOT AVAILABLE](766)Faulk KE, et al. (2020) PLoS ONE, 15: e0230824. [LINK NOT AVAILABLE]
. Academic medical centers experience greater than average trial participation rates (765)Unger JM, et al. (2019) J Natl Cancer Inst, 111: 245. [LINK NOT AVAILABLE], but most patients with cancer are treated at community clinics or hospitals where trials have not been historically available. Unfortunately, more than 75 percent of patients with cancer are ineligible for trials because there are no trials available for their specific disease or they are excluded because of strict eligibility criteria related to other health conditions (765)Unger JM, et al. (2019) J Natl Cancer Inst, 111: 245. [LINK NOT AVAILABLE]. Additional challenges to enrollment in clinical trials include absence of health care facilities in some communities, lack of trust in medical research and institutions, family responsibilities, and costs and time related to participating (767)Nipp RD, et al. (2019) Oncologist, 24: 1048. [LINK NOT AVAILABLE](768)Unger JM, et al. (2016) Am Soc Clin Oncol Educ Book, 35: 185. [LINK NOT AVAILABLE](769)Institute of Medicine. Barriers to patient recruitment and physician participation. Accessed: June 30, 2022. . These factors contribute to a clinical trial population which does not represent the real-world population of patients who may use the new therapies. This discrepancy leaves open questions on safety and efficacy for people from groups that are not adequately represented in clinical trials, and particularly groups that bear disproportionately higher cancer incidence and death.
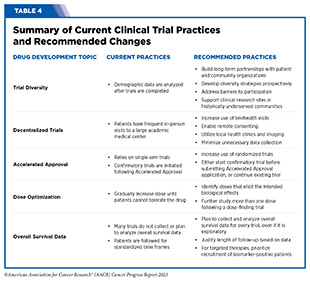
To improve representation in clinical trials, it will be critical that trial sponsors and researchers prospectively integrate diversity strategies with traditional drug development strategy. Historically, diversity has primarily been considered in hindsight, when it is too late to make meaningful changes to clinical trials underpinning regulatory submissions. Included in the federal appropriations omnibus for FY 2023 was a provision that allows FDA to require companies to develop a Diversity Action Plan for registrational trials as well as document their success at meeting diversity goals (770)U.S. Food and Drug Administration. Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials Guidance for Industry. Accessed: July 5, 2023. . The new law also allows FDA to require additional postmarketing clinical trials to follow-up on important signals for specific subgroups of patients identified during premarketing trials. In April 2022, OCE released draft guidance on creating prospective diversity action plans and is now working towards finalizing the guidance (771)U.S. Food and Drug Administration. Decentralized Clinical Trials for Drugs, Biological Products, and Devices. Accessed: July 5, 2023. . In June 2023, AACR, FDA, and several industry partners collaborated on a special article that outlined steps clinical trial sponsors can take to improve diversity in clinical trials, thereby improving access to innovative cancer therapies for all patients (772)Fashoyin-Aje LA, et al. (2023) Clin Cancer Res: OF1. [LINK NOT AVAILABLE]. They called on the entire cancer research community to expand the number of clinical sites involved in research, broaden trial eligibility criteria, address barriers to participation, and continue ongoing conversations among broad stakeholders, including patients. Recommended changes to clinical trials to enhance diversity as well as other improvements are summarized in Table 4.
In the wake of the COVID-19 pandemic, every clinical trial now includes some form of decentralized element, such as consenting patients remotely, using local laboratory test and imaging facilities, and telehealth visits for monitoring side effects. These approaches have greatly reduced the burden of trial participation for patients and health clinics alike. Decentralization also provides value to industry by enabling cost savings, increasing patient enrollments, simplifying trial designs, and promoting faster completion of trials. When the pandemic’s public health emergency designation expired earlier this year, many in the cancer research community were concerned about a lapse in COVID-era guidance that enabled greater decentralization. In response, FDA issued guidance to continue supporting decentralized approaches (771)U.S. Food and Drug Administration. Decentralized Clinical Trials for Drugs, Biological Products, and Devices. Accessed: July 5, 2023. . However, many of these decentralized elements were allowed prior to the COVID-19 pandemic and the limiting factors for widespread adoption revolve around implementation within industry and academic medical centers. As the use of decentralized elements grows, new data demonstrating the value of these changes will hopefully enhance further adoption.
