- What Is Cancer Screening and How Is It Done?
- Recent Advances in Cancer Screening
- Detecting Early Signs of Multiple Types of Cancer from a Single Minimally Invasive Screening Test
- Enhancing the Speed and Accuracy of Interpreting Screening Tests
- Cancer Screening Guidelines
- Who Should Get Screened and When?
- Suboptimal Use of Cancer Screening Tests
Screening for Early Detection
In this section, you will learn:
- Breakthroughs in understanding how cancer develops and progresses are facilitating the development of cancer screening tests that can detect cancer at its earliest stage before it has spread to other sites.
- Professional organizations and government-affiliated agencies carefully evaluate the benefits and harms of cancer screening to make evidence-based recommendations for its use in the clinic.
- Technological advances, such as state-of-the-art DNA sequencing methods, minimally invasive biopsies, artificial intelligence, and cutting-edge imaging are poised to transform early detection in the coming years.
- There are substantial opportunities to save lives by developing evidence-based early detection of cancer types with high mortality rates, such as cancers of pancreas and liver, for which there are currently no screening tests available for the average risk population.
- The COVID-19 pandemic led to significant declines in cancer screening uptake, and ongoing research will be needed to determine the long-term effects of such decline on future cancer outcomes.
Since the signing of the National Cancer Act in 1971, researchers have made significant strides in decoding the underlying causes of cancer development (see Understanding How Cancer Develops). In parallel, technological innovations in DNA sequencing and cellular imaging approaches have enabled reliable and reproducible detection of the genetic, molecular, and cellular events that drive cancer initiation and progression. Collectively, these advances have accelerated the development of screening tests and examinations that can find aberrations before the cancer arises or can identify cancers at an early stage of development.
What Is Cancer Screening and How Is It Done?
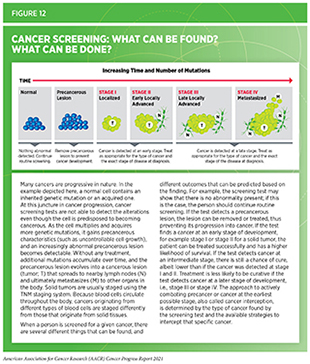
Cancer screening is the evidence-based determination of whether a person has precancerous lesions or cancer before any of its signs or symptoms appear. The key objective is to find an aberration at the earliest possible time during cancer development. Early detection can help health care providers make an informed decision on whether to monitor, treat, or surgically remove precancerous lesions and early-stage cancer before it either progresses to a more advanced stage (see Figure 12).
There are different kinds of cancer screening tests and exams that include visual examination to check for unusual features such as lumps or discolored skin; medical and family history analyses to review an individual’s genetic, behavioral, and environmental risks; laboratory tests to determine the changes in cancer biomarkers in samples of tissues or fluids in the body; and imaging procedures to look for abnormalities inside the body (see sidebar on How Do We Screen for Cancer?).
Recent Advances in Cancer Screening
Screening methods are medical procedures that carry the potential of some harm (see sidebar on Benefits and Potential Harms of Cancer Screening). Thus, a key area of research focus has been to develop screening methods that are minimally invasive, such as liquid biopsies, thereby further reducing the potential of any harm to the person being screened (see Figure 13). Minimally invasive screening tests can potentially increase compliance among individuals who are eligible but forgo recommended cancer screening because of anxiety and/or cultural stigma associated with some screening tests. Additionally, researchers are also investigating whether artificial intelligence (AI), (see Artificial Intelligence: Shaping the Future of Cancer Science and Medicine) which uses machine learning to analyze vast amounts of data and recognize patterns that are otherwise time-consuming and difficult to find, can be harnessed to increase the speed and accuracy of interpreting results from screening tests.
