Advancing the Frontiers of Cancer Science and Medicine
In this section, you will learn:
- Researchers are harnessing the knowledge of the cellular and molecular underpinnings of cancer initiation and progression to develop safer and more effective treatments for cancer.
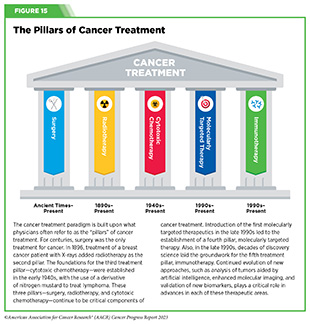
- Advances in novel and innovative approaches to surgery, radiotherapy, chemotherapy, molecularly targeted therapy, and immunotherapy—the five pillars of cancer treatment—are saving and improving lives.
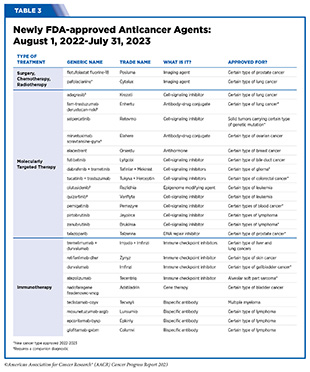
- From August 1, 2022, to July 31, 2023, FDA has approved 14 new anticancer therapeutics and two new imaging agents and has expanded the use of 12 previously approved anticancer therapeutics to treat additional cancer types.
- Included in the FDA approvals are the first antibody–drug conjugate for the treatment of ovarian cancer, several new molecularly targeted therapeutics and immunotherapeutics to treat rare cancers including blood cancers, two new immune checkpoint inhibitors, and a first of a kind gene therapy to treat bladder cancer.
- While these exciting new advances have the potential to transform patient care, much work is needed to ensure equitable access to these treatments for all populations.
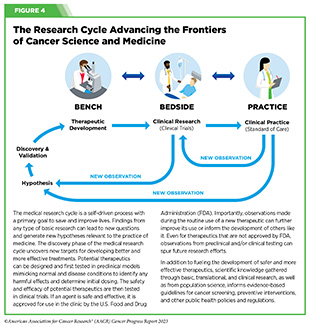
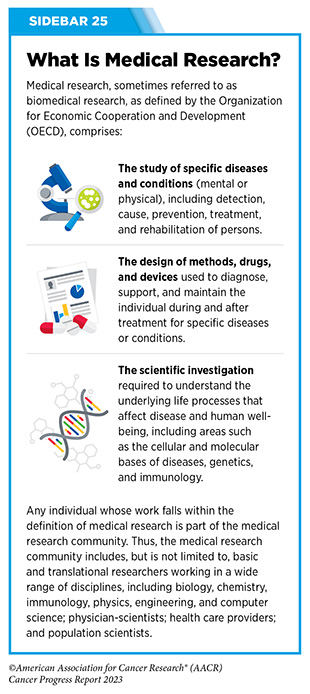
Progress across the continuum of cancer research and patient care improves survival and quality of life for people around the world. In the United States, the annual decline in overall cancer death rate among men, women, children, and adolescents and young adults has accelerated over the past two decades (see Cancer in 2023) (325)Cronin KA, et al. (2022) Cancer, 128: 4251. [LINK NOT AVAILABLE]. This progress is driven by the dedicated efforts of individuals working throughout the cycle of medical research (see Figure 4 and Sidebar 25).
Clinical Research
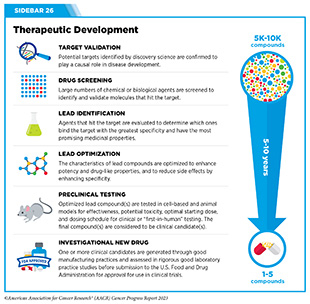
The rapid pace of progress against cancer is attributable in part to the new and effective treatments that are available today, thanks to the discoveries made through decades of research in basic and translational sciences. These discoveries have deepened our understanding of the cellular and molecular underpinnings of cancer initiation and progression and led to the identification of a range of molecular targets that drive cancer (see Understanding the Path to Cancer Development). After a potential therapeutic target is identified, it takes many more years of preclinical research before a candidate therapeutic is developed and ready for testing in clinical trials (see Sidebar 26).
Clinical trials evaluate the safety and efficacy of candidate agents before a preventive intervention or therapeutic can be approved by FDA and used as part of patient care. All clinical trials are critically reviewed and approved by institutional review boards before they can begin and are monitored throughout their duration. There are several types of cancer clinical trials, including prevention trials, screening trials, treatment trials, and supportive or palliative care trials, each designed to answer different research questions (see Sidebar 27). Clinical studies in which participants are randomly assigned to receive experimental treatment or standard of care treatment are called randomized clinical trials and are considered the most rigorous.
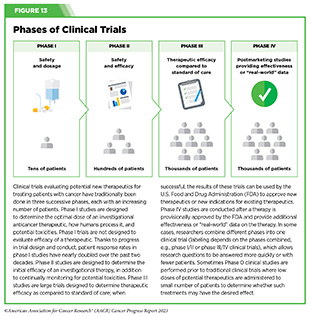
Clinical trials that test candidate therapeutics for patients with cancer have traditionally been done in three successive phases (see Figure 13). Observations made during the real-world use of a drug after it is approved by FDA can also be utilized to further enhance the use of that drug. The multiphase clinical testing process requires many patients, takes years to complete, and has a high rate of failure, making it extremely costly and one of the main barriers to rapid translation of scientific knowledge into clinical advances (326)Arfe A, et al. (2023) J Natl Cancer Inst, 115:917 [LINK NOT AVAILABLE](327)Shadbolt C, et al. (2023) JAMA Netw Open, 6: e2250996. [LINK NOT AVAILABLE]. Identifying and implementing more efficient clinical development strategies are an area of extensive investigation for all stakeholders in medical research (see Sidebar 1).
Advances in the understanding of cancer biology have enabled researchers from academia and the pharmaceutical industry to develop new approaches to designing and conducting clinical trials. Among the new concepts and designs for clinical trials that have emerged in recent years are the adaptive, main protocol, and platform trials designs (329)Li A, et al. (2020) Cancer, 126: 4838. [LINK NOT AVAILABLE]. These designs allow researchers to modify aspects of the trial design, if needed,
by leveraging the accumulating data, thereby increasing the efficiency of the clinical research process. Main protocol, also known as master protocol design, and platform design streamline clinical development and allow the evaluation of multiple new agents by matching the right therapeutics with the right patients earlier, reducing the number of patients who need to be enrolled in the trial, and decreasing the length of time it takes for a new anticancer therapeutic to be tested and made available to patients.
Master protocol can answer multiple clinical questions within a single trial (329)Li A, et al. (2020) Cancer, 126: 4838. [LINK NOT AVAILABLE]. The emergence of this clinical trial design has largely been driven by accumulating knowledge of the genetic mutations that underpin cancer initiation and growth. As one example, I-SPY 2 is one of the longest-running clinical trials that uses a master protocol which provides the regulatory framework to study multiple treatments for breast cancer within a single study (330)Quantum Leap Healthcare Collaborative. The I-SPY Trials. Accessed: July 31, 2023. . The platform design of the I-SPY 2 trial allows new treatments to enter and leave the study with a greater efficiency than traditional clinical trials. The study has led to the FDA approval of several breast cancer treatments, including the molecularly targeted therapeutic neratinib (Nerlynx) (331)Wang H, et al. (2019) Curr Breast Cancer Rep, 11: 303. [LINK NOT AVAILABLE].
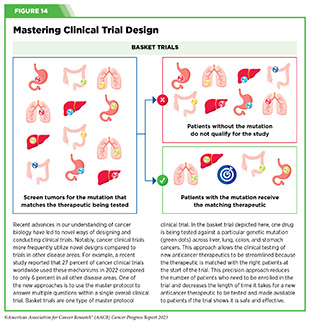
Basket trials are another example of genetic mutation–based master protocol design in clinical trials (see Figure 14). These trials allow researchers to test one anticancer therapeutic on a group of patients who all have the same type of genetic mutation, regardless of the anatomic site of the original cancer. As one example, the combination of molecularly targeted therapeutics dabrafenib and trametinib was shown to work against an array of cancer types characterized by a specific genetic feature, or biomarker, called the BRAF V600E mutation, in two recent basket trials including the NCI MATCH study (see Sidebar 9) (109)O’Dwyer PJ, et al. (2023) Nat Med, 29: 1349. [LINK NOT AVAILABLE]. Based on the data from these trials, the combination treatment received FDA approval in June 2022 and is now benefiting many patients with cancer (1)American Association for Cancer Research. AACR Cancer Progress Report 2022. Accessed: July 5, 2023. . Based on a recent analysis, the use of novel trial designs in clinical cancer research has more than tripled, worldwide, over the past decade (332)IQVIA. Global Oncology Trends 2023. Accessed: July 5, 2023. .
As our understanding of cancer biology continues to evolve and we uncover some of the most elusive questions in cancer medicine (see Cancer Development: Integrating Knowledge) clinical trial designs will need to evolve as well. Additionally, the design and conduct of clinical cancer research need to keep pace with the new wave of technological advances. Novel designs that integrate emerging approaches such as comprehensive tumor profiling (e.g., of genome, transcriptome, proteome, microbiome, and metabolome, among others), artificial intelligence and machine learning, real-world evidence and data, and leverage inputs from patient advocacy communities and social media platforms will be pivotal to advancing the frontier of cancer clinical trials (333)Subbiah V (2023) Nat Med, 29: 49. [LINK NOT AVAILABLE].