Rapidly Delivering Safe and Effective Therapies to Patients
Waiting for definitive evidence of clinical benefit for new therapies before sponsors apply for FDA review can take many years. This delay can mean that patients with deadly diseases who could benefit may die before the drug becomes approved. In 1992, FDA created the Accelerated Approval pathway as a response to the devastating HIV pandemic (773)U.S. Food and Drug Administration. Accelerated Approval. Accessed: July 5, 2023. . Accelerated Approvals leverage early outcome data that are suggestive of “clinical benefit,” but not necessarily a direct measure of clinical benefit for serious diseases that have unmet medical needs. Between 2011 and 2020, 45.6 percent of new cancer drugs were approved through the Accelerated Approval pathway, and cancer therapies accounted for 80 percent of all Accelerated Approvals (774)Wang S, et al. (2022) Drug Discov Today, 27: 1236. [LINK NOT AVAILABLE]. For cancer therapies, progression-free survival, response rate, and side effect rates are early endpoints frequently considered for Accelerated Approval. However, Accelerated Approval also carries a requirement for companies to continue studying the therapy to demonstrate clinical benefit, most often improved overall survival.
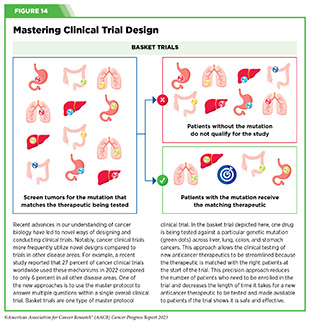
In March 2023, FDA issued draft guidance on considerations for clinical trial designs to support enhanced Accelerated Approval applications (775)U.S. Food and Drug Administration. Clinical Trial Considerations to Support Accelerated Approval of Oncology Therapeutics | FDA. Accessed: July 5, 2023. . A key focus of the guidance was encouraging trial sponsors to conduct more randomized controlled trials instead of the commonly used “single-arm” trials that lack control arms. While single-arm trials have been useful for developing new classes of drugs for diseases that previously had no effective treatment options, the clinical landscape has evolved (see Figure 14). It is now more appropriate to compare new drugs against the current standard of care to ensure that novel treatments provide greater benefits or lower toxicities than what is currently available. The guidance also recommended that trial sponsors either continue and expand the trial used for Accelerated Approval or start a second confirmatory trial before Accelerated Approval is granted. Currently, companies often start confirmatory trials after Accelerated Approval is granted, which leads to delays in granting traditional approval.
Advances in cancer care that prolong the lives of millions of cancer survivors are a major underlying reason for the increasing reliance on Accelerated Approvals for cancer therapies. This is because it is taking more time to demonstrate improvements in overall survival as patients live longer. However, there is ongoing concern that early endpoints do not always correlate with overall survival (776)Merino M, et al. (2023) J Clin Oncol, 41: 2706. [LINK NOT AVAILABLE]. One potential driver of this discordance is the way in which doses of new drugs are selected. Traditionally, cancer drug doses have been determined by escalating the dose given to patients until it is no longer tolerable. This strategy is less useful for targeted therapies and immunotherapies because they may be most effective and result in less severe side effects at doses lower than the “Maximal Tolerated Dose.” To encourage selection of optimal doses of new drugs, FDA issued a draft guidance titled “Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases,” in January 2023 (777)U.S. Food and Drug Administration. Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases. Accessed: July 5, 2023. . Additionally, a workshop in July 2023 co-organized by FDA, AACR, and the American Statistical Association titled “Overall Survival in Oncology Clinical Trials” convened experts from across the cancer research community to discuss best practices to improve how clinical trials collect and assess endpoint data. Some key recommendations for trial sponsors discussed during the workshop included: having a prospective plan to collect and analyze overall survival in all clinical trials, even if it is not the primary endpoint; considering the disease and treatment setting to determine how long data should be collected, instead of a standardized 5-year follow-up; and prioritizing recruitment of patients positive for predictive biomarkers for targeted and immunotherapies. Improvements in how cancer drugs are studied and doses are selected have the potential to accelerate the pace of progress against cancer.