Detecting Early Signs of Multiple Types of Cancer from a Single Minimally Invasive Screening Test
One area of extensive research in the field of cancer screening is the use of blood-based tests, or liquid biopsy tests, to screen for multiple types of cancer at the same time. There is also emerging data that the multicancer early detection (MCED) approach is feasible and can be used for population-based cancer screening in the clinic (344)Liu MC, Oxnard GR, Klein EA, et al. Sensitive and specific multi-cancer detection and localization using methylation signatures in cell-free DNA. Ann Oncol. 2020;31(6):745-759.. MCED tests can detect multiple cancer types by examining specific genetic mutations, epigenetic changes, or other cancer-specific characteristics in the circulating DNA and proteins shed by tumor cells in blood samples. These tests are being investigated for their ability to accurately detect cancers early and to determine whether early detection through these tests can reduce mortality from the cancer for which a person is being screened (345)Liu MC. Transforming the landscape of early cancer detection using blood tests—Commentary on current methodologies and future prospects. Br J Cancer. Published online 2021.. As one example, a recent study found that an MCED test was able to detect signs of cancer across 50 different cancer types with very high specificity (343)Klein EA, Richards D, Cohn A, et al. Clinical validation of a targeted methylation-based multi-cancer early detection test using an independent validation set. Ann Oncol. 2021;0(0).. Researchers estimate that actively treating precancerous lesions and/or early-stage cancers detected by this MCED test may help reduce incidence of late-stage cancer development by 78 percent, resulting in a 26 percent decrease in all cancer-related deaths (346)Hubbell E, Clarke CA, Aravanis AM, Berg CD. Modeled reductions in late-stage cancer with a multi-cancer early detection test. Cancer Epidemiol Biomarkers Prev. 2021;30(3)..
Researchers involved in the development of a second MCED test performed a retrospective study of women with breast cancer and found that adding the MCED test to the routine breast cancer screening by mammography could have identified 11 percent of women who developed breast cancer that was not captured by routine mammography (347)Hathaway C, Paetsch P, Li Y, et al. Association of Breast Cancer Screening Behaviors With Stage at Breast Cancer Diagnosis and Potential for Additive Multi-Cancer Detection via Liquid Biopsy Screening: A Claims-Based Study. Front Oncol. 2021;11.. These studies highlight the enormous promise of liquid biopsies in screening for multiple cancer types in a minimally invasive manner (345)Liu MC. Transforming the landscape of early cancer detection using blood tests—Commentary on current methodologies and future prospects. Br J Cancer. Published online 2021..
Additional research is ongoing to ensure that these approaches are safe and effective for routine cancer screening in the clinic, and to evaluate their impact on overall survival following early detection of cancers (348)Keller L, Belloum Y, Wikman H, Pantel K. Clinical relevance of blood-based ctDNA analysis: mutation detection and beyond. Br J Cancer. 2021;124(2).. One area that requires further investigation is the validation of reliable predictive biomarkers and the development of minimally invasive tests to establish evidence-based screening approaches for early detection of cancer types, such as ovarian and pancreatic cancers for which there are currently no available screening tests for average-risk individuals.
Enhancing the Speed and Accuracy of Interpreting Screening Tests
Different cancer screening tests yield different types of results, some in the form of sequencing data and others as pathology or radiology images. Interpretation of cancer screening test results requires a careful analysis by highly trained health care professionals who include primary health care providers, oncologists, pathologists, and radiologists to ensure an accurate determination of next steps. These deliberate safeguards are in place to maximize benefits of cancer screening while minimizing potential harms (see sidebar on Benefits and Potential Harms of Cancer Screening). However, the process is time-consuming and can sometimes miss signs of cancer.
In recent years, multidisciplinary teams of scientists have been investigating the potential of AI-based approaches in enhancing the accuracy of cancer screening, while simultaneously reducing the time it takes to interpret test results. There are exciting new advances in using AI to improve the accuracy and/or speed of screening for breast, prostate, and lung cancers (349)Svoboda E. Artificial intelligence is improving the detection of lung cancer. Nature. 2020;587(7834).(350)Van Booven DJ, Kuchakulla M, Pai R, et al. A systematic review of artificial intelligence in prostate cancer. Res Reports Urol. 2021;13.(351)Pacilè S, Lopez J, Chone P, Bertinotti T, Grouin JM, Fillard P. Improving Breast Cancer Detection Accuracy of Mammography with the Concurrent Use of an Artificial Intelligence Tool. Radiol Artif Intell. 2020;2(6).. Progress towards routine use of AI in the clinic for cancer screening is underscored by a recent FDA approval of the GI Genius, a medical device that uses AI-based software to assist clinicians in identifying polyps or precancerous lesions during colonoscopy that may not be detectable otherwise (352)The Food and Drug Administration. FDA News Release. FDA authorizes marketing of first device that uses artificial intelligence to help detect potential signs of colon cancer. 2021 Apr 9.. Beyond screening for early cancer detection, researchers are exploring the potential of AI in analyzing large genomic and epigenomic datasets to determine whether a specific pattern of genomic and/or epigenomic changes can predict a specific cancer stage, resolving the structure of proteins that are altered in cancer to find regions of the molecule that can be therapeutically targeted, identifying more effective drugs against cancer-specific targets, and improving the accuracy of cancer diagnosis (353)Dias R, Torkamani A. Artificial intelligence in clinical and genomic diagnostics. Genome Med. 2019;11(1).(354)Callaway E. “It will change everything”: DeepMind’s AI makes gigantic leap in solving protein structures. Nature 2020;588:203–4.(355)Paul D, Sanap G, Shenoy S, Kalyane D, Kalia K, Tekade RK. Artificial intelligence in drug discovery and development. Drug Discov Today. 2021;26(1).(356)Savage N. How AI is improving cancer diagnostics. Nature. 2020;579(7800). (see Looking to the Future).