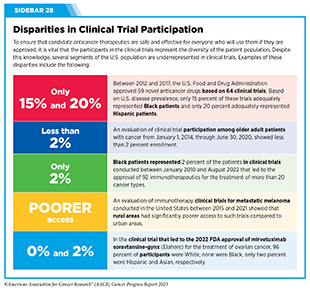
Two of the most pressing challenges that need to be overcome urgently are low participation in cancer clinical trials and a lack of sociodemographic diversity among those who do participate (see Sidebar 28). Low participation in clinical trials means that many trials fail to enroll enough participants to draw meaningful conclusions about the effectiveness of the anticancer therapeutic being tested. Lack of diversity in clinical studies means that the trial participant population does not match the actual demographics of the cancer burden under study (334)In: Bibbins-Domingo K, Helman A, editors. Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups. Washington (DC)2022. [LINK NOT AVAILABLE]. Underrepresentation in clinical trials compromises the applicability of such research findings to the entire U.S. patient population.
Understanding and eliminating barriers to clinical trial participation for all segments of the population is vital if we are to accelerate the pace of progress against cancer for everyone. Numerous studies have investigated the existing barriers that limit participation of racial and ethnic minorities and other medically underserved populations in cancer clinical trials. These studies have identified a range of factors such as lack of awareness of clinical trials, financial challenges, limited health literacy, inadequate or complete lack of insurance, medical distrust, implicit biases among health care providers, lack of trial availability, and narrow eligibility criteria, among others (13)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2022. Accessed: June 30, 2023. . These barriers operate at individual, systemic, and societal levels (340)Kahn JM, et al. (2022) Cancer, 128: 216. [LINK NOT AVAILABLE].
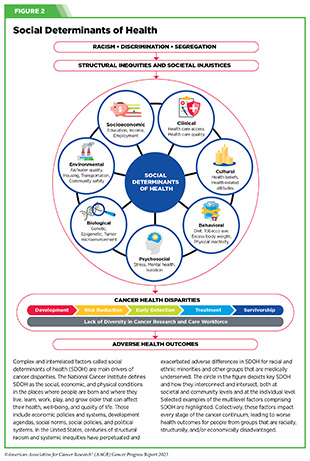
Increased knowledge of the barriers to clinical trial accrual is helping researchers, regulators, and policymakers design and implement evidence-based adaptations that can broaden participant access and promote accrual to clinical research. Such interventions focus on addressing social determinants of health (see Figure 2), and include decentralizing many of the trial activities to ease patient participation, expanding eligibility criteria, improving the efficiency of data collection, including patient reported outcomes (PRO), and enhancing community outreach and patient navigation efforts to raise awareness of trials. One critical area of focus for all stakeholders in medical research is fostering greater diversity, equity, and inclusion within the clinical research workforce so the workforce will resemble the patient populations it serves.
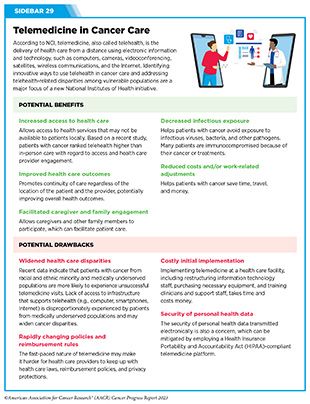
U.S. lawmakers and FDA are working on legislation and guidelines intended to increase the diversity of clinical trial participants (see Diversifying and Decentralizing Trials). These include a diversity action plan which would require researchers and funders of clinical trials to submit concrete goals and needed steps for enrolling specific demographic groups in pivotal studies of new drugs (341)U.S. Food and Drug Administration. Diversity Plans to Improve Enrollment of Participants From Underrepresented Racial and Ethnic Populations in Clinical Trials; Draft Guidance for Industry; Availability. Accessed: July 5, 2023. . COVID-19, despite its adverse effects on all aspects of cancer research and patient care, enabled researchers to decentralize clinical trial designs, so that lifesaving therapeutics could be brought quickly to as many patients as possible (9)American Association for Cancer Research. AACR Report on the Impact of COVID-19 on Cancer Research and Patient Care. Accessed: June 30, 2022. . Adaptations implemented by NCI and FDA during the pandemic, including consenting patients remotely, permitting telehealth for routine clinical assessments (see Sidebar 29), delivering experimental drugs to patients, and allowing the use of local laboratory or imaging facilities accessible to patients have offered a blueprint of success to further revise and reform clinical trials and the drug approval process for the benefit of all patients with cancer.
Progress Across the Clinical Cancer Care Continuum
Research discoveries made as a result of innovative cancer science are continually being translated into new medical products for cancer prevention, detection, diagnosis, treatment, and survivorship. The approval of new medical products, including new anticancer treatments, is not the end of a linear research process. Rather, it is an integral part of the medical research cycle (see Figure 4) because observations made during the routine use of new medical products can be used to accelerate the pace at which similar products are developed and to stimulate the development of new, more effective products.
New FDA-approved medical products are traditionally utilized alongside treatments already in use, including surgery, radiotherapy, and cytotoxic chemotherapy, which continue to be the mainstays of clinical cancer care (see Figure 15). Researchers are also evaluating new ways to refine the use of surgery, radiotherapy, and existing cytotoxic chemotherapeutics to improve survival and quality of life for patients. As one example, a recent clinical trial showed that for patients with early-stage prostate cancer, active monitoring of their disease is a safe alternative to receiving immediate surgery or radiotherapy (346)Hamdy FC, et al. (2023) N Engl J Med, 388: 1547. [LINK NOT AVAILABLE]. In most cases, prostate cancer grows slowly. Therefore, the study directly compared the long-term outcomes of the three approaches, prostate removal surgery, radiotherapy, or active monitoring and found that there was no difference in prostate cancer mortality at the 15-year follow-up between the three groups. These data provide hope for patients with prostate cancer who opt for active monitoring to avoid treatment-related adverse effects, such as sexual and incontinence problems.
The following sections focus on the recent advances across the five pillars of cancer treatment including the 14 new anticancer therapeutics approved by the FDA in the 12 months spanning this report, August 1, 2022, to July 31, 2023 (see Table 3 and Supplemental Table 2). Also highlighted are the 12 previously approved anticancer therapeutics that received FDA approval for treating additional types of cancer in that period.
Not discussed are FDA approvals that expand the use of an anticancer therapeutic previously approved for a given cancer type to include treatment with that therapeutic at different timepoints during the course of clinical care or treatment of a different subtype of the same cancer. For example, the August 2022 FDA approval expanded the use of fam-trastuzumab-deruxtecan-nxki (Enhertu), for the treatment of patients with metastatic HER2-low breast cancer that is not removable by surgery. This expansion occurred nearly three years after the molecularly targeted therapeutic was first approved for treating metastatic HER2-positive breast cancer and was based on results from a phase III clinical trial. The study showed that patients with metastatic breast cancer who were treated with fam-trastuzumab-deruxtecan-nxki lived nearly twice as long without their cancer progressing and lived six months longer overall than those treated with standard chemotherapy (352)Modi S, et al. (2022) N Engl J Med, 387: 9. [LINK NOT AVAILABLE]. Fam-trastuzumab-deruxtecan-nxki is the first treatment approved for patients with HER2-low breast cancer subtype, a newly defined subset of HER2-negative breast cancer.
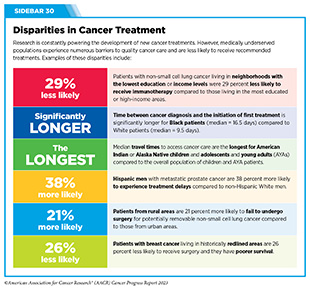
New medical products used across the continuum of clinical cancer care transform lives by improving survival and quality of life. However, not all patients receive the standard of care recommended for the type of cancer with which they have been diagnosed and the stage of cancer at the time of diagnosis (see Sidebar 30). Thus, it is imperative that all stakeholders committed to driving progress against cancer work together to address the challenge of disparities in cancer treatment because these can be associated with adverse differences in survival. Recent studies have shown that disparities in survival for prostate cancer or multiple myeloma between Black patients and White patients can be eliminated when both population groups have equivalent access to care and to standard treatments (13)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2022. Accessed: June 30, 2023. .
Advances in Cancer Treatment with Surgery
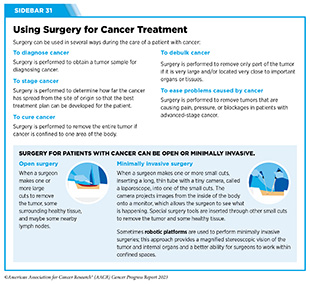
For many years, surgery was the only pillar of cancer treatment (see Figure 15). Today, it remains the foundation of curative treatment for many patients. Surgery is used in several ways during the care of a patient with cancer (see Sidebar 31).
