- Understanding the Biology of SARS-CoV-2 Infection and COVID-19
- State of the COVID-19 Epidemic
- Detecting SARS-CoV-2 and COVID-19
- Clinical Management of COVID-19
- Cancer in the Midst of COVID-19
- Cancer Researchers Working to Combat the COVID-19 Pandemic
- Adapting Cancer Research and Medicine in the COVID-19 Era
In this section, you will learn:
- As of July 31, 2020, there were 17,622,478 confirmed cases of Coronavirus Disease 2019 (COVID-19) and 680,165 deaths from the disease globally; there were 4,566,275 cases and 153,391 deaths in the United States.
- Older adults, males, and individuals of any age with certain underlying medical conditions are at an increased risk for severe COVID-19 illness.
- Racial and ethnic minorities have been disproportionately impacted by COVID-19 for many of the same reasons that they shoulder a disproportionate burden of cancer.
- The COVID-19 pandemic has disrupted cancer care for many people, causing concerns that the delays in screening, diagnosis, and treatment will cause thousands of additional deaths from cancer in the future.
- Cancer researchers are uniquely positioned to respond to many of the challenges posed by COVID-19.
The year 2020 will be inextricably linked to Coronavirus Disease 2019 (COVID-19), the disease that affected more than 17.6 million people worldwide and taken more than 680,000 lives by July 31, 2020 (61)COVID-19 Map – Johns Hopkins Coronavirus Resource Center [Internet]. [cited 2020 Jul 15]..
At the end of 2019 a disease presenting as pneumonia with unknown origins was identified in Wuhan, a city in the Hubei Province of China (62)Ge H, Wang X, Yuan X, Xiao G, Wang C, Deng T, et al. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis [Internet]. Springer; 2020 [cited 2020 Aug 1];39:1011–9.. In early January 2020, the cause of the disease was identified as a novel coronavirus by the Chinese Center for Disease Control and Prevention. The International Committee on Taxonomy of Viruses termed the virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and in February 2020, the World Health Organization (WHO) designated the disease caused by SARS-CoV-2 as Coronavirus Disease 2019, or COVID-19. The ensuing global health crisis, which was declared a pandemic by the WHO on March 11, 2020, continues to exact an immense toll on people and countries around the world.
This Special Feature on COVID-19 and Cancer will provide an overview of the basic biology of SARS-CoV-2, the epidemiology of COVID-19, the influence of cancer research on the detection and treatment of the disease, and the opportunities and challenges ahead for the cancer community due to the COVID-19 pandemic.
Understanding the Biology of SARS-CoV-2 Infection and COVID-19
Named because of their crown-like appearance, coronaviruses constitute a family of hundreds of viruses most commonly found in birds and small mammals (for example, bats and rodents), but occasionally in humans. There are seven coronaviruses, including SARS-CoV-2, that are known to infect humans; four result in cold-like illnesses while the other three are responsible for the deadly, global outbreaks of the respiratory illnesses severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), and COVID-19.
Viruses generally cannot live very long outside a host organism. They have no means of independent reproduction or metabolism and are composed primarily of genetic material, either DNA or RNA, encased in a protein “shell” called a capsid or nucleocapsid. The capsid may or may not be enclosed in an envelope; most viruses that infect animals, including SARS-CoV-2, have this envelope.
To multiply, a virus must attach to and enter an appropriate host cell, where it hijacks the host’s genetic material and cellular machinery to produce viral genomes and capsid and envelope proteins. These capsids are assembled around new genomes and transported to the host cell surface where they meet up with new envelope proteins and exit the host in a process called budding.
