Combatting Cancer Through Science-based, Patient-centered Policies
In this section you will learn:
- Federal funding for medical research, most specifically through the NIH, NCI, and CDC has a significant impact on our nation’s health and the United States economy.
- Regulatory science initiatives at the FDA are vital to accelerating the progress against cancer.
- Policies and federally funded public health programs, many of which are supported by the CDC, ensure that individuals have access to preventive services, screening, and coverage for cancer treatment.
- Tobacco control policies improve public health and reduce cancer risk.
- Newly passed legislation aims to improve outcomes for children and adolescents who are diagnosed with cancer.
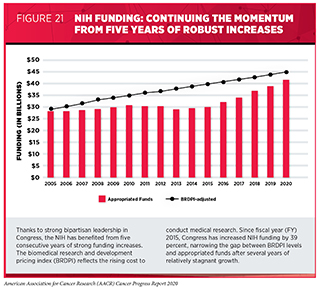
This is both an exciting and uncertain time for cancer research. On the one hand, it is an extraordinary time, as new discoveries are changing the way we prevent, detect, diagnose, and treat cancer, bringing hope to patients and their loved ones. This progress would not be possible without years of public investment in medical research through the NIH. Congress has made a strong commitment to advancing medical science over the past five years, increasing NIH funding by 39 percent from FY 2015 to FY 2020. In particular, Senator Roy Blunt (R-MO), Senator Patty Murray (D-WA), Congresswoman Rosa DeLauro (D-CT), and Congressman Tom Cole (R-OK) have demonstrated remarkable leadership in their respective roles on the Labor-Health and Human Services (HHS)-Education Appropriations Subcommittees in the Senate and House, respectively.
Unfortunately, in 2020, the COVID-19 health and economic crisis has had a significant negative impact on medical research including cancer research. Across the country, many researchers had to put their work on hold as laboratories closed, accrual to clinical trials slowed, and resources were diverted to the COVID-19 response (see Special Feature on Covid-19 and Cancer).
During these challenging times, we are fortunate that Congress continues to express its commitment for the critical role of NIH-funded research to fuel progress against cancer and other diseases. Similarly, the FDA has received strong bipartisan support in recent years. Funding for the FDA, including the Oncology Center of Excellence (OCE), is essential to ensure that research breakthroughs can be translated into safe and effective new treatments. Meanwhile, increased CDC funding is crucial to bringing evidence-based public health interventions, including cancer screening, to communities across the country.
According to NIH Director Francis Collins, MD, PhD, the coronavirus pandemic has caused over $10 billion in lost research, not to mention the additional unforeseen medical research needs posed by this virus. Therefore, in addition to robust annual budget increases, supplemental funding will be needed to reignite the research efforts that drive progress against cancer and other diseases.
Medical Research: A Wise Investment for America
Annual investments in the NIH are the bedrock of the U.S. scientific enterprise, leading to discoveries that save lives, as discussed by Congressman Peter King. NIH-funded research grants have played a role in many of the major medical breakthroughs that are benefiting patients today, including much of the exciting progress described in this report (29). Thanks to strong bipartisan leadership in both the House and Senate, Congress has made medical research a top priority, increasing the NIH budget by a total of $11.6 billion over the last five years (see Figure 21).
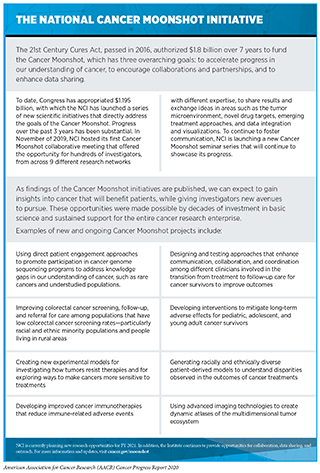
The 21st Century Cures Act, bipartisan legislation passed by Congress in 2016, has also provided funding for the National Cancer Moonshot Initiative through the NIH Innovation Fund (see sidebar on The National Cancer Moonshot Initiative). The National Cancer Moonshot Initiative is accelerating progress against cancer in specific priority areas where there is significant opportunity.