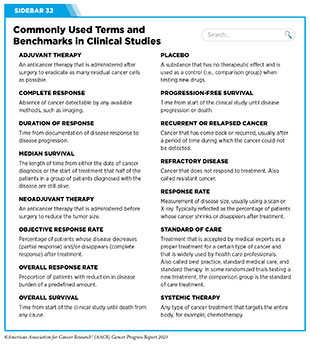
Sometimes, additional therapy is given before, after, or around the time of surgery based on specifics of a patient’s situation (see Sidebar 32). Researchers have found that this approach not only improves the surgeon’s ability to remove the tumor (for example by shrinking the tumor when given before the surgery), but also increases the patient’s overall survival and/or quality of life (359)Burotto M, et al. (2019) Semin Oncol, 46: 83. [LINK NOT AVAILABLE].
Improving Quality of Life After a Cancer Surgery
Despite the immense benefits of surgery for the treatment of cancer, complications are common and can negatively affect patient quality of life. Enhanced recovery after surgery (ERAS) programs are emerging as one approach to address this issue. These comprehensive programs focus on optimizing patient care before, during, and after surgery using strategies that ensure the patient is as physically and emotionally fit for surgery as possible; alleviate the stress of surgery; promote recovery; and reduce the time before patients with cancer can begin adjuvant treatment. Providing patients with an individualized plan that includes exercise, nutrition, stress reduction, and smoking cessation to optimize their physical fitness before surgery is one strategy included in some ERAS programs (360)Gustafsson UO, et al. (2019) World J Surg, 43: 659. [LINK NOT AVAILABLE](361)Santa Mina D, et al. (2017) PM R, 9: S305. [LINK NOT AVAILABLE]. The components of ERAS programs can vary depending on the type of surgery being performed and the center at which the surgery is being performed, but overall, these programs have been promising.
One study found that among patients undergoing surgery for tumors that have metastasized to the spine, those who participated in an ERAS program had reduced blood loss, shorter hospitalization, and significant reduction in opioid pain reliever utilization compared to those who did not participate (362)Chakravarthy VB, et al. (2022) Cancer, 128: 4109. [LINK NOT AVAILABLE]. Another study showed that among patients undergoing surgery for colorectal cancer, those who participated in an individualized plan that included exercise, nutritional intervention, and psychological support had fewer medical complications and better recovery postsurgery than those who did not participate in such programs (363)Molenaar CJL, et al. (2023) JAMA Surg, 158: 572. [LINK NOT AVAILABLE].
Other approaches to reducing the complications during and after surgery and improving quality of life postprocedure are to perform less extensive and minimally invasive surgeries, such as robotic surgeries or to identify a subset of patients who could skip surgery altogether.
As one example, data from a recent clinical trial showed that for certain patients with early-stage non–small cell lung cancer (NSCLC), surgical removal of only part of the affected lobe of lung is an effective treatment option (364)Altorki N, et al. (2023) N Engl J Med, 388: 489. [LINK NOT AVAILABLE]. The study, which compared the outcomes of patients who had their entire lobes removed to those who had only the tumor-affected regions removed, showed that the 5-year overall survival was similar in the two groups. While the study participants represent only a select subgroup of patients with lung cancer, these data are important considering that removal of less lung tissue can preserve lung function, especially for older adults and those with compromised lung capacity, such as patients with a prior lung cancer.
Studies have shown that less invasive surgeries may benefit patients since they can minimize postprocedural complications without compromising and sometimes improving long-term outcomes (365)Topal H, et al. (2022) JAMA Netw Open, 5: e2248147. [LINK NOT AVAILABLE](366)Son SY, et al. (2022) JAMA Surg, 157: 879. [LINK NOT AVAILABLE](367)Di Benedetto F, et al. (2023) JAMA Surg, 158: 46. [LINK NOT AVAILABLE]. As one example, in a recent clinical trial, patients with locally advanced stomach cancer who underwent a minimally invasive procedure had significantly lower long-term complications after surgery, but similar 5-year overall and relapse-free survival rates compared to those who had open surgeries (366)Son SY, et al. (2022) JAMA Surg, 157: 879. [LINK NOT AVAILABLE]. Additionally, two retrospective analyses showed improved disease-free and overall survival for patients with pancreatic cancer and reduced morbidity during surgery for patients with liver cancer who underwent minimally invasive surgeries compared to those who received open surgeries (365)Topal H, et al. (2022) JAMA Netw Open, 5: e2248147. [LINK NOT AVAILABLE](367)Di Benedetto F, et al. (2023) JAMA Surg, 158: 46. [LINK NOT AVAILABLE]. Yet another report from an early-stage clinical trial showed that a selected subset of patients with breast cancer who responded remarkably well to neoadjuvant chemotherapy could potentially forgo surgery without risking tumor recurrence (368)Kuerer HM, et al. (2022) Lancet Oncol, 23: 1517. [LINK NOT AVAILABLE].
Recent studies have also identified subsets of patients who could skip surgery altogether without compromising outcomes. In a clinical trial, women who had early-stage(368)Kuerer HM, et al. (2022) Lancet Oncol, 23: 1517. [LINK NOT AVAILABLE]reast cancer with defined clinical characteristics had equally good overall survival whether they received radiotherapy delivered to the lymph nodes in their underarms (axillary radiotherapy), or an invasive surgical procedure to remove these lymph nodes (axillary lymph node dissection) (369)Bartels SAL, et al. (2023) J Clin Oncol, 41: 2159. [LINK NOT AVAILABLE]. Notably, axillary lymph node dissection is associated with a significantly higher rate of morbidity, particularly lymphedema, which causes swelling in the arms that can cause pain and problems in functioning. These risks are drastically reduced if radiotherapy is given instead and suggests radiation rather than surgery should be the preferred approach in these patients.
While less invasive approaches to surgery such as those described above are promising, before they can become standard of care, it is vital that they are shown in rigorous, well-designed, larger clinical trials to have no adverse effect on long-term patient survival.
Visualizing Lung Cancers More Precisely During Surgery
Lung cancer is the leading cause of cancer deaths in the United States with an estimated 127,070 deaths predicted in 2023 (28)American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . While surgery is the standard treatment and provides the best chance to cure early-stage lung cancer, up to 55 percent of people with lung cancer who undergo surgery with curative intent have a recurrence (370)Uramoto H, et al. (2014) Transl Lung Cancer Res, 3: 242. [LINK NOT AVAILABLE]. Therefore, it is vital that the entire tumor is removed during surgery. Surgeons rely on either imaging tumors before surgery, visually inspecting tumors under normal white light during surgery, or examining tumors by touch to identify cancerous tissue. Unfortunately, some lung lesions can be difficult to visualize, particularly if they are small, beneath the surface of the lung, or a type of lesion characterized by increased opacity of the lung called ground glass opacity, which is being increasingly diagnosed as the rates of lung cancer screenings rise (371)Migliore M, et al. (2018) Ann Transl Med, 6: 90. [LINK NOT AVAILABLE](372)Huang C, et al. (2019) J Cancer, 10: 6888. [LINK NOT AVAILABLE].
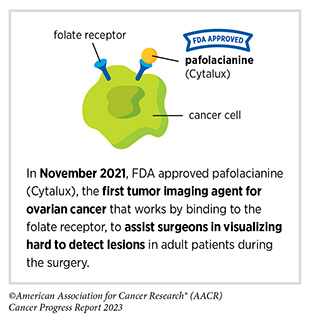
In December 2022, the FDA approved pafolacianine (Cytalux), a folate receptor–targeted fluorescent agent, as the first and only targeted molecular imaging agent that illuminates lung cancers and enhances surgeons’ ability to see cancer in real time as they operate. Molecular imaging using pafolacianine during surgery enables the detection of lung lesions that may have otherwise been missed. Pafolacianine was previously approved to assist surgeons in visualizing hard to detect lesions in adult patients with ovarian cancer during surgery (1)American Association for Cancer Research. AACR Cancer Progress Report 2022. Accessed: July 5, 2023. . Pafolacianine binds to folate receptors, a protein that is commonly found on the surface of many cancers and illuminates tumor cells under near-infrared light. The agent is administered via intravenous infusion within 24 hours before surgery and assists surgeons in visually identifying additional malignant tissue to be removed during the procedure.
The approval in lung cancer was based on a clinical trial that evaluated the utility of pafolacianine in visualizing tumors in the lungs that may otherwise be undetected with conventional visualization under white light (373)Sarkaria IS, et al. (2023) J Thorac Cardiovasc Surg. [LINK NOT AVAILABLE]. Molecular imaging using pafolacianine during surgery identified in 19 percent of patients primary lung nodules that surgeons could not find using white light and palpation; additionally, pafolacianine revealed in eight percent of patients additional lesions that were completely missed using white light. The expanded approval of pafolacianine represents a significant advancement in the treatment of lung cancer by enhancing detection of lung tumors during surgery, improving the ability to remove them completely, and reducing the probability of leaving behind cancerous tissue.
Improvements in Radiation-based Approaches to Cancer Care
Radiotherapy is the use of high-energy rays (e.g., gamma rays and X-rays) or particles (e.g., electrons, protons, and carbon nuclei) to control or eradicate cancer. Discovery of X-rays in 1895 allowed visualization of internal organs at low doses, and the effective use of X-rays at high doses to treat a breast cancer patient a year later established radiotherapy as the second pillar of cancer treatment (see Figure 15). Radiotherapy plays a central role in the management of cancer and works primarily by damaging DNA, leading to cancer cell death. The use of radiotherapy in treatment and management of cancer continues to increase, as indicated by a 16.4 percent increase in radiation facilities across the United States between 2005 and 2020 (374)Maroongroge S, et al. (2022) Int J Radiat Oncol Biol Phys, 112: 600. [LINK NOT AVAILABLE].