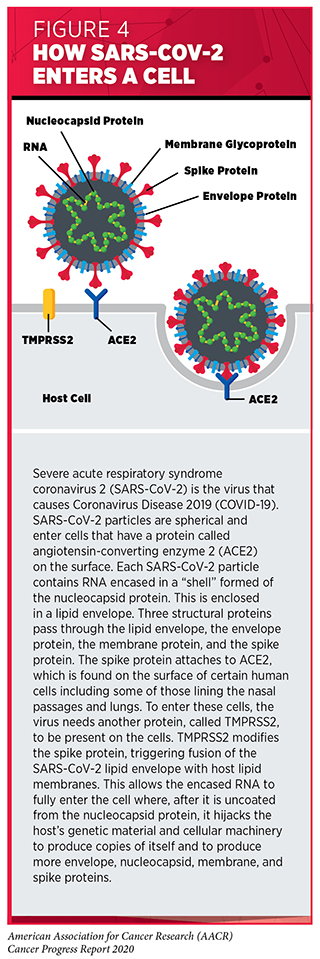
SARS-CoV-2 uses RNA as its genetic material and has four major structural proteins: the spike, nucleocapsid, membrane, and envelope proteins (63)Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature [Internet]. Nature Publishing Group; 2020 [cited 2020 Aug 1];583:459–68. (see Figure 4). To infect a human, the spike protein attaches to a protein called angiotensin-converting enzyme 2 (ACE2), which is found on the surface of certain human cells in the nasal passages, lungs, and gastrointestinal tract (64)Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell [Internet]. Elsevier; 2020 [cited 2020 Aug 1];181:271-280.e8.. To enter these cells, the virus needs another protein, called TMPRSS2, to be present on the cells. TMPRSS2 is naturally found in several tissues of the human body including the prostate, lung, gastrointestinal tract, and urinary tract, and it is frequently found together with ACE2 on cells in the nasal passages and lungs.
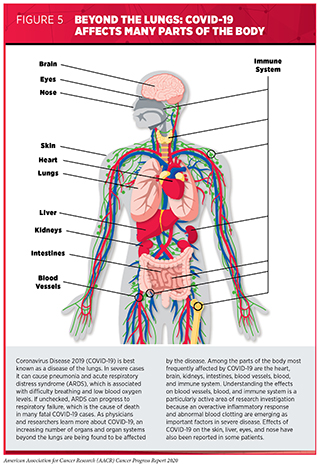
Once infected with SARS-CoV-2, individuals can begin shedding and transmitting virus particles within two to three days, often before experiencing disease symptoms (65)He X, Lau EHY, Wu P, Deng X, Wang J, Hao X, et al. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med [Internet]. Nature Publishing Group; 2020 [cited 2020 Aug 3];26:672–5.. For most people, it takes about four to five days after infection with SARS-CoV-2 for symptoms to appear, but for others it can take up to 14 days (66)Clinical Care Guidance for Healthcare Professionals about Coronavirus (COVID-19) | CDC [Internet]. [cited 2020 Aug 1].. Among the symptoms of COVID-19 are fever, dry cough, loss of taste and/or smell, fatigue, muscle pain/body aches, and difficulty breathing. As the disease progresses, moving from the upper to lower respiratory tract and throughout the body, the disease can cause damage to nearly every organ and system in the body (67)Gupta A, Madhavan M V., Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med [Internet]. Nature Publishing Group; 2020 [cited 2020 Jul 15];26:1017–32.(68)Wadman M. How does coronavirus kill? Clinicians trace a ferocious rampage through the body, from brain to toes. Science (80- ) [Internet]. 2020 [cited 2020 Jul 15]; (see Figure 5).
One possible explanation for the widespread damage seen in some patients who have COVID-19 is an overactive inflammatory response (69)Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol [Internet]. Nature Publishing Group; 2020 [cited 2020 Aug 1];20:363–74.(67)Gupta A, Madhavan M V., Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med [Internet]. Nature Publishing Group; 2020 [cited 2020 Jul 15];26:1017–32.. This can lead to a condition known as cytokine-release syndrome and severe, systemic inflammation. Inflammation in the lungs can progress to acute respiratory distress syndrome (ARDS), causing difficulty breathing and low blood oxygen levels. If unchecked, ARDS can progress to respiratory failure, which is the cause of death in many fatal COVID-19 cases. In addition, uncontrolled cytokine-release syndrome can lead to failure of other organs, most notably the heart, liver, and kidneys. Another explanation for the widespread damage in some patients who have COVID-19 is that cells lining the blood vessels can become damaged by the virus and/or the inflammatory response, which triggers abnormal blood clotting (67)Gupta A, Madhavan M V., Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med [Internet]. Nature Publishing Group; 2020 [cited 2020 Jul 15];26:1017–32.. This, in turn, can cause widespread organ damage.