Addressing Cancer Drug Shortages
In addition to ensuring that new cancer therapies are safe and effective, FDA is responsible for regulating the supply of drugs so that patients can get the medications they need. Unfortunately, there have been record shortages of cancer drugs this year. In December 2022, there were 295 prescription medications in short supply (778)The Washington Post. Cancer patients are confronting widespread shortages of chemotherapy drugs. Accessed: July 5, 2023. . Shortages of cisplatin and carboplatin are particularly impacting care options for patients, which are estimated to be prescribed for 10 to 20 percent of patients with cancer (779)National Cancer Institute. Discovery – Cisplatin and The Treatment of Testicular and Other Cancers. Accessed: July 5, 2023. . The underlying causes of the shortages are varied and incredibly complex, and include supply chain disruptions, manufacturing quality issues, limited economic incentives to manufacture generic drugs, and increasing reliance on fewer manufacturers. To help alleviate the shortage of cisplatin, FDA issued a temporary authorization to import cisplatin from China. Congress has held several hearings this year to better understand the issue and identify potential solutions in coordination with the White House, FDA, and cancer-focused professional organizations (780)The New York Times. Drug Shortages Near an All-Time High, Leading to Rationing. Accessed: July 5, 2023. .
FDA-NCI Collaborations to Promote Innovative Clinical Research
FDA and NCI have recently established several collaborations to promote innovation in clinical research. In February 2023, the Clinical Trials Innovation Unit (CTIU) was established to fund novel trial designs, with a focus on expanding access to historically underserved communities (781)National Cancer institute. Clinical Trials Innovation Unit. Accessed: July 5, 2023. . This collaboration pairs funding from NCI and trial design advice from FDA with the goal of expanding trial eligibility, investigating new endpoints, utilizing streamlined data collection strategies, and other novel methods and technologies to improve equity in cancer research. Another collaboration created in 2019, called the Connecting Awardees to Regulatory Experts (CARE) program, is focused on small businesses funded by NCI to begin developing new therapies (782)Pond MA, et al. (2023) Clin Transl Sci, 16: 412. [LINK NOT AVAILABLE]. The CARE program provides additional opportunities for NCI-funded small businesses to meet with FDA to discuss their development plans and promote successful regulatory submissions. From 2019 to 2022, 141 small businesses have participated in CARE, with 90 percent recommending the program to other companies.
Advancing Policy to Strengthen Cancer Prevention and Screening Programs
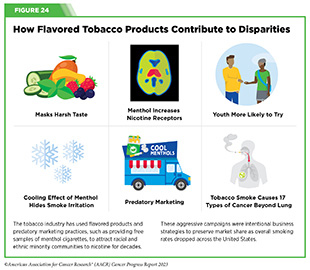
Approximately 40 percent of cancer cases in the United States can be attributed to preventable risk factors, such as tobacco use, infections, and UV exposure (see Reducing the Risk of Cancer Development and Figure 24). Routine screenings using evidence-based approaches to detect common cancers greatly improve treatment options and outcomes (see Screening for Early Detection). However, inequities remain in access to cancer screenings and follow-up care, which are major contributors to delayed diagnoses among underinsured and uninsured populations. CDC’s National Breast and Cervical Cancer Early Detection Program and Colorectal Cancer Control Program provide routine cancer screenings for medically underserved populations across the United States. Funding limitations prevent these crucial programs from providing screening services to all eligible people. According to the most recent data available, 6.8 percent of eligible people receive cervical cancer screenings and 15 percent of eligible people received breast cancer screenings through these programs (783)American Cancer Society Cancer Action Network. Congress Should Provide Higher Funding for CDC Cancer Programs and the National Breast and Cervical Cancer Early Detection Program. Accessed: July 5, 2023. (784)Centers for Disease Control and Prevention. About the National Breast and Cervical Cancer Early Detection Program | CDC. Accessed: July 5, 2023. . Medicaid expansion has helped increase cancer screenings in medically underserved populations (785)Healio. Colorectal cancer screening for ‘vulnerable’ patients higher in Medicaid expansion states. Accessed: July 5, 2023. and is another substantive approach for advancing health equity (786)Friedman AS, et al. (2022) Am J Public Health, 112: 1630. [LINK NOT AVAILABLE]. Thus, additional federal investment for these programs would improve equity in cancer screening, follow-up care, and advance the goals of the Cancer Moonshot, as underscored by Senator Robert P. Casey, Jr.