There are many types and uses of radiotherapy (see Sidebar 33). However, it is important to note that radiotherapy may also have harmful side effects, partly because of the radiation-induced damage to healthy cells surrounding the tumor tissue (375)Wang K, et al. (2021) CA Cancer J Clin, 71: 437. [LINK NOT AVAILABLE].
Researchers are continuously working on making radiotherapy safer and more effective and identifying when radiotherapy can be avoided without affecting the chances of survival for patients. As one example, a recent clinical trial showed that older adult patients with small, early-stage breast cancer may forgo radiation
after breast conserving surgery without compromising their overall survival (376)Kunkler IH, et al. (2023) N Engl J Med, 388: 585. [LINK NOT AVAILABLE]. Traditionally, in these patients, surgery has been followed with radiotherapy to reduce the risk of cancer recurrence. However, radiotherapy can lead to a range of potential side effects including pain, minor risks of organ damage and secondary cancer, as well as time and financial losses. Adverse effects are especially challenging for older adults, many of whom have other comorbidities. The new evidence provides these patients with the option for a less aggressive course of action.
Another clinical trial showed that radiation therapy before initial surgery may not be needed for patients with locally advanced rectal cancer that has spread locally within the rectum but not to other organs (377)Schrag D, et al. (2023) N Engl J Med, 389: 322. [LINK NOT AVAILABLE]. Traditionally, these patients receive radiation combined with chemotherapy, also known as chemoradiotherapy, before surgical removal of their tumors. Chemoradiotherapy shrinks the tumor making it easier to remove and helping to prevent recurrence. Data from the recent clinical trial showed that chemotherapy alone before surgery was just as effective as chemoradiotherapy at keeping the cancer at bay (377)Schrag D, et al. (2023) N Engl J Med, 389: 322. [LINK NOT AVAILABLE].
Researchers are also designing novel radiotherapeutics, to be used alone or in combination with other treatments, to target more cancer types and benefit more patients. Additionally, technological innovations, such as the development of advanced imaging and sophisticated computer analytic programs assisted by AI, are helping optimize the delivery of the radiation to the tumor while minimizing exposure to normal tissues (378)Santoro M, et al. (2022) Applied Sciences, 12: 3223. [LINK NOT AVAILABLE]. As one example, Magnetic Resonance Imaging (MRI)-guided radiotherapy (MRgRT) is a novel technology with the potential to transform radiotherapy for many patients including those with prostate cancer (379)Ng J, et al. (2023) Front Oncol, 13: 1117874. [LINK NOT AVAILABLE]. MRgRT provides the ability to image tumors and internal organs with MRI and adapt the radiotherapy plan in real-time while the patient is undergoing the procedure. Unlike traditional radiotherapy, MRgRT allows monitoring of changes in tumor size and positional changes of internal organs during each treatment to achieve a more accurate delivery of the radiation dose. This is particularly critical for rapidly changing tumors and body regions, such as the prostate, where there could be dramatic changes in organ position during each treatment.
Imaging Prostate Cancer More Clearly
Prostate cancer is the most common type of cancer in men in the United States. In 2023, an estimated 288,300 new cases will be diagnosed and 34,700 men will die from the disease.
Prostate cancer that is confined to the prostate is usually treated with surgery or radiation therapy. Unfortunately, many patients with primary prostate cancer have detectable metastases in their pelvic lymph nodes, which are correlated with a risk for cancer recurrence. Surgical procedures known as pelvic lymph node dissection or pelvic lymphadenectomy are used to detect pelvic node lesions, but their use is imprecise and limited to a planned surgical area. An ideal detection method for metastatic prostate cancer would locate tumors in pelvic nodes as well as more distant sites. The more precise a patient’s diagnosis, the easier it is for a health care provider to tailor the treatment to ensure that it is as effective and safe as possible. Notably, despite surgery or radiotherapy many patients with prostate cancer have local or distal recurrences within 10 years.
Among the tools physicians use to make cancer diagnoses is positron emission tomography–computed tomography (PET–CT or PET), a form of imaging that can help physicians precisely locate the position of a patient’s cancer within the body and determine the extent to which the cancer may have spread. Before a PET scan, patients are injected with a radioactive imaging agent. The PET scan detects cancer by identifying where in the body the radioactive agent accumulates.
In May 2023, FDA approved flotufolastat fluorine-18 (Posluma) for PET imaging of PSMA-positive lesions in patients with prostate cancer with suspected metastasis or with suspected recurrence based on elevated serum PSA level. PSA is a secreted biochemical marker that is used to screen individuals for prostate cancer and for predicted recurrence of the disease among patients who have received treatment. PSMA is a protein that is present in abundance on the surface of more than 90 percent of primary and metastatic prostate cancer cells. Flotufolastat F-18 contains a short peptide sequence that binds to PSMA and is internalized by cells that express PSMA. Flotufolastat F-18 also contains the radioisotope fluorine-18 which enables PET imaging of the prostate and other areas of the body where prostate cancer may have spread. Clinicians can use this information to decide which patient should receive treatment and spare others from unnecessary procedures.
Findings from two clinical trials that FDA used to approve flotufolastat F-18 indicate that detection of prostate cancers using this approach may help physicians make the best treatment decisions for patients (380)The ASCO Post Staff. FDA Approves Flotufolastat Fluorine-18 Injection, First Radiohybrid PSMA-Targeted PET Imaging Agent for Prostate Cancer. Accessed: July 5, 2023. . One study demonstrated a higher specificity of flotufolastat F-18 for the detection of pelvic lymph node metastasis, compared to standard histopathology, in patients with PSMA-positive lesions. Flotufolastat F-18 provided valuable information that would likely result in changes in clinical management for these patients. In the second study, flotufolastat F-18 demonstrated high prostate cancer recurrence detection rates in patients who had suspected disease recurrence based on elevated PSA levels.
Advances in Treatment with Cytotoxic Chemotherapy
Cytotoxic chemotherapy—use of chemicals to kill cancer cells—was first introduced as a pillar of cancer treatment in the early to mid-20th century (349)DeVita VT, Jr., et al. (2008) Cancer Res, 68: 8643. [LINK NOT AVAILABLE]. Chemotherapy remains a backbone of cancer treatment and its use is continually evolving to minimize potential harms to patients with cancer, while maximizing its benefits.
As with surgery, chemotherapy is more commonly used to treat cancer in combination with one or more additional types of treatments. Furthermore, FDA continues to grant approvals to newer and more effective chemotherapeutics. FDA also routinely expands the use of previously approved chemotherapeutics for additional cancer types through review of new clinical trials as well as by monitoring of current real-world use of such agents. The FDA Project Renewal leverages expertise of clinical researchers to review existing published literature on drug utilization and maintain updated labeling of older, commonly prescribed anticancer therapeutics. As one example of this approach, in December 2022, FDA approved updated labeling for the chemotherapeutic capecitabine (Xeloda) which included new indications and dosing regimens for capecitabine tablets.
Treatment with cytotoxic chemotherapeutics can have adverse effects on patients. These effects can occur during treatment and continue in the long term, or they can appear months or even years later. Health care providers and researchers are investigating different approaches to make chemotherapeutics safer for patients. Areas of ongoing investigation include designing modifiable chemotherapeutics, e.g., with “on” and “off ” switches, that are selectively delivered to tumors while sparing healthy tissue; evaluating less aggressive chemotherapy regimens which can allow patients the chance of an improved quality of life without compromising survival; and identifying biomarkers such as circulating tumor DNA to correctly predict which patients will or will not benefit from chemotherapy, among other approaches (381)East P, et al. (2022) Nat Commun, 13: 5632. [LINK NOT AVAILABLE](382)Rais R, et al. (2022) Sci Adv, 8: eabq5925. [LINK NOT AVAILABLE](383)Kotani D, et al. (2023) Nat Med, 29: 127. [LINK NOT AVAILABLE].
Notably, due to complex reasons, the United States is amid a significant chemotherapeutic shortage. The situation is affecting many patients and disrupting clinical research nationwide. It is imperative that all stakeholders in health care come together and identify ways to address these shortages at the earliest possible time (see Addressing Cancer Drug Shortages).
Advances in Treatment with Molecularly Targeted Therapy
Remarkable advances in our understanding of the biology of cancer, including the identification of numerous genetic mutations that fuel tumor growth, set the stage for a new era of precision medicine, an era in which the standard of care for many patients is changing from a one-size-fits-all approach to one in which greater understanding of the individual patient and the characteristics of his or her cancer dictates the best treatment option for the patient (see Understanding the Path to Cancer Development).
Therapeutics directed to molecules influencing cancer cell multiplication and survival target tumor cells more precisely than cytotoxic chemotherapeutics, which generally target all rapidly dividing cells, and thereby limit damage to healthy tissues. The greater precision of these molecularly targeted therapeutics tends to make them more effective and less toxic than cytotoxic chemotherapeutics. As a result, they are not only saving the lives of patients with cancer, but also allowing these individuals to have a higher quality of life. Unfortunately, because of multilevel barriers to health care, there are disparities in the utilization of molecularly targeted treatments among patients from racial and ethnic minorities and other medically underserved populations (13)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2022. Accessed: June 30, 2023. . It is vital that ongoing research and future public health policies are aimed to ensure equitable access to precision cancer medicine including tumor genetic testing and the receipt of molecularly targeted therapeutics for all patients.