Cancer Screening Guidelines
Cancer screening has the potential to save lives by detecting cancer early when it is easier to treat and chances of survival are the highest. As an example, a recent study evaluated the benefit of breast cancer screening over a period of 20 years in more than 150,000 women between the ages of 35 and 64 who had no family or personal history of breast cancer. The results showed a nearly 30 percent relative reduction in breast cancer mortality among women age 50 and older who underwent routine mammography, compared to those who did not (357)Mittra I, Mishra GA, DIkshit RP, et al. Effect of screening by clinical breast examination on breast cancer incidence and mortality after 20 years: Prospective, cluster randomised controlled trial in Mumbai. BMJ. 2021;372.. However, it is important to note that some screening tests are invasive medical procedures that can potentially cause harm (see sidebar on Benefits and Potential Harms of Cancer Screening). Because of the potential harms, the risks and benefits of cancer screening are carefully considered for each individual.
Guidelines for cancer screening help individuals decide whether they should be screened for cancer, at what age they should start screening, how frequently they should get screened, and by which method. In the United States, an independent group of experts convened by the Agency for Healthcare Research and Quality of U.S. Department of Health and Human Services evaluates data regarding the benefits and potential harms of different approaches to disease prevention, including cancer screening tests, genetic testing, and preventive therapeutics, to make evidence-based recommendations about the use of these in the clinic. These volunteer experts form U.S. Preventive Services Task Force (USPSTF). (see Figure 14)
USPSTF recommendations on cancer screening tests fall into several categories, including recommendations for screening certain individuals at certain intervals, recommendations against screening, and deciding that there is insufficient evidence to make a recommendation. In addition to considering evidence regarding potential new screening programs, USPSTF reevaluates existing recommendations as new research becomes available and can revise the recommendations if necessary (see Figure 14).
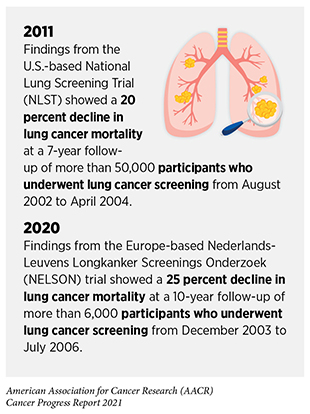
As an example, USPSTF reviewed 223 recent studies that analyzed data from more than 86,000 participants before updating its prior guidelines for lung cancer screening in 2021. The new guidelines recommend that former or current smokers start screening annually for lung cancer at an earlier age (50 instead of 55 years) (see sidebar on Consensus Cancer Screening Recommendations)(360)Krist AH, Davidson KW, Mangione CM, et al. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA – J Am Med Assoc. 2021;325(10):962-970.. The new guidelines also reduce the smoking history from 30 pack-years to 20 pack-years; a pack-year equals smoking one pack per day for one year and is a way to measure the amount a person has smoked over a long period of time. The guidelines were revised, in part, because new evidence showed that the previous eligibility criterion for lung cancer screening—adults ages 55 to 80 with a history of 30 pack-years of smoking—was too stringent for African American smokers, who are at a higher risk of developing lung cancer but typically have a smoking history of fewer pack-years, making them ineligible for screening (361)Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD. Evaluation of USPSTF Lung Cancer Screening Guidelines among African American Adult Smokers. JAMA Oncol. 2019;5(9)..
Several professional societies also convene panels of experts to evaluate data regarding the benefits and potential harms of cancer screening tests, and each society then makes its own evidence-based recommendations about the use of these tests. Not all professional organizations issue screening guidelines for all cancer types. In addition, because the representatives on each panel are often different, and different groups give more weighting to certain benefits and potential harms than other groups do, this can result in differences in recommendations from distinct groups of experts. Despite certain differences in screening recommendations by various subject matter expert panels for the five cancers for which screening is most conducted, there are more commonalities in the guidelines than there are differences (see sidebar on Consensus Cancer Screening Recommendations).