The level of enthusiasm for cancer research has perhaps never been higher, as demonstrated by an almost 50 percent increase in NCI grant applications over the past five years (542)Lowy: Paylines are dropping because NCI is drawing more applicants who would’ve previously gone elsewhere – The Cancer Letter [Internet]. [cited 2020 Aug 3].. While this tremendous interest is promising for the field of cancer research, it also represents a unique challenge for the NCI compared with other NIH institutes and centers, which have seen relatively stable application numbers over the same period. Given that the NCI’s budget has not increased at the same rate as its investigator-initiated RPG applications, there has been a recent trend of falling paylines and declining success rates for these grants. For example, in FY 2018, the payline for NCI R01 grants was 8 percent. The FY 2018 success rate for NCI applications was 12 percent, compared with 22 percent for the rest of NIH (543)Annual Plan and Budget Proposal FY2021 – National Cancer Institute [Internet]. [cited 2020 Aug 3].. Such low rates serve as a disincentive for researchers, particularly those earlier in their careers, to continue submitting grant applications to the NCI or to remain in the field of cancer research.
Congressional leaders did act to address this issue in the FY 2020 budget by providing a 5 percent funding increase for NCI, specifically including funds to increase the number of research grants funded in the year. Based on this allocation, the NCI announced that the payline for R01 grants would increase to 10 percent, the first time this figure has been in double digits since FY 2017 (544)FY 2020 Budget Boost for NCI – National Cancer Institute [Internet]. [cited 2020 Aug 3].. While this was an important step, we also know that many promising proposals that could change the landscape of cancer are still being left unfunded. Continued funding increases for the NCI — and specifically for investigator-initiated RPG awards — is critical to ensure continued progress. The NCI Director’s FY 2021 Professional Judgement Budget Proposal calls for a total of $6.928 billion for the NCI, which would allow for a payline increase to 15 percent.
Most funds appropriated to the NIH by Congress are awarded to scientists in all 50 states and the District of Columbia through a competitive review process. Investments in the NIH and NCI also extend well beyond the laboratory and the clinic. As the single largest public funder of medical research in the world, NIH-funded research in communities across the U.S. supported nearly 476,000 jobs and generated more than $81 billion in economic activity in FY 2019 (545)NIH’s Role in Sustaining the U.S. Economy – United For Medical Research [Internet]. [cited 2020 Aug 3]..
Congress has made a clear and impactful commitment to medical research over the last five years, returning the NIH to a trajectory of steady funding growth. However, this is no time to stop. With so many opportunities to make progress against cancer and other diseases, it is as important as ever for our elected leaders to continue providing robust, sustained, and predictable increases for medical research funding.
Supporting a Strong, Diverse Research Workforce
Continued progress against cancer requires investment in the recruitment, training, and ongoing support of the next generation of cancer researchers. Early-career scientists are not only key to ensuring a strong pipeline of cancer researchers, but they are also responsible for bringing fresh ideas and innovative research questions to the field. To realize the full potential of our medical research enterprise, we must also ensure that the cancer research workforce reflects the diversity of our country, including diversity in race, ethnicity, gender, geography, and scientific discipline. The NIH and NCI play a large role in supporting young researchers who will become the scientific and clinical leaders of the future.
With the support of Congress, the NIH has prioritized advancing the careers of early-career researchers, including through the Next Generation Research Initiative. This program is focused on ensuring the long-term stability and strength of the U.S. medical research enterprise by supporting early-stage investigators and mid-career investigators through specific funding efforts.
The NCI has also implemented several additional policies and programs to support early-stage investigators. For example, for FY 2020 grant applications, the NCI has established a payline in the 15th percentile for early-stage investigators, compared with 10 percent for the general applicant pool. In addition, the NCI continues to convert the most meritorious R01 applications from early-stage investigators to Method to Extend Research in Time (MERIT) (R37) awards. This program, which was introduced in 2018, provides the opportunity to extend funding for an additional two years beyond the initial award period, allowing more time for these researchers to establish their careers before submitting renewal applications (544)FY 2020 Budget Boost for NCI – National Cancer Institute [Internet]. [cited 2020 Aug 3]..