In the 12 months spanning August 1, 2022, to July 31, 2023, FDA approved seven new molecularly targeted anticancer therapeutics (see Table 3). During this period, FDA also approved nine previously approved molecularly targeted anticancer therapeutics for treating additional types of cancer.
Expanding Treatment Options for Patients with Lung Cancer
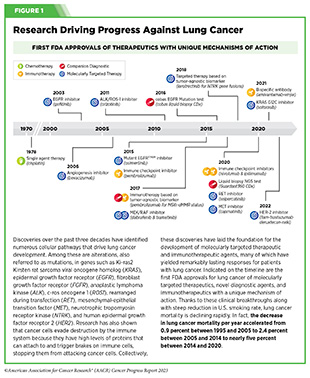
Lung cancer is the second most diagnosed cancer in both men and women and the most common cause of cancer death. More than 127,000 deaths are estimated to occur from the disease in 2023 in the United States (28)American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . Decades of basic and translational research have significantly increased our understanding of the genetic changes that drive lung cancer growth and have fueled the development of therapeutics that target these changes (see Figure 1) (28)American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . Two recent FDA decisions have the potential to drive more progress against lung cancer because they have provided new molecularly targeted therapeutic options for certain patients with the disease.
About 81 percent of lung cancers diagnosed in the United States are classified as non–small cell lung cancers (NSCLC) and approximately 25 percent of patients with NSCLC carry mutations in the gene that is responsible for producing KRAS, an essential protein needed for growth and survival of normal lung cells, but which can contribute to cancer if mutated (28)American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. (384)Janne PA, et al. (2022) N Engl J Med, 387: 120. [LINK NOT AVAILABLE]. Mutated KRAS represents one of the most common genetic alterations responsible for the development and progression of human cancers. Patients with NSCLC harboring KRAS mutations often develop resistance to standard treatments such as chemotherapy, radiation therapy, and immunotherapy, and only 25 percent of these patients live five years or more after diagnosis (438)Voruganti T, et al. (2023) JAMA Oncol, 9: 334. [LINK NOT AVAILABLE]. The most common KRAS mutation in patients with NSCLC is known as KRAS G12C, an alteration that is more frequently found in individuals who smoke currently or have smoked previously. The G12C mutation causes KRAS protein to prefer an “on” or “active” state, leading to uncontrollable cell growth that can form tumors.
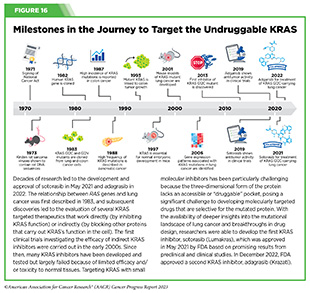
Historically, KRAS has been considered an undruggable target because of the difficulties in designing a therapeutic that could selectively bind and inhibit KRAS in cancers. Despite major breakthroughs in selective targeting of a range of other genetic drivers of NSCLC, no effective treatment options were available for patients with KRAS G12C until two years ago. Thanks to enhanced understanding of KRAS biology and unprecedented progress in structural biology and drug development, in May 2021, sotorasib (Lumakras) became the first ever molecularly targeted therapeutic approved by the FDA for the treatment of patients with NSCLC with the KRAS G12C mutation (see Figure 16) (4)American Association for Cancer Research. AACR Cancer Progress Report 2021. Accessed: June 30, 2023. .
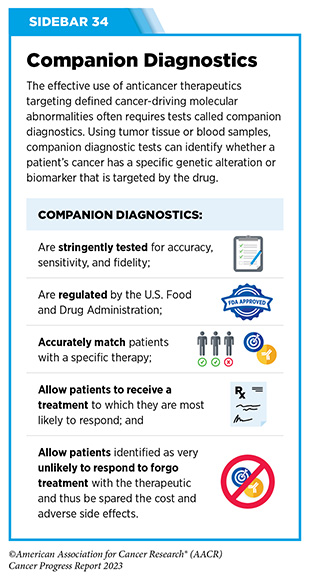
In December 2022, FDA approved a new molecularly targeted therapeutic, adagrasib (Krazati), for adult patients with locally advanced or metastatic NSCLC that has the KRAS G12C mutation, as determined by an FDA-approved test, and who have received at least one prior systemic treatment such as chemotherapy or immunotherapy. The FDA also approved companion diagnostics (see Sidebar 34), QIAGEN therascreen KRAS RGQ PCR kit (tumor tissue-based) and Agilent Resolution ctDx FIRST Assay (blood-based) to help identify patients with NSCLC carrying the KRAS G12C mutation. Both sotorasib and adagrasib bind to KRAS G12C protein and lock it in an inactive state thus blocking tumor growth.
The FDA approval of adagrasib was granted after it was shown in a phase II clinical trial that 43 percent of the patients who received the targeted therapeutic had complete or partial tumor shrinkage and continued to respond for a median of 8.5 months without their cancer progressing (384)Janne PA, et al. (2022) N Engl J Med, 387: 120. [LINK NOT AVAILABLE]. A critical finding from the clinical trial was that adagrasib was able to reach the brains of patients with NSCLC and shrink tumors that had metastasized to the brain (385)Kotecha R, et al. (2022) N Engl J Med, 387: 1238. [LINK NOT AVAILABLE]. While additional research is needed to confirm therapeutic activity in the brain, these data are extremely encouraging considering recent findings that 27 to 42 percent of patients with NSCLC whose tumors harbor the KRAS G12C mutation may have central nervous system (CNS) metastases at diagnosis, and such metastases are associated with a poor prognosis (384)Janne PA, et al. (2022) N Engl J Med, 387: 120. [LINK NOT AVAILABLE].
Another major advance against lung cancer during the 12 months covered in this report is the FDA approval of fam-trastuzumab deruxtecan-nxki (Enhertu) for adult patients with surgically unremovable or metastatic NSCLC whose tumors have a type of mutation in the human epidermal growth factor receptor 2 (HER2) gene, called an activating mutation, as detected by an FDA-approved test, and who have received a prior systemic therapy. The FDA also approved Oncomine Dx Target Test (tissue-based) and Guardant360 CDx (blood-based) as companion diagnostics to test patients for activating HER2 mutations.
HER2-mutated NSCLC, which accounts for three percent of all NSCLC cases, is associated with female sex, never-smoking history, and a poor prognosis. Furthermore, this type of NSCLC has a higher incidence of brain metastases than NSCLC without HER2 mutations or with other mutations (386)Li BT, et al. (2022) N Engl J Med, 386: 241. [LINK NOT AVAILABLE](387)Riudavets M, et al. (2021) ESMO Open, 6: 100260. [LINK NOT AVAILABLE].
Fam-trastuzumab deruxtecan-nxki is a type of molecularly targeted therapeutic called an antibody–drug conjugate. It comprises a cytotoxic agent, deruxtecan, attached to the HER2-targeted antibody, trastuzumab (Herceptin), by a linker. When the antibody attaches to HER2 protein on the surface of lung cancer cells, the antibody–drug conjugate is internalized by the cells. This leads to deruxtecan being released from the linker and the antibody. Once free, the deruxtecan is toxic to the cancer cells, which ultimately die.
The approval of fam-trastuzumab deruxtecan-nxki was primarily based on the results of a phase II clinical trial in which treatment with the HER2-targeted therapeutic shrank tumors in nearly 60 percent of the study participants (388)National Cancer Institute. Enhertu Approved for Lung Cancer. Accessed: July 5, 2023. . Among patients whose tumors shrank, the treatment kept their lung cancer at bay for nearly 9 months. While fam-trastuzumab deruxtecan-nxki has previously been approved for the treatment of patients with HER2-driven breast and gastric cancers (4,389), this was the first approval of a HER2-targeted therapeutic for NSCLC and provides new hope for patients with NSCLC who carry an activating HER2 mutation.
Like most cancer treatments, fam-trastuzumab deruxtecan-nxki can have adverse effects, some of which can be severe. One of the most concerning and, in the case of NSCLC, life threatening, is interstitial lung disease which causes stiffness in the lungs, making it difficult to breathe and get oxygen to the bloodstream. Therefore, FDA approved fam-trastuzumab deruxtecan-nxki with a warning for interstitial lung disease and recommends that patients being treated with the molecularly targeted therapeutic be monitored for signs and symptoms of interstitial lung disease, including cough, dyspnea (difficult or labored breathing), fever and other new or worsening respiratory symptoms. If interstitial lung disease is suspected, further testing and intervention must be considered.
While FDA approvals of sotorasib, adagrasib and fam-trastuzumab deruxtecan-nxki are significant advances for patients with NSCLC, all stakeholders in public health need to work together to ensure that every patient has access to and benefits from the latest developments in precision cancer medicine. Patients with lung cancer who receive molecularly targeted therapies have better survival compared to those who do not receive targeted therapies (390)Goulart BHL, et al. (2021) Clin Lung Cancer, 22: e723. [LINK NOT AVAILABLE](391)Lemmon CA, et al. (2023) JCO Precis Oncol, 7: e2200294. [LINK NOT AVAILABLE]. Unfortunately, according to recent data, many patients with advanced NSCLC do not receive appropriate molecular tests or the appropriate molecularly targeted treatments due to gaps in the delivery of clinical care (392)Osazuwa-Peters OL, et al. (2023) Clin Lung Cancer, 24: 305. [LINK NOT AVAILABLE](393)Sadik H, et al. (2022) JCO Precis Oncol, 6: e2200246. [LINK NOT AVAILABLE].