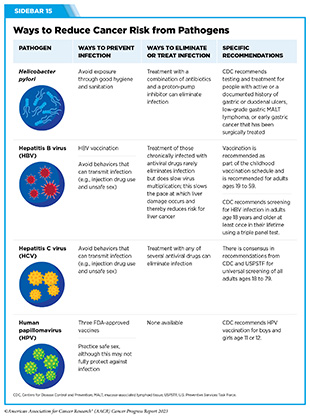
HPV infections can lead to six types of cancer, including nearly every case of cervical cancer (see Prevent and Eliminate Infection from Cancer-causing Pathogens) (787)Centers for Disease Control and Prevention. How many cancers are linked with HPV each year? Accessed: July 14, 2022. . There are effective, evidence-based strategies that can prevent HPV-attributed cancers, such as guideline-concordant HPV vaccination, cervical cancer screenings, and timely follow-up care (see Sidebar 15). Although HPV vaccination coverage increased among adolescents 13 to 17 years old in 2021 (215)Pingali C, et al. (2022) MMWR Morb Mortal Wkly Rep, 71: 1101. [LINK NOT AVAILABLE], there is still progress to be made to reach the Healthy People 2030 full vaccination goal of 80 percent in adolescents (217)Healthy People 2030. Increase the proportion of adolescents who get recommended doses of the HPV vaccine — IID‑08. Accessed: July 5, 2023. . Eliminating HPV-attributed cancers will only be achieved by coordinated strategies among all stakeholders to build confidence in vaccination and improve screening and treatment for HPV-related lesions.
Leveraging Policy to Reduce Tobacco-related Illness
Decades of public health awareness campaigns and effective tobacco control policies have resulted in historically low smoking rates among adults in the United States. In 2021, 18.7 percent of U.S. adults regularly used any tobacco product (32)Cornelius ME, et al. (2023) MMWR Morb Mortal Wkly Rep, 72: 475. [LINK NOT AVAILABLE], and 11.5 percent of adults regularly smoked cigarettes. Despite the progress made against tobacco use among adults, tobacco product use during adolescence is still cause for great concern as this greatly increases the risk of nicotine dependence later in life. Results from the most recent National Youth Tobacco Survey indicate that 16.5 percent of high school and 4.5 percent of middle school students currently use any tobacco product (788)U.S. Food and Drug Administration. Results from the Annual National Youth Tobacco Survey | FDA. Accessed: July 5, 2023. . E-cigarettes remain the top choice for youth under the age of 18, with 14.1 percent of high school students reporting e-cigarette use and 3.3 percent of middle school students reporting e-cigarette use. Additional tobacco control policies across all levels of government remain important to continue reducing tobacco-related cancers as smoking remains the number one preventable cause of cancer (see Figure 24).
In September 2022, FDA Commissioner Robert Califf, M.D., MACC requested an independent, operational evaluation of the FDA Center for Tobacco Products (CTP) to ensure the Center is prepared to address the impending challenges associated with youth tobacco use, tobacco-attributed disease, and death.
The report from the Reagan-Udall Foundation for the Food and Drug Administration provided 15 recommendations, across multiple areas, to strengthen CTP’s response towards the rapidly changing tobacco product landscape (790)U.S. Food and Drug Administration. Operational evaluation of certain components of FDA’s tobacco program. Accessed: July 5, 2023. . In alignment with report recommendations, FDA and NIH awarded funding for a new Center for Rapid Surveillance of Tobacco, which will enhance CTP’s and the research community’s ability to understand, track, and assess changes in tobacco use and the product marketplace (791)U.S. Food and Drug Administration. FDA and NIH Award Funding for New Center for Rapid Surveillance of Tobacco. Accessed: July 5, 2023. .
Further policies that could reduce tobacco-related illness include expanding flavor prohibitions to all tobacco products beyond e-cigarettes; increasing restrictions on tobacco product advertising and promotions; and increasing funding for awareness and cessation programs within FDA, NCI, and CDC’s Office on Smoking and Health.