Not everyone who becomes infected with SARS-CoV-2 goes on to develop symptoms of COVID-19; these people are said to be asymptomatic. Even among people who develop symptoms, there is a wide diversity in the severity of the disease. Gaining a deeper understanding of how infection with SARS-CoV-2 causes severe disease and what determines how severe disease will be for an individual are areas of intensive research investigation.
State of the COVID-19 Epidemic
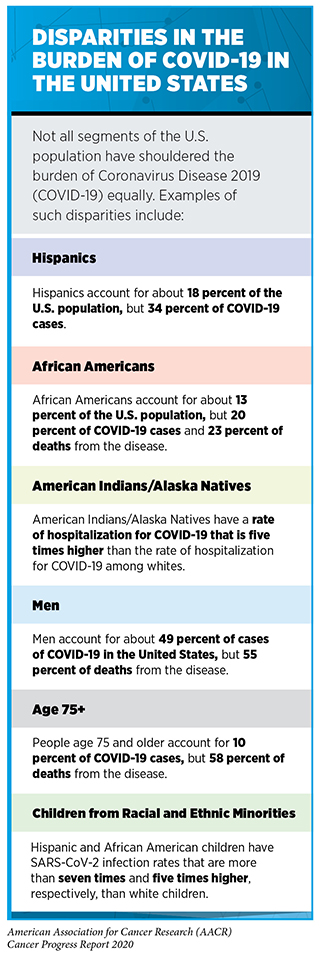
As of July 31, 2020, 17,622,478 people worldwide have been diagnosed with COVID-19, and more than 680,165 people have died from the disease (61)COVID-19 Map – Johns Hopkins Coronavirus Resource Center [Internet]. [cited 2020 Jul 15].. The United States accounts for more than one in every four recorded cases of COVID-19 and almost one in every four recorded deaths from the disease. As with the burden of cancer, certain segments of the U.S. population have shouldered a disproportionate burden of COVID-19 (see sidebar on Disparities in the Burden of COVID-19 in the United States).
COVID-19 has spread swiftly around the world. The virus that causes the disease is predominantly spread through close contact from person to person when an infected person coughs, sneezes, or talks, releasing droplets that contain the virus into the air (75)How Coronavirus Spreads | CDC [Internet]. [cited 2020 Aug 1].. These droplets can land in the mouths or noses of people who are nearby or possibly be inhaled into the lungs. Researchers continue to learn more about the ways in which the virus can be spread, with some data suggesting that in a few cases it might be possible that a person can become infected after touching a surface or object that has the virus on it and then touching his or her own mouth, nose, or possibly eyes (75)How Coronavirus Spreads | CDC [Internet]. [cited 2020 Aug 1].. Infected individuals can spread the virus even before they develop symptoms of the disease or even if they never develop symptoms (76)Moghadas SM, Fitzpatrick MC, Sah P, Pandey A, Shoukat A, Singer BH, et al. The implications of silent transmission for the control of COVID-19 outbreaks. Proc Natl Acad Sci U S A [Internet]. National Academy of Sciences; 2020 [cited 2020 Aug 1];117:17513–5.(77)Furukawa NW, Brooks JT, Sobel J. Evidence Supporting Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 While Presymptomatic or Asymptomatic. Emerg Infect Dis [Internet]. 2020 [cited 2020 Aug 1];26.. Given current knowledge of how SARS-CoV-2 is spread, the CDC recommends the following prevention strategies: washing hands frequently; avoiding close contact with people who don’t live in your household by staying six feet apart; covering your mouth and nose with a cloth face cover when around other people; covering your mouth and nose when you cough and sneeze; cleaning and disinfecting frequently touched surfaces daily; and monitoring your health daily (78)How to Protect Yourself & Others | CDC [Internet]. [cited 2020 Aug 1].. In the absence of a SARS-CoV-2 vaccine, these prevention strategies are critical to limiting infection with the virus and, therefore, the morbidity and mortality of COVID-19, with one study estimating that mandates requiring the use of face masks in public had prevented from 230,000 to 450,000 cases of COVID-19 by May 22, 2020 (79)Lyu W, Wehby GL. Community Use Of Face Masks And COVID-19: Evidence From A Natural Experiment Of State Mandates In The US. Health Aff [Internet]. Health Affairs; 2020 [cited 2020 Aug 1];10.1377/hlthaff..