Notably, the COVID-19 crisis presents an enormous challenge for early-career researchers. Many young scientists have had their studies, fellowships, and initial projects severely disrupted by the pandemic, and are at risk of leaving the medical research field. As Congress considers both annual appropriations and supplemental funding, it will be vitally important to invest additional resources in support of these young researchers, on whom we are clearly depending for future breakthroughs against deadly diseases such as cancer.
Advancing Regulatory Science and Policy
The FDA is a crucial part of the medical research enterprise. To fulfill its public health mission of assessing the safety and efficacy of medical products, the agency must stay abreast of the ever-accelerating rate of innovation demonstrated by cancer research. Achieving this mission will require consistent, robust support from Congress through annual appropriations. User fee agreements are a necessary and essential source of support to the agency, but appropriated dollars support vital regulatory science programs that advance regulatory policies and culminate in the scientifically informed, efficient, and expeditious review of oncology medical products.
The support that FDA receives is also vital to ensuring that the agency is able to keep pace with the technological advancements that are taking place throughout the industries that it is charged with regulating. Therefore, in 2019, the FDA unveiled the Technology Modernization Action Plan (TMAP). This plan outlines strategies for modernizing the agency’s technological infrastructure, developing new technologies to support regulatory efforts, and communicating with stakeholders to drive interoperable technological progress. Through the execution of this plan, the agency aims to build an infrastructure that will support increased use of new technologies and data collection methods, enable the agency to better evaluate novel sources of data, and ensure that the potential benefits from new technologies and approaches are translated to patients in an even more expeditious time frame.
Additionally, the FDA has been preparing for the increased use of AI, both within the agency and in the applications for drugs and devices it reviews. AI algorithms have been explored to complement the analysis of scans collected to detect and monitor cancer. Combined with the experience of radiologists, these algorithms have the potential to improve the efficiency of cancer detection and diagnosis. AI has applications in helping scientists identify new cancer therapy candidates and clinical decision software used to support oncologists and other physicians. Many of these applications must be reviewed by the FDA to ensure the benefits they provide outweigh any risks. To successfully integrate and review AI, the FDA will need a modern technology infrastructure and a robust workforce with expertise in AI and related concepts. Such a workforce is vital to the agency’s efforts to keep pace with modern technology.
As mandated by the 21st Century Cures Act and other legislation, the FDA is also seeking to understand and explore potential uses for real-world evidence in regulatory decision-making and has included it as an important aspect of the TMAP infrastructure revitalization effort. Real-world evidence is clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of data sources such as electronic health records, insurance claims data, and wearable health devices. Real-world evidence has been used to supplement randomized controlled trial data, particularly in cases of rare cancers or other diseases. Efforts to incorporate real-world evidence into regulatory decision-making are likely to take center stage as groups conducting cancer clinical trials and the FDA work together to overcome the impact of COVID-19 and consider ways to overcome the challenges posed by the lack of certain data from clinical trials that were adversely affected by the pandemic.
FDA is also taking steps to reorganize its workforce to allow its staff to become more efficient and to better understand the diseases and drugs that fall in their respective areas of review. For example, during the fall of 2019, the FDA Office of New Drugs increased the number of clinical review divisions from 19 to 27. A key piece of this reorganization was the restructuring of the Office of Hematology and Oncology Products into the Office of Oncology Diseases (OOD) and expanding from three clinical review divisions to six.
Established in 2017, the OCE was created to streamline the review of anticancer therapeutics and increase regulatory efficiency through collaboration between agency staff with oncology expertise from the medical product centers of the FDA — the Center for Drug Evaluation and Research (CDER), the Center for Biologics Evaluation and Research (CBER), and the Center for Devices and Radiological Health (CDRH). In 2019, the OCE approved 11 new anticancer therapeutics. In addition, despite the unprecedented effects of the COVID-19 pandemic and the resulting need for agency staff to engage in maximum telework for personal safety, the OCE approved over 40 new anticancer indications, including ten new anticancer therapeutics from March to July 2020.