Targeting Cancers Based on a Common Genetic Feature, Not Tissue of Origin
Chromosomal translocations that involve the RET gene and lead to the production of activating RET fusion proteins (see Sidebar 7) are rare alterations observed mostly in patients with certain types of thyroid cancer and lung cancer (394)Duke ES, et al. (2023) Clin Cancer Res: OF1. [LINK NOT AVAILABLE]. In 2020, FDA approved the RET-targeted therapeutic, selpercatinib (Retevmo), for treating patients with metastatic NSCLC and certain thyroid cancers that test positive for chromosomal translocations involving the RET gene (389)Sengupta R, et al. (2020) Clin Cancer Res, 26: 5055. [LINK NOT AVAILABLE].
In solid tumors other than lung cancer and thyroid cancer, RET gene fusions are rarer, observed in less than one percent of patients (394)Duke ES, et al. (2023) Clin Cancer Res: OF1. [LINK NOT AVAILABLE]. However, this is a distinct patient population since RET gene fusions are mutually exclusive of other genetic alterations and provide a unique opportunity for therapeutic intervention (394)Duke ES, et al. (2023) Clin Cancer Res: OF1. [LINK NOT AVAILABLE]. A recent decision from FDA offers a new treatment option for these patients who until this approval had no molecularly targeted therapeutics available for their cancer.
In September 2022, FDA expanded the approval of selpercatinib for the treatment of adult patients with locally advanced or metastatic solid tumors with a RET gene fusion that have progressed on or following prior systemic treatment or who have no satisfactory alternative treatment options. The approval of selpercatinib was based on the results of a phase I/II basket clinical trial (see Figure 14) in which treatment with the RET-targeted therapeutic shrank tumors in nearly 44 percent of the study participants (395)Subbiah V, et al. (2022) Lancet Oncol, 23: 1261. [LINK NOT AVAILABLE]. Patients with a range of cancer types including pancreatic adenocarcinoma, colorectal, salivary gland, unknown primary, breast, soft tissue sarcoma, bronchial carcinoid, ovarian, small intestine, and cholangiocarcinoma responded to selpercatinib, emphasizing the importance of basket clinical trial designs in driving progress in precision medicine.
Delivering a Cytotoxic Drug Precisely to Ovarian Cancer Cells
In 2023, an estimated 19,710 new cases of ovarian cancer will be diagnosed in the United States, and 13,270 women will die from the disease (28)American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . Many patients with ovarian cancer are diagnosed at an advanced stage. Patients with advanced disease are usually treated with platinum-based chemotherapeutics. Although most patients respond initially to platinum-based treatment, nearly 80 percent will experience relapse. Unfortunately, patients with recurrent ovarian cancer are resistant to platinum-based treatments and have a poor prognosis.
Folate receptor alpha (FRα) is a cell surface protein that binds to and transports folate (also known as vitamin B9) into cells. Research has shown that FRα is expressed at much higher levels in advanced ovarian cancer cells, compared to healthy adult tissues (396)Dilawari A, et al. (2023) Clin Cancer Res, OF1. [LINK NOT AVAILABLE]. There is also emerging evidence, including clinical data, that elevated FRα expression may be associated with lack of response to standard chemotherapy in ovarian cancer (397)Matulonis UA, et al. (2023) J Clin Oncol, 41: 2436. [LINK NOT AVAILABLE]. These attributes make FRα a promising target for therapeutic intervention in ovarian cancer.
The molecularly targeted therapeutic mirvetuximab soravtansine-gynx (Elahere) targets FRα and, in November 2022, received FDA approval for adult patients with FRα positive, platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer, who have received one to three prior systemic treatment regimens. FDA also approved the companion diagnostic VENTANA FOLR1 RxDx Assay to identify patients eligible for the therapy.
Mirvetuximab soravtansine-gynx is an antibody–drug conjugate designed to deliver a cytotoxic drug to cells that express FRα. It is the first antibody–drug conjugate to be approved by FDA to treat platinum-resistant ovarian cancer and marks the first FDA approval since 2014 for platinum chemotherapy-resistant ovarian cancer, which is associated with a poor prognosis. The approval was based on results from a phase III clinical trial that enrolled 106 patients. Nearly 32 percent of patients responded to mirvetuximab soravtansine-gynx, with a median duration of response of 6.9 months (396)Dilawari A, et al. (2023) Clin Cancer Res, OF1. [LINK NOT AVAILABLE](397)Matulonis UA, et al. (2023) J Clin Oncol, 41: 2436. [LINK NOT AVAILABLE]. The approval of mirvetuximab soravtansine-gynx is great news for patients, such as Jaclyn (Jackie) VanRaaphorst. There is preliminary evidence that mirvetuximab soravtansine-gynx also improves overall survival for this FRα-positive ovarian cancer patient population (398)Angelergues A, et al. (2023) Journal of Clinical Oncology, 41: LBA5507. [LINK NOT AVAILABLE].
A common adverse effect of mirvetuximab soravtansine-gynx is ocular toxicity—changes that affect the structure or function of the eye. Therefore, FDA approved mirvetuximab soravtansine-gynx with a warning that patients being treated with the molecularly targeted therapeutic be monitored and treated for signs and symptoms of vision impairment and corneal disorders.
Improving Outcomes for Patients with Metastatic Breast Cancer
Despite major advances in the treatment of breast cancer, it remains the second leading cause of cancer-related death for women in the United States ((28)American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . Recent FDA decisions have the potential to power more progress against breast cancer because they have provided new therapeutic options for certain patients with the disease.
For patients with breast cancer, one factor determining what treatment options should be considered is the presence or absence of three tumor biomarkers, estrogen and progesterone hormone receptors, which drive tumor growth upon engagement with their respective hormones, and the protein HER2. About 70 percent of breast cancers diagnosed in the United States are characterized as hormone receptor–positive and HER2-negative (3)Giaquinto AN, et al. (2022) CA Cancer J Clin, 72: 524. [LINK NOT AVAILABLE]. Potential treatment options for these patients include the combination of an antihormone therapeutic such as tamoxifen, which works by preventing the hormone estrogen from attaching to its receptor; or letrozole, which works by lowering the level of estrogen in the body; or fulvestrant, which works by destroying estrogen receptors (ER) with a cyclin-dependent kinase 4/6 inhibitor. Treatment with antihormone therapeutics is also called endocrine therapy.
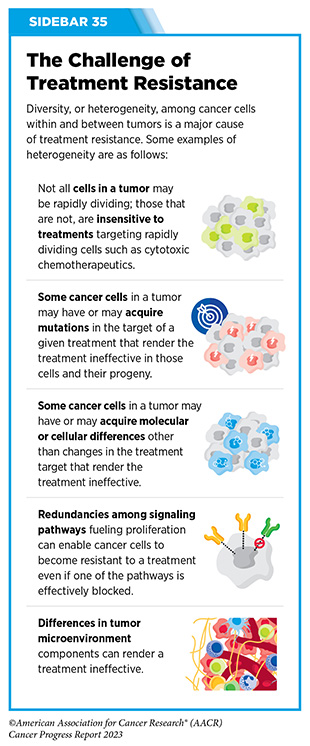
Unfortunately, most advanced, hormone receptor-positive breast cancers that initially respond to endocrine therapy eventually become treatment resistant (see Sidebar 35). Resistance to fulvestrant commonly develops due to mutations in ESR1, the gene that encodes the ER protein. Until recently, fulvestrant was the only available FDA-approved treatment that worked by destroying ER. Therefore, patients whose tumors become resistant to it were left with limited treatment options.
The FDA approval of elacestrant (Orserdu) in January 2023 brings new hope to these patients. Elacestrant, which also works by destroying the ER, was approved for postmenopausal women or adult men with ER-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer with disease progression following at least one line of endocrine therapy. Unlike fulvestrant, which is delivered through intramuscular injections, elacestrant is administered orally, making it more convenient for patients to receive the treatment. The approval was based on results from a phase III, randomized clinical trial showing that among patients with ESR1 mutations, those treated with elacestrant had a 45 percent lower risk of death or disease progression than those treated with other endocrine therapies (399)Bidard FC, et al. (2022) J Clin Oncol, 40: 3246. [LINK NOT AVAILABLE].
Personalizing Treatment for Patients with a Rare Solid Tumor
Rare cancer is defined by the National Cancer Institute as cancer that occurs in fewer than 15 out of 100,000 people each year. Rare cancers can be challenging for researchers to study and for physicians to treat (see Sidebar 36). During the 12 months covered by this report, August 1, 2022, to July 31, 2023, the FDA approved molecularly targeted therapeutics and immunotherapeutics for treating several rare cancers, bringing the promise of precision medicine to patients, such as Isabella (Bella) Snow Fraser, p. 110, and Alexis Browning, p. 112, who often have few treatment options.