The presentation of disease experienced by individuals who are infected with SARS-CoV-2 covers a wide spectrum, from no symptoms, to mild disease, to severe disease, to critical disease and even death. Advanced age (age 65 and older), sex (male), and having certain chronic health conditions, such as chronic kidney disease, chronic obstructive pulmonary disease (COPD), obesity, heart failure, coronary artery disease, sickle cell disease, diabetes, and a weakened immune system, increase a person’s risk of severe COVID-19. Other health conditions, including asthma and high blood pressure, have also been linked to an increased risk of severe COVID-19, but additional research is needed to confirm these associations. One recent study showed that patients with COVID-19 who had underlying chronic health conditions were six times more likely to be hospitalized and 12 times more likely to die compared with those who had no underlying chronic health conditions (73)Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, et al. Coronavirus Disease 2019 Case Surveillance — United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep [Internet]. 2020 [cited 2020 Aug 2];69:759–65.. However, healthy patients of any age and sex can develop severe disease (80)Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. Solomon CG, editor. N Engl J Med [Internet]. Massachusetts Medical Society; 2020 [cited 2020 Aug 2];NEJMcp2009575..
Research has shown that patients with cancer who develop COVID-19 seem to be at increased risk for severe disease and for death from the disease (81)Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov [Internet]. American Association for Cancer Research; 2020 [cited 2020 Jul 16];10:935–41.(82)Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with Cancer Appear More Vulnerable to SARS-CoV-2: A Multicenter Study during the COVID-19 Outbreak. Cancer Discov [Internet]. American Association for Cancer Research; 2020 [cited 2020 Jul 16];10:783–91.(83)Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature [Internet]. Springer US; 2020;(84). Patients who have a blood cancer appear to be at greatest risk. Research is underway to understand whether differences in COVID-19–related deaths among patients with cancer are a result of the cancer, the cancer treatment, or other factors. It is also important that patients with cancer are adequately represented in clinical trials assessing the safety and effectiveness of vaccines and treatment for COVID-19. Without such research, we cannot ensure that these agents will benefit patients with cancer.
Detecting SARS-CoV-2 and COVID-19
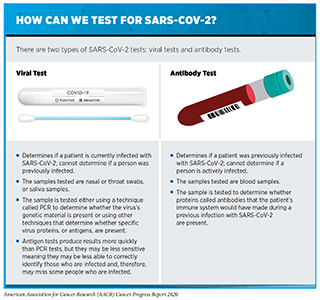
Timely testing to identify those who are or have been infected with SARS-CoV-2 is a crucial step in understanding and controlling the COVID-19 pandemic (see sidebar on How Can We Test for SARS-CoV-2?). Without knowledge of who is infected, it is challenging to implement appropriate measures to prevent further spread of the virus and to understand when such measures can be eased.
New technologies in the form of symptom and contact tracing apps for smartphones are being used to track new COVID-19 cases and identify “hot spots” of infection in real time (85)Servick K. COVID-19 contact tracing apps are coming to a phone near you. How will we know whether they work? Science (80- ) [Internet]. 2020 [cited 2020 Aug 2];. Having this information has the potential to allow local hospitals and health care systems to better prepare for surges in new cases; however, it remains to be determined whether these apps will be effective at controlling disease spread. Large sets of data collected through these apps can also be used by researchers to deepen scientific understanding of SARS-CoV-2 and the symptoms related to COVID-19, as well as to identify potential risk factors and disparities related to infection with the virus and disease severity (86)Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med [Internet]. Nature Publishing Group; 2020 [cited 2020 Aug 2];26:1037–40.(87)Drew DA, Nguyen LH, Steves CJ, Menni C, Freydin M, Varsavsky T, et al. Rapid implementation of mobile technology for real-time epidemiology of COVID-19. Science [Internet]. American Association for the Advancement of Science; 2020 [cited 2020 Aug 2];368:1362–7..