Who Should Get Screened and When?
Many factors can contribute to a person’s risk of developing cancer, and each person has his or her unique cancer risks. Thus, the decision of whether someone should be screened for cancer, at what age, and for which cancer type(s) is different for each person. It is important that people consult with their health care providers to develop a personalized cancer screening plan that considers their risk of developing a cancer and their tolerance for the potential harms of a screening test.
Individuals at Average Risk of Developing Cancer
Individuals are considered at an average risk of developing cancer if they do not have a family or personal history of cancer and are without any known risk factors that can cause cancer (see Preventing Cancer: Identifying Risk Factors). Health care providers consider two key characteristics—age and gender—when recommending a cancer screening test to a person who is at an average risk. Because cancer is predominantly a disease of aging, the probability of developing cancer increases with advanced age. In fact, 80 percent of all cancer cases in the United States are diagnosed among those age 55 or older (3)American Cancer Society. American Cancer Society: Cancer Facts and Figures 2021. Atlanta Am Cancer Soc. Published online 2021:13-15.. Ongoing research is assessing the optimal age range during which screening individuals who are at an average risk of developing cancer can have maximal benefit. For instance, according to a new report, commencing mammography at age 40 or 41, instead of the currently recommended age of 50, reduced breast cancer mortality by 25 percent in the first 10 years after the start of screening (362)Duffy SW, Vulkan D, Cuckle H, et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): final results of a randomised, controlled trial. Lancet Oncol. 2020;21(9).. Thus, it is imperative that individuals continually evaluate—and update as needed—their cancer screening plans in routine consultation with their health care providers.
Individuals at High Risk of Developing Cancer
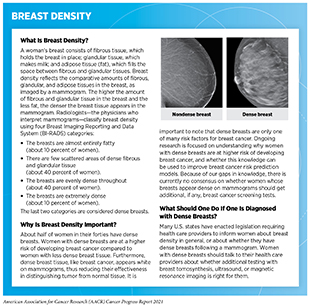
Some individuals are at a higher risk for developing certain type(s) of cancer. The reason for an increased risk includes exposure to one or more cancer risk factors (see Preventing Cancer: Identifying Risk Factors), unique tissue makeup, and/or a family history of cancer. As an example, smoking—an established risk factor for several cancer types—places individuals at a higher risk for developing cancer. According to CDC, people who smoke cigarettes are 15 to 30 times more likely to develop lung cancer or die from it than people who do not smoke. Another reason why an individual could be at a higher risk is because of the individual’s cellular or tissue makeup. For instance, women who have extremely dense breasts have an increased risk of developing breast cancer compared to women with less dense breasts (363)Nazari SS, Mukherjee P. An overview of mammographic density and its association with breast cancer. Breast Cancer. 2018;25(3). (see sidebar on Breast Density).
All individuals at high risk of developing cancer should routinely consult their health care provider team and develop a personalized risk-reducing plan. Some may be able to reduce their risk through behavioral changes, for example, quitting smoking or reducing alcohol consumption. Others may need to increase the frequency of cancer screening or use a test not recommended for average-risk individuals. Some may even consider taking medicine or undergoing surgery to reduce their risk of developing cancer (see Table 4 and Supplemental Table 1).
Individuals with Inherited Cancer Susceptibility Syndromes
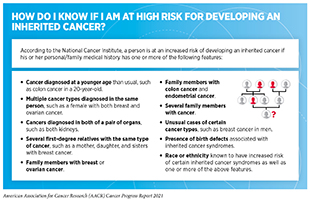
Some individuals have inherited cancer susceptibility syndromes, a category of disorders in which there is a higher-than-average risk of developing cancer. Also called hereditary cancer syndromes, these disorders are caused by inherited genetic mutations that can predispose an individual to develop certain types of cancer (see Table 2). If a person thinks that he or she is at a high risk for inheriting a cancer-predisposing genetic mutation, he or she should consult a health care provider and consider genetic testing (see sidebar on How Do I Know If I Am at High Risk for Developing an Inherited Cancer?). As researchers learn more about inherited cancer risk (364)Brown GR, Simon M, Wentling C, Spencer DM, Parker AN, Rogers CA. A review of inherited cancer susceptibility syndromes. JAAPA. 2020;33(12)., there will be new genetic mutations to test for and changes to the recommendations about who should be offered genetic testing. Therefore, individuals at a high risk of developing inherited cancers should keep an ongoing and informed dialogue with health care providers to make shared decisions about suitability of genetic counseling and genetic testing.