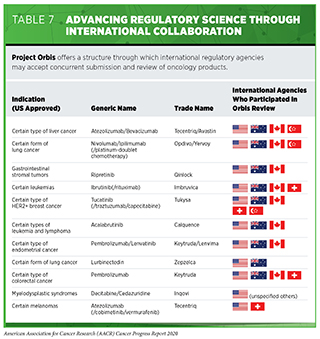
Beyond coordinating the reviews of anticancer therapeutics, the OCE is focused on bringing stakeholders together to discuss, learn, and collaborate with the goal of advancing regulatory science. In September 2019, the OCE announced Project Orbis, a valuable initiative that provides a framework for drug approval applications to be submitted and reviewed concurrently by multiple international regulatory agencies. The FDA oncology products review division has long held regular, confidential teleconferences with other regulatory agencies to exchange information related to applications they are reviewing, and Project Orbis represents an extension of that collaboration to speed global drug review (see Table 7).
In addition, the OCE is working to make patient-reported outcomes (PROs) from cancer clinical trials more accessible to the public. Although the FDA often receives and reviews PRO data as part of the drug approval process, it is rarely included in product labeling. Through a pilot website unveiled in June 2020, Project Patient Voice is a platform to provide patient-reported symptom data to the public in a standardized, easily digestible format (546)Project Patient Voice | FDA [Internet]. [cited 2020 Aug 16].. The OCE will refine the presentation of these data over time by incorporating stakeholder feedback.
Advancing Effective Cancer Prevention, Treatment, and Control Efforts
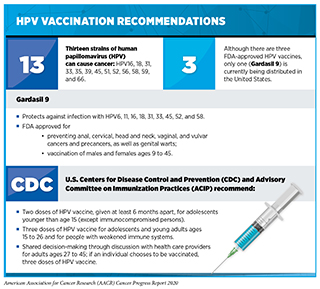
To achieve the greatest benefits to public health, new medicines and technologies must reach all members of society. Public health policies and programs play an important role in supporting equitable access to effective cancer prevention methods such as screening, early treatment, and HPV vaccinations. For example, it is estimated that in the United States, HPV infection accounts for about 34,000 cases of cancer each year, including almost all cases of cervical cancer (see Prevent and Eliminate Infection with Cancer-causing Pathogens). HPV vaccination is recommended for girls and boys ages 11 or 12 (see sidebar on HPV Vaccination Recommendations). Although HPV vaccination rates among U.S. adolescents have risen in recent years, they remain significantly below the national goal of 80 percent set by the U.S. Department of Health and Human Services in Healthy People 2020 (121)Elam-Evans LD, Yankey D, Singleton JA, Sterrett N, Markowitz LE, Williams CL, et al. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years — United States, 2019. MMWR Morb Mortal Wkly Rep [Internet]. 2020 [cited 2020 Aug 20];69:1109–16.. Therefore, continued funding for screening programs such as CDC’s National Breast and Cervical Cancer Early Detection Program is essential. The elimination of HPV-related cancers in the United States will only be possible through concerted efforts by all stakeholders to enhance public awareness of the importance of cancer prevention and screening, to increase vaccination rates, and to improve screening and treatment of pre-cancerous HPV-related lesions.
The cancer advocacy and scientific communities continue to work with members of Congress, the NIH, the CDC, and other federal agencies to support and accelerate the elimination of HPV-related cancers in the United States and globally through public policy. Despite advances in cancer research and care, there are persistent disparities in health outcomes for certain segments of the U.S. population, including racial and ethnic minorities, individuals of low socioeconomic status, and residents of rural areas (see sidebars Which U.S. Population Groups Experience Cancer Health Disparities? and U.S. Cancer Health Disparities). Many drivers of cancer health disparities have been identified, and policy solutions are needed to help achieve health equity. State-level vaccination mandates to attend public schools have greatly reduced the incidence of diseases like measles, mumps, and pertussis. Unfortunately, only Hawaii, Rhode Island, Virginia, Puerto Rico, and Washington, DC require HPV vaccination for school attendance. Connecticut and New York are pursuing bills to mandate HPV vaccines in 2020, but further efforts will be needed to achieve the goal of an 80 percent vaccination rate in the United States.