Clinical Management of COVID-19
Infection with SARS-CoV-2 causes a wide range of symptoms and disease severity (see Understanding the Biology of SARS-CoV-2 Infection and COVID-19). More than 80 percent of people who are diagnosed with COVID-19 have mild symptoms and do not require hospitalization (73)Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, et al. Coronavirus Disease 2019 Case Surveillance — United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep [Internet]. 2020 [cited 2020 Aug 2];69:759–65.(81)Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer Discov [Internet]. American Association for Cancer Research; 2020 [cited 2020 Jul 16];10:935–41.. Among those patients who require hospitalization, about 16 percent have critical disease and require admission to the intensive care unit (73)Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, et al. Coronavirus Disease 2019 Case Surveillance — United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep [Internet]. 2020 [cited 2020 Aug 2];69:759–65.. In some cities, the number of patients requiring hospitalization has overwhelmed the capacity of hospitals to care for them.
As of July 31, 2020, no therapeutics have been approved by the FDA to treat patients who have COVID-19. Until such therapeutics are available, the clinical management of COVID-19 centers around treating symptoms (80)Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. Solomon CG, editor. N Engl J Med [Internet]. Massachusetts Medical Society; 2020 [cited 2020 Aug 2];NEJMcp2009575.. For example, patients with low blood oxygen levels are first placed on their sides in the prone position. If this does not improve blood oxygen levels, patients are given supplemental oxygen or receive invasive mechanical ventilation. Those patients who experience kidney failure receive dialysis and those who have abnormal blood clotting are treated with anticoagulants such as heparin (88)N T, H B, X C, J G, D L, Z S. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost [Internet]. J Thromb Haemost; 2020 [cited 2020 Aug 2];18..
Although no therapeutics have been approved by the FDA for treating patients who have COVID-19, the agency authorized the emergency use of an investigational antiviral therapeutic called remdesivir for treating patients who have severe COVID-19 in May 2020 (89)Emergency Use Authorization | FDA [Internet]. [cited 2020 Aug 2].. Remdesivir was already under development as a potential treatment for infection with several viruses, including Ebola virus. The emergency use was granted based on results from a clinical trial that showed that treatment with remdesivir reduced the median recovery time for patients who had severe COVID-19 from 15 days to 11 days (90)Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the Treatment of Covid-19 — Preliminary Report. N Engl J Med [Internet]. Massachusetts Medical Society; 2020 [cited 2020 Aug 2];NEJMoa2007764.. Unfortunately, there is a limited supply of remdesivir. Therefore, the NIH recommends that remdesivir be prioritized for use in hospitalized patients with COVID-19 who require supplemental oxygen but who are not on high-flow oxygen, noninvasive ventilation, mechanical ventilation, or extracorporeal membrane oxygenation (91)What’s new | Coronavirus Disease COVID-19 [Internet]. [cited 2020 Aug 2]..
The NIH also recommends the use of dexamethasone, which is a steroid that is frequently used to dampen inflammation, as a treatment for patients with COVID-19 who are mechanically ventilated and patients with COVID-19 who require supplemental oxygen but who are not mechanically ventilated (91)What’s new | Coronavirus Disease COVID-19 [Internet]. [cited 2020 Aug 2].. This recommendation is based on results from a large clinical trial that showed that a 10-day course of dexamethasone reduced the risk of death by about a third (36 percent) among hospitalized patients with COVID-19 who were mechanically ventilated and by about a fifth (18 percent) among hospitalized patients with COVID-19 who required supplemental oxygen but who were not mechanically ventilated (92)Group TRC. Dexamethasone in Hospitalized Patients with Covid-19 — Preliminary Report. N Engl J Med [Internet]. Massachusetts Medical Society; 2020 [cited 2020 Aug 2];NEJMoa2021436..
There are many other therapeutics being investigated as potential treatments for COVID-19 because the global research community has responded robustly to this pandemic. These investigational therapeutics work in a wide variety of ways to combat COVID-19 (see sidebar on What Types of Treatment Are Being Investigated for COVID-19?). As of July 31, 2020, the FDA reported that there were more than 570 drug development programs in planning stages and that the agency had reviewed more than 270 trials of potential treatments for COVID-19 (93)Coronavirus Treatment Acceleration Program (CTAP) | FDA [Internet]. [cited 2020 Aug 2].. One area of active research investigation is the repurposing of therapeutics that are already under investigation or have been approved by the FDA for other uses because we already have information about dosage, toxicity, and adverse effects for these therapeutics, which can accelerate the pace of therapeutic development and approval relative to the development of novel therapeutics.