Accelerating Progress Against Pediatric Cancer
Cancer is the leading disease-related cause of death in children in the U.S. Each year, more than 15,000 children and adolescents under 19 years of age are diagnosed with cancer (792)American Childhood Cancer Organization. US Childhood Cancer Statistics. Accessed: July 5, 2023. . Due to advances in treatments, about 80 percent of children survive their diagnoses. The overall cancer death rate for children under 14 years of age has declined an average of 1.5 percent per year between 2015 and 2019 (325)Cronin KA, et al. (2022) Cancer, 128: 4251. [LINK NOT AVAILABLE]. Nonetheless, cancer diagnosis continues to be devastating news for affected children and their families. Chronic conditions resulting from pediatric cancer treatments can continue to impact survivors into adulthood, and families of young people with cancer frequently endure emotional and financial hardship.
An important development in the fight against pediatric cancer is the Childhood Cancer Survivorship, Treatment, Access and Research (STAR) Act. Enacted in 2018, the Childhood Cancer STAR Act aims to improve health outcomes of young patients with cancer and advance pediatric cancer research by:
- Awarding grants to state cancer registries to improve surveillance for pediatric cancer.
- Supporting efforts to expand collection of biospecimens and clinical information on pediatric cancer patients to support research on childhood cancer and survivorship.
- Creating programs to explore innovative care models for childhood cancer survivors.
In January 2023, President Biden signed into law the Childhood STAR Reauthorization Act (793)Congerss.gov. S.4120 – 117th Congress (2021-2022): Childhood Cancer STAR Reauthorization Act. Accessed: July 5, 2023. , allowing programs from the STAR Act to continue through FY 2028 at its fully authorized level of $30 million, as discussed by Congressman Michael T. McCaul, p. 164. The reauthorization also supports funding of the Childhood Cancer Data Initiative (CCDI) at $50 million, which helps children’s hospitals, clinics, and other facilities share clinical and research data. Created in 2019, the CCDI works to improve understanding of pediatric cancer biology, prevention, treatment, and survivorship through enhanced data sharing.
However, the Childhood Cancer STAR Reauthorization Act only provides legal authority for programs created by the law to continue. As a next step, pediatric cancer advocates must ensure that Congress fully funds the Childhood Cancer STAR Act and the CCDI during the FY 2024 appropriations process.
Another program crucial to advancing progress against childhood cancer is the Gabriella Miller Kids First Pediatric Research Program (Kids First). Established in 2015, Kids First aims to help researchers make new discoveries into the biology of pediatric cancer as well as the relationship between structural birth defects and certain types of childhood cancer. Between 2015 and 2022, Kids First selected 63 pediatric cancer and structural birth defects cohorts for whole genome sequencing that entails more than 20,000 patients and 55,000 genomes (794)National Institutes of Health. Gabriella Miller Kids First Pediatric Research Program. Accessed: July 5, 2023. .
Kids First is set to sunset at the conclusion of FY 2023. To build on the program’s success in accelerating childhood cancer research, a bipartisan group of lawmakers introduced the Gabriella Miller Kids First Research Act 2.0 (H.R. 3391) (795)Congress.gov. H.R.3391 – 118th Congress (2023-2024): Gabriella Miller Kids First Research Act 2.0. Accessed: July 5, 2023. . In addition to reauthorizing Kids First through FY 2027, the Gabriella Miller Kids First Research Act 2.0 would nearly double funding for the program to $25 million annually. The House passed an earlier version of the Gabriella Miller Kids First Research Act 2.0 in July 2022, but insufficient support in the Senate and a limited Congressional calendar prevented the legislation from moving forward. Fortunately, lawmakers from both parties have reintroduced the Gabriella Miller Kids First Research Act 2.0 in Congress, breathing new life into this important vehicle for improving the lives of the nation’s youngest patients with cancer.