As with other cancer screening tests, the decision of whether to undergo genetic testing, which test to perform, and what follow-up steps should be taken is a complex one. Subject matter expert panels (for instance, USPSTF) and professional organizations (for example, American College of Obstetricians and Gynecologists) issue evidence-based recommendations for individuals with certain cancer susceptibility syndromes to provide guidance for an informed and shared decision-making process (150)Jiang Q, Isquith J, Ladel L, et al. Inflammation-driven deaminase deregulation fuels human pre-leukemia stem cell evolution. Cell Rep. 2021;34(4).(151)Rozenblatt-Rosen O, Regev A, Oberdoerffer P, et al. The Human Tumor Atlas Network: Charting Tumor Transitions across Space and Time at Single-Cell Resolution. Cell. 2020;181(2). (see Figure 15). Furthermore, several government agencies, including FDA, Centers for Medicare and Medicaid Services, and Federal Trade Commission (FTC), provide regulatory oversight to ensure safety of a genetic test for the individual. It is important to note that there are direct-to-consumer genetic tests that individuals can use without a prescription from a physician, but there are many factors to weigh when considering whether to use one of these tests. Because of the complexities of these tests, FDA and FTC recommend involving a health care professional in any decision to use such testing, as well as to interpret the results.
Individuals from Certain Racial and Ethnic Minorities
The rapid pace of progress in understanding the genetic and epigenetic basis of precancerous and cancerous aberrations is laying the groundwork for precision cancer prevention (365)Kassem N, Stout LA, Hunter C, Schneider B, Radovich M. Precision Prevention: The Current State and Future of Genomically Guided Cancer Prevention. JCO Precis Oncol. 2020;(4).. At the same time, advances in cancer genetics and epigenetics are also identifying gaps in our knowledge that require additional research to achieve evidence-based health equity for all. One such gap is in our understanding of how mechanisms of cancer onset and progression may differ for individuals from different racial and ethnic population groups.
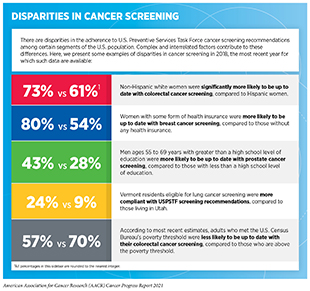
Accruing evidence shows that a breast cancer diagnosis at a younger age is more common in African American women compared to white women. Furthermore, African American women are more likely to be diagnosed with biologically aggressive forms of the disease at all ages (366)Yedjou CG, Sims JN, Miele L, et al. Health and Racial Disparity in Breast Cancer. In: Advances in Experimental Medicine and Biology. Vol 1152. ; 2019.. This knowledge has raised the possibility that disparities in breast cancer outcomes for African American women can be eliminated by changing the recommendations for mammography and genetic testing based on race and/or ethnicity (367)Rebner M, Pai VR. Breast cancer screening recommendations: African American women are at a disadvantage. J Breast Imaging. 2020;2(5).. Another example of cancer health disparity is that African American men tend to develop lung cancer at earlier ages and after fewer pack-years of smoking compared to white men (361)Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD. Evaluation of USPSTF Lung Cancer Screening Guidelines among African American Adult Smokers. JAMA Oncol. 2019;5(9).. Studies investigating cancer health disparities—such as the examples above—are providing much needed information to develop cancer screening guidelines that are tailored for specific racial and ethnic population groups and are thus more effective in improving outcomes through early detection. However, disparities in the uptake of cancer screening among the underserved segments of the U.S. population remain (see sidebar on Disparities in Cancer Screening). These disparities underscore the urgent need for action that includes targeted efforts such as culturally sensitive interventions to raise awareness of the benefits of cancer screening among underserved population groups. In addition, it is important to educate and train clinical researchers and coordinators involved in designing and conducting clinical trials about best practices on how to recruit individuals from diverse and underrepresented population groups, as well as to encourage racial and ethnic minorities to participate in clinical studies on cancer etiology, prevention, and early detection.