In addition to developing therapeutics for treating patients with COVID-19, stakeholders in the global research community are working collaboratively to develop SARS-CoV-2 vaccines. In the United States, on April 17, 2020, the NIH announced a public-private partnership—Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)—to develop a coordinated research strategy for prioritizing and accelerating the development of the most promising vaccines and therapeutics against COVID-19. ACTIV brings together the NIH and other U.S. government agencies; the European Medicines Agency (EMA); and representatives from academia, philanthropic organizations, and numerous biopharmaceutical companies. Also in the United States, the National Institute of Allergy & Infectious Diseases (NIAID) has founded a new clinical trial network, the COVID-19 Prevention Trials Network (COVPN), to address the challenges of patient enrollment in clinical trials and the need to recruit volunteers from parts of the country that are experiencing a severity of the COVID-19 epidemic. COVPN comprises the HIV Vaccine Trials Network, the HIV Prevention Trials Network, the Infectious Diseases Clinical Research Consortium, and the AIDS Clinical Trials Group and will leverage existing infrastructure to engage communities to facilitate the enrollment of the thousands of volunteers needed for late-stage clinical trials of promising vaccines against SARS-CoV-2.
Cancer in the Midst of COVID-19
The COVID-19 pandemic has created many challenges across the continuum of cancer care, with individual health care systems and institutions adjusting cancer screening, diagnosis, treatment, and follow-up care to respond to the rapidly evolving situation. Some cancer care has continued, some was altered, and some was suspended. Individuals who are scheduled for any form of cancer care should consult with their health care provider before their appointment.
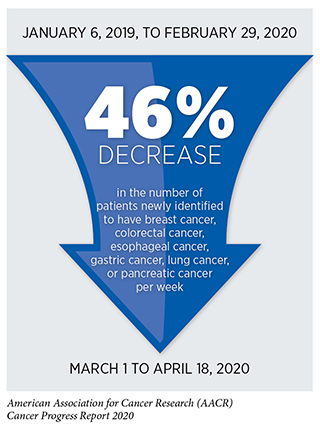
There is deep concern about the consequences that delays in cancer screening, diagnosis, and treatment will have on outcomes for patients with cancer (10)Sharpless NE. COVID-19 and cancer. Science [Internet]. American Association for the Advancement of Science; 2020 [cited 2020 Jun 29];368:1290.(94)Hoehn RS, Zureikat AH. Cancer disparities in the COVID-19 era. J Surg Oncol [Internet]. Wiley-Blackwell; 2020 [cited 2020 Jul 16];. Data from electronic medical records from 190 hospitals spanning 23 states show that the number of screening tests for early detection of cervical, breast, and colon cancer conducted in the United States plummeted by 85 percent or more after the first COVID-19 case was reported in the United States on January 20, 2020 (95)EPIC Health Research Network. Preventive Cancer Screenings during COVID-19 Pandemic. 2020;1–5., and a recent survey of patients with cancer found that 79 percent of those who are actively undergoing treatment had to delay some aspect of their care as a result of COVID-19, including 17 percent who reported delays to their cancer treatment (96)Cancer Action Netwrok. COVID-19 Pandemic Impact on Cancer Patients and Survivors: Survey Findings Summary. 2020;1–5.. It will take years to determine the consequences of all the delays, but researchers at the NCI have estimated that there will be at least 10,000 additional deaths from breast cancer and colorectal cancer over the next decade in the United States as a result of the negative impact that the COVID-19 pandemic has had on screening and treatment for these two types of cancer (10)Sharpless NE. COVID-19 and cancer. Science [Internet]. American Association for the Advancement of Science; 2020 [cited 2020 Jun 29];368:1290..