Bile duct cancer, also known as cholangiocarcinoma, is a rare but aggressive disease in which cancer arises from cells in the bile ducts. Cholangiocarcinoma is often diagnosed at an advanced stage. There are two types of bile duct cancer: intrahepatic, where cancer forms in the bile ducts inside the liver; and extrahepatic, where cancer forms in the bile ducts outside the liver. Less than 8,000 new cases of bile duct cancer are estimated to be diagnosed in the United States in 2023 and only a small number of bile duct cancers are intrahepatic (28)American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . While rare, the incidence of intrahepatic cholangiocarcinoma is increasing worldwide (400)Goyal L, et al. (2023) N Engl J Med, 388: 228. [LINK NOT AVAILABLE]. Surgery is the main curative treatment option for patients with intrahepatic cholangiocarcinoma. However, up to two thirds of patients have disease recurrence and patients with intrahepatic cholangiocarcinoma have a 5-year overall survival rate of less than eight percent (400)Goyal L, et al. (2023) N Engl J Med, 388: 228. [LINK NOT AVAILABLE].
Alterations in fibroblast growth factor receptor 2 (FGFR2), a protein involved in many cellular processes including multiplication, migration, and survival, are associated with several cancers including bile duct cancers. Nearly 14 percent of patients with intrahepatic cholangiocarcinoma have fusions or rearrangements in the FGFR2 gene (see Sidebar 7) (400)Goyal L, et al. (2023) N Engl J Med, 388: 228. [LINK NOT AVAILABLE]. FDA had previously approved two molecularly targeted therapeutics, pemigatinib (Pemazyre) and infigratinib (Truseltiq), which block the function of FGFR2 proteins, for the treatment of patients with cholangiocarcinoma with confirmed FGFR2 fusions or rearrangements (389)Sengupta R, et al. (2020) Clin Cancer Res, 26: 5055. [LINK NOT AVAILABLE](401)Sengupta R, et al. (2021) Clin Cancer Res, 27: 5757. [LINK NOT AVAILABLE]. While these agents are benefiting many patients with bile duct cancer, their efficacy has been somewhat limited due to the development of treatment resistance (400)Goyal L, et al. (2023) N Engl J Med, 388: 228. [LINK NOT AVAILABLE].
In September 2022, the FDA granted approval to a third FGFR2-targeted therapeutic, futibatinib (Lytgobi) for adult patients with previously treated, unresectable, locally advanced or metastatic intrahepatic cholangiocarcinoma that tests positive for FGFR2 fusions or other rearrangements. The approval was based on the results of a phase I/II clinical trial that showed that futibatinib shrank tumors in 42 percent of patients (400)Goyal L, et al. (2023) N Engl J Med, 388: 228. [LINK NOT AVAILABLE]. The median duration of response was 9.7 months. Futibatinib works differently than pemigatinib and infigratinib and preliminary data indicate that it may mitigate the challenge of treatment resistance since patients who had disease progression after prior FGFR-targeted therapy with other inhibitors maintained sustained clinical benefit with futibatinib (400)Goyal L, et al. (2023) N Engl J Med, 388: 228. [LINK NOT AVAILABLE].
Combining Molecularly Targeted Therapeutics to Block Tumor Growth
The BRAF enzyme has a critical role in controlling cell growth. The BRAF gene is altered in approximately six percent of all human cancers, including melanoma and colorectal cancer (402)Dankner M, et al. (2018) Oncogene, 37: 3183. [LINK NOT AVAILABLE]. Most cancer-related changes in the BRAF gene cause the protein to continuously stay active, thus helping cancer cells grow faster than normal cells. One of the most common cancer-related changes is the BRAF gene is called the BRAF V600E mutation. Presence of the BRAF V600E mutation is associated with poor outcomes for patients with certain types of cancer.
The first time FDA approved the use of two molecularly targeted therapeutics as a combination treatment for cancer was in January 2014 (67)Arteaga CL, et al. (2014) Clin Cancer Res, 20: S1. [LINK NOT AVAILABLE]. The approval was for the use of dabrafenib (Tafinlar) and trametinib (Mekinist) for treating patients with metastatic melanoma that tests positive for activating BRAF V600E and BRAF V600K mutations. The two therapeutics target different components of the BRAF signaling pathway. Dabrafenib targets altered BRAF proteins containing V600 mutations, while trametinib targets MEK1 and MEK2, which are two proteins that mediate the function of BRAF. By blocking both BRAF and MEK, the combination therapy can more completely and effectively shut down the signaling pathway. The combination was approved after it was shown to almost double the length of time before disease progression compared with dabrafenib alone (403)Flaherty KT, et al. (2012) N Engl J Med, 367: 1694. [LINK NOT AVAILABLE].
In March 2023, the same combination of molecularly targeted therapeutics was approved for pediatric patients one year of age and older with low-grade glioma with a BRAF V600E mutation who require systemic therapy. The FDA also approved new oral formulations of dabrafenib and trametinib for pediatric patients who are unable to swallow pills.
Brain and other nervous system tumors are the second most diagnosed cancers in children. Low-grade glioma is the most common type of pediatric brain cancer. Research has demonstrated that BRAF signaling pathway activation is common in pediatric low-grade gliomas. Therefore, the March approval of dabrafenib and trametinib combination therapy brings hope to many parents and families whose children are diagnosed with the disease. FDA approved the combination therapy based on data from a clinical trial showing that a significantly higher percentage of patients who received dabrafenib and trametinib had their tumors shrink compared to those who received the standard of care chemotherapy (47 percent vs. 11 percent, respectively) (404)Hargrave DR, et al. (2022) J Clin Oncol, 40: 2009. [LINK NOT AVAILABLE]. Patients treated with dabrafenib and trametinib also had a 69 percent lower risk of disease progression, with a progression-free survival of 20 months, compared to seven months among patients receiving chemotherapy (404)Hargrave DR, et al. (2022) J Clin Oncol, 40: 2009. [LINK NOT AVAILABLE].
The FDA approval of a second combination therapy during the 12 months covered in the report provides a new and first of a kind treatment option for certain patients with colorectal cancer. The combination of tucatinib (Tukysa) and trastuzumab (Herceptin), both HER2-targeted therapeutics, was approved by FDA in January 2023 for patients with HER2-positive unresectable or metastatic colorectal cancer that has progressed following at least two standard treatments, including chemotherapy. To be eligible to receive the new combination, patients’ tumors must also not have driver mutations in the RAS group of genes.
Colorectal cancer is the second most common cause of cancer death in the United States. An estimated 153,020 people are expected to be diagnosed with colorectal cancer in the United States in 2023 (28)American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . Excessive production of the HER2 protein which leads to tumor cell multiplication, invasion, and metastasis is found in approximately three to five percent of patients with metastatic colorectal cancers (405)Ahcene Djaballah S, et al. (2022) Am Soc Clin Oncol Educ Book, 42: 1. [LINK NOT AVAILABLE] such as that of Brian Beck. The approval of the tucatinib and trastuzumab combination for this patient population was based on a phase II clinical trial which showed that 38 percent of patients who received the drug combination had their tumors shrink or disappear (406)Strickler JH, et al. (2023) Lancet Oncol, 24: 496. [LINK NOT AVAILABLE].
Considering that prior treatment options for patients with HER2-positive colorectal cancer that has returned or started growing again after receiving standard treatments were not very effective, the approval of tucatinib and trastuzumab represents a significant breakthrough for this subset of patients with metastatic colorectal cancer. Ongoing studies are evaluating whether addition of tucatinib and trastuzumab to standard treatment regimens could be used earlier on as the initial treatment for metastatic HER2-positive colorectal cancer.
Adding Precision to the Treatment of Blood Cancers
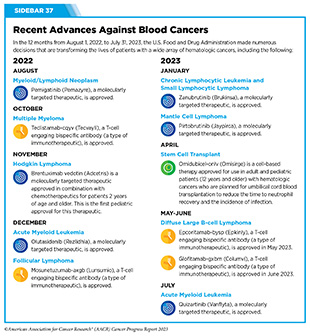
Cancers that arise in blood-forming tissues, such as the bone marrow, or in cells of the immune system, are called blood cancers, or hematologic cancers. In the 12 months covered by this report, FDA has made numerous decisions that are transforming the lives of patients with a wide array of hematologic cancers (see Sidebar 37).
Acute myeloid leukemia (AML) is the most commonly diagnosed leukemia in the United States, with 20,380 new cases anticipated in 2023 (28)American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . AML has only 32 percent overall 5-year relative survival rate, the lowest among leukemias (5)Surveillance, Epidemiology, and End Results (SEER) Program. Accessed: July 5, 2023. . Research has substantially increased our understanding of the biology of AML, in particular the different types of genetic mutations that promote AML development. This knowledge is fueling the emergence of molecularly targeted therapeutics for defined groups of patients with the disease.
One of the genes known to be mutated in about seven to 14 percent of AML cases is IDH1 (407)de Botton S, et al. (2023) Blood Adv, 7: 3117. [LINK NOT AVAILABLE]. Mutation in IDHI gene results in an altered IDH1 protein, which can drive cancer formation by interfering with normal cellular maturation and promote uncontrolled cell multiplication. This knowledge led to the development of ivosidenib (Tibsovo), the first therapeutic to target IDH1, which was approved by FDA in 2018.
In December 2022, FDA approved a second IDH1-targeted agent, olutasidenib (Rezlidhia), for adult patients with AML that has not responded to or has relapsed after other treatments, and that harbors an IDH1 mutation as detected by an FDA-approved test. At the same time that the molecularly targeted therapeutic was approved, FDA also approved the companion diagnostic, Abbott RealTime IDH1 Assay, to identify patients with AML with an IDH1 mutation.