Aside from medical research, health care coverage remains an important issue for children facing cancer. Nearly half of all children in the U.S. have health insurance coverage through Medicaid or the Children’s Health Insurance Program (CHIP) (796)Georgetown University Health Policy Inititative. Millions of Children May Lose Medicaid: What Can Be Done to Help Prevent Them From Becoming Uninsured? Accessed: July 5, 2023. . However, millions of children face the prospect of losing their insurance coverage as flexibilities permitted because of the COVID-19 pandemic expire. Since March 2020, the federal government has provided state Medicaid offices additional Medicaid funding in exchange for ensuring continuous coverage of Medicaid enrollees and suspending eligibility determination processes. These emergency coverage protections for Medicaid enrollees ended on April 1, 2023, and the federal government has allowed state Medicaid agencies up to 14 months to redetermine the eligibility of enrollees, including children. While outcomes of the redetermination process will vary from state to state, an estimated 6.7 million children could lose their coverage and face prolonged periods without insurance (796)Georgetown University Health Policy Inititative. Millions of Children May Lose Medicaid: What Can Be Done to Help Prevent Them From Becoming Uninsured? Accessed: July 5, 2023. . These coverage losses would be especially devastating to children undergoing cancer treatment and their families. Thus, it is crucial for policymakers to ensure that state Medicaid agencies have the guidance and resources available to limit coverage losses and connect children who have lost Medicaid or CHIP coverage with new coverage options.
Building Health Equity by Addressing Cancer Disparities
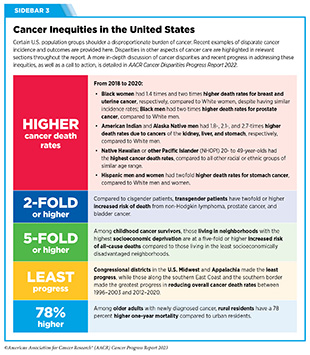
As described in the AACR Cancer Disparities Progress Report 2022 (13)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2022. Accessed: June 30, 2023. , systemic disadvantages greatly contribute to poorer health outcomes for medically underserved communities. Centuries of discriminatory policies that restrict housing, education, and employment opportunities for racial and ethnic minorities have led to lower health insurance coverage rates, lower utilization of preventive health services, poor nutrition, and inadequate access to quality health care. Additionally, the lack of high-quality health care facilities in low-income neighborhoods and rural communities results in a lower quality of care even for those who can afford it. Eliminating cancer disparities will require a long-term, multipronged approach that supports individuals, communities, health care centers, and federal agencies, as well as local, tribal, and state governments. Recent policy developments related to cancer screening, nutrition, and health insurance have demonstrated that work in addressing health disparities is underway, but there is still more progress that must be made (see Sidebar 3).
Routine cancer screenings are necessary to detect precancerous and cancerous lesions as early as possible in cancer development; however, variability in cancer screening contributes to cancer disparities. Unfortunately, follow-up care is less likely to occur for patients from rural areas or from racial and ethnic minority backgrounds for many historic and systemic reasons, including being uninsured or underinsured, decreased access to care, health care system bias, and miscommunication with health care providers (see Suboptimal Uptake of Cancer Screening).
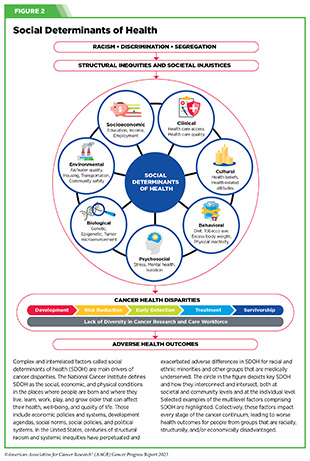
Several additional initiatives organized by NIH, NCI, the National Institute on Minority Health and Health Disparities (NIMHD), and CDC are designed to address cancer disparities. For example, NIH’s All of Us program aims to improve precision medicine research by building one of the largest and most diverse health databases. To date, over 661,000 people have joined the research program. The NCI Community Oncology Research Program is a national network that brings cancer clinical trials and care delivery studies to people in their own communities. Additionally, the NCI Center to Reduce Cancer Health Disparities supports disparities research within NCI and reinforces training a diverse cancer research workforce. NIMHD is NIH’s core institute to support research on the many factors that contribute to disparate health outcomes, including socioeconomics, politics, discrimination, culture, and environment (see Figure 2). This work needs to continue and be adequately funded to urgently address cancer disparities. All research and clinical trials should be conducted with consideration for every patient. This means reaching out to and involving individuals in previously neglected regions of the country.
.
Next Section: Conclusion Previous Section: Envisaging the Future of Cancer Science and Medicine