Several decisions made by the FDA since the onset of the pandemic have provided certain patients with cancer alternative treatment options that have the potential to reduce the need for frequent patient visits to health care facilities, which is an important consideration for patients during the COVID-19 pandemic. In April 2020, the agency approved an alternative dosing schedule for the immunotherapeutic pembrolizumab (Keytruda), which is approved for treating a wide array of cancer types (see Releasing the Brakes on the Immune System. Pembrolizumab is administered intravenously, meaning that patients must travel to a health care facility to receive the treatment. The new dosing regimen of 400 mg of pembrolizumab every six weeks provides an alternative to the standard 200 mg every three weeks that can reduce the number of times a patient must visit a health care facility for treatment. In July 2020, the FDA approved a tablet form of the epigenetic therapeutic decitabine for treating certain patients with myelodysplastic syndrome (MDS) (see Using Epigenetic Therapy to Treat Cancer). Given that decitabine is normally given intravenously, the new tablet provides patients with an option that can reduce the number of health care–facility visits. In July 2020, the FDA also approved a version of the commonly used combination of HER2-targeted therapeutics trastuzumab (Herceptin) and pertuzumab (Perjeta) that can be given subcutaneously (under the skin), rather than given intravenously as normal. Although this still needs to be administered by a health care provider, it can be given at a patient’s home.
Cancer Researchers Working to Combat the COVID-19 Pandemic
Cancer researchers are uniquely positioned to respond to many of the challenges posed by COVID-19, and many have refocused their expertise to combat the unprecedented global pandemic. For example, the NCI is lending its expertise and cutting-edge resources to conduct research that will contribute to the global effort to address COVID-19 (98)NCI’s COVID-19 Research Initiatives – National Cancer Institute [Internet]. [cited 2020 Aug 2].. The NCI has a world-class serology facility that works to standardize human papillomavirus (HPV) antibody testing, the HPV Serology Laboratory. This laboratory is now being used to advance COVID-19 serological testing and is part of a broader effort within the NIH to increase our understanding of SARS-CoV-2 infection and the immune response to the SARS-CoV-2 virus. Other researchers at the NCI are part of an initiative that includes several NIH institutes to better understand the impact of a person’s genome on COVID-19 outcomes and to screen compounds for use as potential COVID-19 treatments. Yet others at the NCI are building a large longitudinal cohort of people with cancer and COVID-19, the NCI COVID-19 in Cancer Patients Study (NCCAPS), to gain information that will support better treatment management for people with cancer and COVID-19 (99)NCI COVID-19 in Cancer Patients Study (NCCAPS) – National Cancer Institute [Internet]. [cited 2020 Aug 2]..
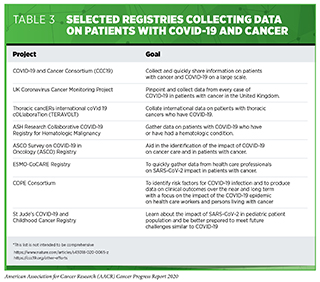
In addition, cancer researchers around the world are working together to leverage their experience to accelerate SARS-CoV-2 vaccine and therapeutic development, and to repurpose treatments used in cancer care for the benefit of patients with COVID-19. An area in which cancer researchers have vast expertise is the collection and sharing of “big data.” This expertise is being harnessed to better understand the effect of COVID-19 on patients with cancer. The goal is to obtain clinical and other patient-related information on a large enough scale to answer questions about the epidemiology of COVID-19 among patients with cancer as well as clinical data on the effectiveness of COVID-19 diagnostics, vaccines, and treatments in these patients. Several cancer organizations as well as multi-institutional teams have already launched initiatives to catalyze data sharing (see Table 3).
Adapting Cancer Research and Medicine in the COVID-19 Era
As highlighted throughout this report and in previous AACR Cancer Progress Reports, the past decade has been an incredible time in cancer research, leading to unprecedented progress against cancer. However, the COVID-19 pandemic forced cancer research laboratories to shutter or to refocus to work on COVID-19-related projects instead of cancer. Attention diverted away from cancer research due to the pandemic, although necessary, will come at a real cost to progress against cancer in the future.