Racial and ethnic populations continue to be underrepresented in clinical trials for developing new anticancer therapeutics. Barriers to patient participation in clinical trials that need to be addressed include financial barriers, restrictive eligibility criteria, and lack of recruitment and information about and access to clinical trials. Public policies are also needed to support continued innovation and greater access to treatment and diagnostic options for all patients with cancer. Notably, the COVID-19 pandemic has adversely affected the conduct of cancer clinical trials. According to the NCI, the effect of the COVID-19 pandemic on trials varies. In regions with high numbers of COVID-19 cases, some sites halted enrolling new patients, while other sites had to seek approval for changes to the clinical trial protocols to accommodate patients and continue care (547)Coronavirus (COVID-19) Update: FDA Issues Guidance for Conducting Clinical Trials | FDA [Internet]. [cited 2020 Aug 16]..
Supporting Public Health Policies to Reduce the Use of Tobacco Products
Thanks to the implementation of nationwide comprehensive tobacco control initiatives, the smoking rate among U.S. adults declined from 20.9 percent in 2005 to 13.7 percent in 2018 (116). However, the use of e-cigarettes has increased dramatically over the past few years. Data have shown that the use of e-cigarettes increased from about 1 percent in 2011 to nearly 28 percent in 2019 (140)Cullen KA, Gentzke AS, Sawdey MD, Chang JT, Anic GM, Wang TW, et al. e-Cigarette Use Among Youth in the United States, 2019. JAMA [Internet]. American Medical Association; 2019 [cited 2020 Jun 30];322:2095.. According to the 2019 National Youth Tobacco Survey (NYTS), more than 5 million middle and high school students reported having used e-cigarettes in the past 30 days and nearly one million reported daily use. This is especially concerning because data suggest that youth and young adults who use e-cigarettes are more likely to try combustible cigarettes later (137)Stratton K, Kwan LY, Eaton DL, Effects H, Delivery N, Health P, et al. Public Health Consequences of E-Cigarettes [Internet]. National Academies Press; 2018.. The 2019 NYTS also indicated that current e-cigarette users reported that Juul was their usual brand. Cartridge-based e-cigarettes, such as Juul, are available in very high nicotine content; have appealing flavors; and can be easily concealed and used discreetly. Many public health experts believe that youth and young adult e-cigarette use has reached an epidemic proportion. Given the alarming rates of use, many organizations have called for more action to protect youth and young adults from addiction to nicotine and the detrimental health effects of e-cigarette use. In December 2019, the President signed legislation raising the federal minimum age for sale of tobacco products, including e-cigarettes, from 18 to 21 years. While the passing of “Tobacco 21” was an important step, public health advocates want more to be done.
In January 2020, the FDA issued a final guidance outlining the agency’s enforcement priorities for electronic nicotine delivery systems (ENDS). Under this policy, companies were ordered to cease the manufacture, distribution, and sale of flavored cartridge-based e-cigarettes with the exceptions of tobacco and menthol flavors. Unfortunately, disposable ENDS products were not included in the flavor ban and are still available in flavors targeted towards children.
Nearly all tobacco use begins in youth and young adulthood, and 95 percent of adult smokers began smoking before they turned 21. Therefore, we recognize that Tobacco 21 is one among several important federal policy changes that are important to address the public health crisis of e-cigarette use among U.S. youth and young adults. An additional important step involved a July 2019 order by the U.S. District Court for the District of Maryland requiring manufacturers of e-cigarettes, cigars, and other new tobacco products that were on the market as of August 8, 2016, to submit applications to the FDA for premarket review by May 12, 2020. While the FDA pushed back the deadline because of the coronavirus pandemic, therefore delaying the deadline to September 2020, tobacco companies are required to submit a Premarket Tobacco Product Application (PMTA) for any new tobacco product, including e-cigarettes, that demonstrates the product is appropriate for the protection of public health. Other potential actions to protect public health include prohibiting the manufacture and sale of all flavored tobacco products for disposable and open-tank devices available to youth (unless they are FDA-approved to aid in tobacco cessation for adult users); strongly supporting the actions that FDA’s Center for Tobacco Products is taking to regulate the manufacturing, distribution, and marketing of tobacco products; and increasing funding for the prevention and cessation activities that are supported by the CDC Office on Smoking and Health.