Olutasidenib was approved for the treatment of AML after it was shown in a phase I/II clinical trial that 32 percent of patients treated with the molecularly targeted therapeutic had complete remission, meaning that there was no evidence of disease and full recovery of blood counts (407)de Botton S, et al. (2023) Blood Adv, 7: 3117. [LINK NOT AVAILABLE]. Not only does the approval of olutasidenib increase treatment options for patients with IDH1-mutated AML, but there is also preliminary evidence that patients may respond longer to olutasidenib compared to the other IDHI-targeted therapy (407)de Botton S, et al. (2023) Blood Adv, 7: 3117. [LINK NOT AVAILABLE]. A potential side effect observed among patients treated with olutasidenib is differentiation syndrome. The condition is caused by a large, rapid release of immune molecules called cytokines from leukemia cells and can lead to fever, cough, troubled breathing, build-up of excess fluid around the heart and lungs, low blood pressure, and kidney failure, but is generally readily treated with full resolution. The FDA approval is accompanied by a warning highlighting the risk of this potentially fatal adverse effect.
In July 2023, the FDA approved a second new molecularly targeted therapeutic, quizartinib (Vanflyta), for the treatment of AML. Quizartinib was approved for treating adults who have newly diagnosed AML that tests positive for a mutated FLT3 gene known as FLT3 internal tandem duplication (ITD). Mutations in the FLT3 gene promote the multiplication and survival of AML cells in 25 to 30 percent of cases, and patients with this type of AML have particularly poor outcomes (408)Yanada M, et al. (2005) Leukemia, 19: 1345. [LINK NOT AVAILABLE]. The approval was based on results from a phase III clinical trial showing that patients who received quizartinib had a 22 percent reduced risk of death compared to those who received standard chemotherapy during the course of the clinical study (409)Erba HP, et al. (2023) Lancet, 401: 1571. [LINK NOT AVAILABLE]. Quizartinib can cause several cardiac adverse effects and is therefore available only through a restricted program.
At the same time that the FDA made the decision about quizartinib, it expanded the use of the LeukoStrat CDx FLT3 Mutation Assay as a companion diagnostic to identify patients with FLT3 ITD mutation–positive AML who are eligible for treatment with the new molecularly targeted therapeutic.
Myeloid/lymphoid neoplasm (MLN) with fibroblast growth factor receptor 1 (FGFR1) rearrangement is a rare, aggressive disease characterized by higher-than-normal levels of certain white blood cells. MLNs do not respond well to standard chemotherapy and can rapidly progress to AML (410)Verstovsek S, et al. (2018) Ann Oncol, 29: 1880. [LINK NOT AVAILABLE]. FGFR1 is a cell surface protein that stimulates cellular proliferation upon binding with specific extracellular molecules. In rare instances, the FGFR1 gene fuses with another gene (an alteration known as a genetic rearrangement) resulting in a fusion protein that drives the development of MLNs.
Pemigatinib (Pemazyre) inhibits the function of FGFR1 to suppress the growth of FGFR1-driven cancers (410)Verstovsek S, et al. (2018) Ann Oncol, 29: 1880. [LINK NOT AVAILABLE] and in August 2022, it was approved by FDA for adults with MLNs with FGFR1 rearrangement who have not responded to or have relapsed after other treatments. The approval was based on results from a phase II clinical trial that showed that 79 percent of patients had a complete response to pemigatinib. Therefore, the FDA approval of pemigatinib for adult patients with relapsed or refractory MLNs with FGFR1 alteration is a major milestone for the treatment of patients who are diagnosed with the disease.
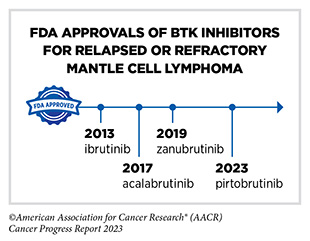
Non-Hodgkin lymphoma (NHL) is the most commonly diagnosed blood cancer in the United States. In 2023, 77,240 people in the United States are expected to be newly diagnosed with the disease (28)American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . Notably, NHL encompasses many different types of cancer, most of which arise in immune cells called B cells. Two molecularly targeted therapeutics, recently approved by FDA for treating different subtypes of NHL arising in B cells— pirtobrutinib (Jaypirca) and zanubrutinib (Brukinsa)—target a protein called Bruton tyrosine kinase (BTK). BTK was first identified in 1993. Since its discovery, the role of BTK has been studied extensively in blood cancers and inflammatory diseases. Researchers have found that BTK is a key component of a signaling pathway that promotes the survival and expansion of NHL B cells. Consequently, BTK inhibitors have revolutionized the treatment of NHL arising in B-cells, particularly chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) and mantle cell lymphoma (MCL) as well as certain inflammatory diseases (411)Alu A, et al. (2022) J Hematol Oncol, 15: 138. [LINK NOT AVAILABLE].
Ibrutinib was the first BTK inhibitor approved by FDA. The approval, in 2013, was for the treatment of patients with relapsed and refractory mantle cell lymphoma (MCL). While the approval of ibrutinib was a major milestone in personalized treatment for B-cell cancers, researchers soon discovered that in patients on continuous treatment with BTK-targeted therapy, cancer cells can acquire mutations in the BTK gene that render the therapeutic ineffective. Since then, newer and more sophisticated BTK inhibitors with improved specificities and thus reduced toxicities have been developed to mitigate the challenge of acquired resistance (see Sidebar 35).
Pirtobrutinib (Jaypirca) is a new BTK targeted therapeutic that FDA approved in January 2023 for treating MCL. The agent was approved for patients with relapsed or refractory MCL that has not responded to or has relapsed after another treatment, including a BTK inhibitor. Pirtobrutinib has a unique mechanism of action that makes it effective even against mutated forms of BTK that are resistant to other BTK-targeted therapeutics (412)Zhang J, et al. (2022) Biomark Res, 10: 17. [LINK NOT AVAILABLE]. The approval of pirtobrutinib was based on results from a phase I/II clinical trial, which showed that 50 percent of MCL patients treated with the molecularly targeted therapeutic had tumor shrinkage, with 13 percent having their tumors disappear.
Zanubrutinib, another BTK-targeted therapeutic, was approved for treating patients with MCL in November 2019 (413)Sengupta R, et al. (2020) Cancer Epidemiol Biomarkers Prev, 29: 1843. [LINK NOT AVAILABLE]. In January 2023, FDA approved zanubrutinib for treating adults who have CLL or SLL, which are slow-growing types of NHL. CLL and SLL are essentially the same disease but have different names depending on where in the body the NHL cells accumulate. CLL cells are found mostly in the blood and bone marrow, whereas SLL cells are found mostly in the lymph nodes.
The approval of zanubrutinib to treat CLL and SLL was based on results from two phase III clinical trials. The first trial which evaluated the efficacy of zanubrutinib in previously untreated patients with CLL/SLL showed that patients who received zanubrutinib lived a longer time without their cancer worsening compared with patients who received standard treatments. In the second trial, which compared zanubrutinib to ibrutinib in CLL/SLL patients whose disease did not respond to or came back after prior treatments, a greater percentage of patients receiving zanubrutinib were alive during the course of the study with no growth of their cancer, compared to patients taking ibrutinib (414)Brown JR, et al. (2023) N Engl J Med, 388: 319. [LINK NOT AVAILABLE].
Blocking Progression of Metastatic Prostate Cancers
Prostate cancer is the most commonly diagnosed cancer among men living in the United States. In 2023 alone, more than 288,000 men are expected to be diagnosed with the disease (28)American Cancer Society. Cancer Facts and Figures. Accessed: July 5, 2023. . Research has shown that up to 30 percent of prostate cancers have mutations in genes that influence the homologous recombination repair (HRR) pathway (e.g., BRCA, ATM), a cellular process in which a group of proteins work together to repair DNA damage (415)de Bono J, et al. (2020) N Engl J Med, 382: 2091. [LINK NOT AVAILABLE]. Changes in the HRR pathway may result in the inability to repair DNA and lead to accumulation of mutations and cancer.
Poly-ADP ribose polymerase (PARP) proteins are central to a second type of DNA repair pathway called base excision repair. Researchers have found that breast, ovarian, pancreatic, and prostate cancers with genetic mutations that lead to HRR deficiency are responsive to PARP-targeted therapeutics because disruption of these two DNA repair pathways leads to pervasive DNA damage that kills cancer cells. In July 2023, FDA approved a PARP-targeted therapeutic, talazoparib (Talzenna) for treating certain groups of men with metastatic prostate cancer carrying mutations in genes that influence the homologous recombination DNA repair pathway.
Men, such as Colbert English, p. 96, who are diagnosed with metastatic prostate cancer are often treated initially with therapeutics that target androgens, the hormones that fuel prostate cancer growth. When the cancer stops responding to these treatments, it is referred to as castration-resistant prostate cancer. Talazoparib was approved in combination with the androgen-targeted therapeutic enzalutamide (Xtandi) for patients with HRR gene-mutated metastatic castration-resistant prostate cancer. Mutations in HRR genes such as BRCA1, BRCA2, and ATM were assessed prospectively using tumor tissue and/or blood-based DNA sequencing assays. The approval was based on results from a phase III clinical trial that showed that treatment with talazoparib significantly improved progression-free survival compared with treatment with enzalutamide alone (416)Agarwal N, et al. (2023) Lancet, 402: 291. [LINK NOT AVAILABLE].
Next Section: Spotlight on Immunotherapy Previous Section: Screening for Early Detection