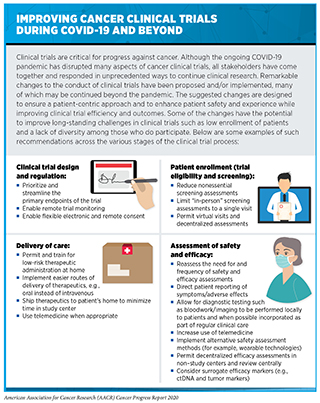
One area of research that has been significantly disrupted during the COVID-19 pandemic is the conduct of all types of clinical trials, including cancer clinical trials. One report estimated that globally, there was a 74 percent decrease in the number of new patients enrolling in clinical trials during the first two weeks of May 2020 compared with the same period in 2019 (100)Gewin V. The career cost of COVID-19 to female researchers, and how science should respond. Nature [Internet]. 2020 [cited 2020 Aug 15];583:867–9.. More recent analysis from the same group indicates that enrollment in clinical trials has increased since the first two weeks of May 2020, but it remains 30 percent lower than before the COVID-19 pandemic (101)Langin K. The pandemic is hitting scientist parents hard, and some solutions may backfire. Science (80- ) [Internet]. 2020 [cited 2020 Aug 15];. In the United States, it was estimated that the number of patients enrolled each week in NCI-sponsored clinical trials more than halved between early March and early April 2020 (102)Woolston C. ‘It’s like we’re going back 30 years’: how the coronavirus is gutting diversity in science. Nature [Internet]. 2020 [cited 2020 Aug 15];. A separate report found that only 20 percent of the U.S. institutions that were surveyed for the report were continuing to enroll patients in cancer clinical trials at pre-COVID-19 rates (103)Medidata. COVID-19 and Clinical Trials: The Medidata Perspective. Release 5.0. 2020;. Among the challenges to clinical trial enrollment and conduct are a decrease in the ability or willingness of patients to go to health care facilities and the limited availability of services such as radiology, surgery, and cardiology (104). All stakeholders are working together to adapt clinical trial practices to ensure that ongoing and new clinical trials can safely continue (104)(105)Unger JM, Blanke CD, LeBlanc M, Hershman DL. Association of the Coronavirus Disease 2019 (COVID-19) Outbreak With Enrollment in Cancer Clinical Trials. JAMA Netw Open [Internet]. American Medical Association; 2020 [cited 2020 Aug 2];3:e2010651.(106)Upadhaya S, Yu JX, Oliva C, Hooton M, Hodge J, Hubbard-Lucey VM. Impact of COVID-19 on oncology clinical trials. Nat Rev Drug Discov [Internet]. 2020 [cited 2020 Aug 2];19:376–7.. Many of the adjustments to cancer clinical trial practice already made and being considered in response to the COVID-19 pandemic have the potential to lead to long-term positive changes (see sidebar on Improving Cancer Clinical Trials during COVID-19 and Beyond).
There are many other challenges in cancer research posed as a result of the COVID-19 pandemic. One of the most concerning is that cancer research laboratories that were shuttered or were refocused to work on COVID-19-related projects instead of cancer will require significant time and resources to reopen and reestablish. It will be critical to provide support to the cancer research that has been interrupted, particularly that conducted by early-career, minority, and female investigators for whom losses to productivity due to the pandemic could have the potential to be career ending (107)Waterhouse DM, Harvey RD, Hurley P, Levit LA, Kim ES, Klepin HD, et al. Early Impact of COVID-19 on the Conduct of Oncology Clinical Trials and Long-Term Opportunities for Transformation: Findings From an American Society of Clinical Oncology Survey. JCO Oncol Pract [Internet]. American Society of Clinical Oncology; 2020 [cited 2020 Aug 2];16:417–21.(108)Doherty GJ, Goksu M, de Paula BHR. Rethinking cancer clinical trials for COVID-19 and beyond. Nat Cancer [Internet]. Nature Publishing Group; 2020 [cited 2020 Aug 2];1:568–72.(109)Lowy: COVID-19 pandemic inspired new “shortcuts” in NCI clinical trials – The Cancer Letter [Internet]. [cited 2020 Aug 2]..
In addition, the COVID-19 pandemic has had an adverse impact across the continuum of cancer care (see Cancer in the Midst of COVID-19. Addressing the delays in cancer screening, diagnosis, and treatment that have occurred since the beginning of the COVID-19 pandemic will require considerable time and innovative solutions. One approach being recommended by some professional organizations to overcome barriers to colorectal cancer screening during the ongoing COVID-19 pandemic is to increase the use of noninvasive stool-based tests because these can be undertaken at home, rather than requiring travel to a health care facility as is needed for a colonoscopy. Another approach being used to help ensure continuity of care for patients with cancer is the use of telemedicine in place of in-person visits. It is imperative that racial and ethnic minorities and other the underserved populations have access to the solutions to the cancer care challenges posed by the COVID-19 pandemic so as not to exacerbate existing cancer health disparities.