Suboptimal Use of Cancer Screening Tests
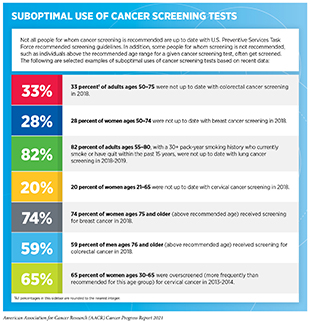
Even though the benefits of screening for breast, cervical, colorectal, and lung cancer outweigh the potential risks for defined groups of individuals (see sidebar on Consensus Cancer Screening Recommendations), many of those for whom screening is recommended do not get screened (see sidebar on Suboptimal Use of Cancer Screening Tests). Individuals who are not up to date with cancer screening recommendations are disproportionately found in medically underserved segments of the U.S. population (see sidebar on Disparities in Cancer Screening).
In addition to the suboptimal uptake among those individuals who should get screened, some people for whom screening is not recommended, such as individuals below or above the recommended age range for a given cancer screening test or those with limited life expectancy, are screened even though the evidence indicates that the benefits of screening are unlikely to outweigh the potential harms for them (see sidebar on Suboptimal Use of Cancer Screening Tests).
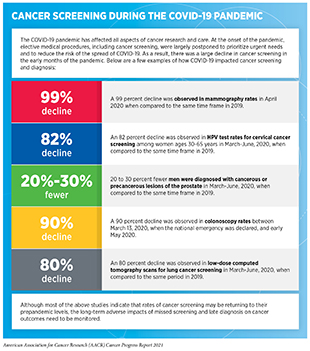
These challenges have been exacerbated in the past two years due to the ongoing COVID-19 pandemic, which led to a sharp decline in cancer screening during its initial peak. It will be important to continue monitoring whether the substantial decrease in cancer screening leads to any long-term changes in U.S. cancer mortality (372)Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh Q-DD. Cancer Screening Tests and Cancer Diagnoses During the COVID-19 Pandemic. JAMA Oncol. 2021;7(3):458-460.(373)Epic Health Research Network. Delayed cancer screenings. 2020 May 4; [updated 2020 Jul 17]. (see sidebar on Cancer Screening During the COVID-19 Pandemic).
The suboptimal use of cancer screening tests, especially among racial and ethnic minorities, underscores the requirement for new approaches and public policies to increase cancer screening awareness, accessibility, and affordability. As an example, findings from a new study show that individuals who received a series of text messages about the importance of colorectal screening, along with free at-home kits for a fecal immunochemical test, had a nearly 10-fold increase in screening completion, compared to those individuals who only received one text reminding them of an overdue screening test (377)Huf SW, Asch DA, Volpp KG, Reitz C, Mehta SJ. Text Messaging and Opt-out Mailed Outreach in Colorectal Cancer Screening: a Randomized Clinical Trial. J Gen Intern Med. Published online 2021.. Another recent study evaluated the preference of average-risk individuals for different colorectal cancer screening tests (see sidebar on How Do We Screen for Cancer?). Nearly two thirds of all participants, and half of Hispanic and non-Hispanic African American participants, preferred stool-based tests over colonoscopy. Preference for stool-based tests was also higher among younger individuals (ages 45 to 54 years) and among those without insurance because the test is less expensive than colonoscopy (378)Zhu X, Parks PD, Weiser E, et al. National survey of patient factors associated with colorectal cancer screening preferences. Cancer Prev Res. 2021;14(5).. It is important to note that any abnormality identified by a stool-based test still requires follow-up confirmatory tests, such as through colonoscopy, as well as the removal of any precancerous lesions. Nonetheless, these findings underscore the need to develop cancer screening outreach strategies that are based on sociodemographic characteristics, awareness and use of minimally invasive screening methods, and access to preventive health care services.
At the federal level, NCI and CDC play important roles in raising awareness for cancer screening. The NCI’s colorectal cancer outreach and screening initiative—Screen to Save—is one example of how government agencies can help increase overall cancer screening rates and reduce cancer health disparities. The Screen to Save initiative aims to provide culturally tailored, evidence-based colorectal cancer information, education, and screening resources within racially and ethnically diverse and rural communities through recruitment of community health educators. Government-mandated health insurance is another effective mechanism to increase the utilization of preventive health services. For example, a recent study found that health insurance coverage mandated by the 2010 Affordable Care Act increased the probability of being up-to-date on colorectal cancer screening by three percent (382)Preston MA, Ross L, Chukmaitov A, et al. Health insurance coverage mandates: Colorectal cancer screening in the Post-ACA Era. Cancer Prev Res. 2021;14(1).. Although each of the approaches, programs, and policies discussed here is raising awareness of and accessibility to cancer screening, a concerted and coordinated effort to maximize their impact is needed to achieve the vision of cancer health equity.