Policies that Stimulate Progress against Pediatric Cancer
Cancer remains the second leading cause of death among U.S. children ages 1 to 14. Research-fueled advances against pediatric cancer have increased the five-year relative survival rate for children diagnosed with cancer from 63 percent in the mid-1970s to 85 percent (2)Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2017, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2 [Internet]. [cited 2020 Jun 29].. Despite the progress, almost 1,200 children are expected to die of cancer in 2020. In addition, children who survive cancer often face health issues later in life. Recently enacted federal policies and programs are playing a key role in addressing the challenges faced by children with cancer and their families.
A recent initiative that has received strong support from Congress is the Childhood Cancer Data Initiative (CCDI), which focuses on the critical need to collect, analyze, and share data to address the burden of cancer in children, adolescents, and young adults (AYAs). The initiative supports maximizing the use and benefit of data from childhood and AYA cancer research for patients and survivors, and aims to make it easier for researchers to learn from each of the approximately 16,000 children and adolescents diagnosed with cancer in the United States each year. The CCDI is a federal investment of $50 million proposed to be extended in equal amounts per year for the next 10 years. The first year of the initiative was funded in December 2019.
On August 18, 2017, key provisions of the Research to Accelerate Cures and Equity (RACE) for Children Act were signed into law as part of the FDA Reauthorization Act of 2017. The RACE Act requires that drug developers study molecularly targeted therapeutics that they developed for adult populations in pediatric populations. This applies to therapeutics that target molecules that fuel cancer development in both pediatric and adult patients. To facilitate this process, the RACE Act obligated the development of a Pediatric Molecular Targets List detailing molecular targets that do and do not meet the criteria. As of August 18, 2020, applications submitted to the FDA for therapies meeting RACE Act criteria must have agency-approved pediatric study plans.
The Childhood Cancer Survivorship, Treatment, Access, and Research (STAR) Act, signed into law in June 2018, is the most comprehensive childhood cancer legislation passed by Congress to date. This legislation includes provisions to improve childhood cancer surveillance, enhance research on the late effects of childhood cancers, and increase research opportunities by expanding the collection of biospecimens for childhood cancer patients.
The STAR Act provisions are being implemented across multiple agencies. For example, the NCI has already issued two requests for applications for research proposals focused on improving care and health-related quality of life for childhood, adolescent, and young adult cancer survivors. Numerous grants have been supported in the first round of funding, which was issued in 2019, with the second round to be funded in 2020. Meanwhile, the CDC has expanded support for childhood cancer surveillance in 10 states and is working to partner with more states to improve collection of this vital information. The Government Accountability Office is currently conducting an extensive report on barriers that impede access to care for childhood cancer survivors, and the Agency for Healthcare Research and Quality is developing national standards of care for childhood cancer survivors based on research of best practices.
All of these components are critical to increasing our understanding of childhood cancers and the best ways to support survivors as they transition to adulthood and beyond. Further progress will depend on Congress continuing to appropriate annual funding for STAR Act implementation in accordance with the legislation ($300 million total over 10 years).
The Gabriella Miller Kids First Pediatric Research Program (Kids First) at the NIH is supporting new discoveries in the biology of childhood cancer and the links to birth defects. Funding for this program was established in the Gabriella Miller Kids First Research Act, passed by Congress in 2014. Since that time, $75 million have been invested in pediatric research. The Gabriella Miller Kids First Research Act 2.0 was introduced in April 2020 by Reps. Jennifer Wexton (D-VA), Tom Cole (R-OK), Peter Welch (D-VT), and Gus Bilirakis (R-Fl). This legislation redirects certain penalties against pharmaceutical companies for specified violations to the 10-Year Pediatric Research Initiative Fund, an existing fund that supports pediatric disease research. The NIH will make allocations from this fund to support lifesaving pediatric research that does not duplicate existing activities.