- Investments in Research Fuel a Healthier Future
- Building a Diverse Cancer Research Workforce Drives Innovation
- Improving Regulatory Science to Ensure Safety and Efficacy of Cancer Therapies
- Advancing Policy to Strengthen Cancer Prevention and Screening Programs
- Accelerating Progress Against Pediatric Cancers
- Building Health Equity by Addressing Cancer Disparities
- Learning from COVID-19 to Strengthen Digital Health Infrastructure for Cancer Care
Impacting the Future of Cancer Research and Patient Care Through Evidence-Based Policies
In this section, you will learn:
- Continued funding for NIH and NCI is vital to accelerate the pace of new scientific breakthroughs against cancer and build programs focused on the training and retention of a diverse cancer research workforce.
- Federal policy advancements from FDA, CDC, and NCI improve diversity and access to clinical trials, access to cancer screening, and cancer outcomes.
- Disparities in the cancer burden must be addressed through equitable access to health care, insurance, optimal nutrition, and physical activity.
- Public health resources are essential for understanding the impact of cancer health disparities, and these systems need additional investment to truly support the communities they serve.
Investments and policies enacted by Congress and programs implemented by federal agencies including NIH, NCI, CDC, and FDA are essential to making progress against the collection of diseases we know as cancer.
The 21st Century Cures Act, which was signed into law in December 2016, authorized $1.8 billion to fund the Cancer Moonshot, an initiative led by NCI with the goal of accelerating the pace of progress against cancer through prevention, screening, scientific discovery, collaboration, and data sharing, over a seven-year period. Congress has continued to appropriate full funding for the Cancer Moonshot (see sidebar on The Cancer Moonshot).
The 21st Century Cures Act also established the FDA’s Oncology Center of Excellence (OCE). OCE was established to support the development of anticancer therapies with an emphasis on facilitating active collaboration between OCE and other FDA centers. These efforts have focused on diversifying and decentralizing clinical trials to improve minority representation when developing new therapeutic options with the goal of achieving patient-centered regulatory decision making through innovation and collaboration. OCE plays a crucial role in reviewing new breakthrough treatments to ensure they are safe and effective for patients with cancer.
In February 2022, President Joseph R. Biden, Jr., announced a reignited Cancer Moonshot with a mission to “reduce the death rate from cancer by at least 50 percent over the next 25 years and to improve the experience of people and their families living with and surviving cancer—and, by doing this and more, end cancer as we know it today.” The Cancer Moonshot, along with many other cancer-based initiatives, marks the continued commitment of Congress and the Executive Branch to cancer research and improving patient outcomes. To realize the goals of the reignited Cancer Moonshot, the full reach of the federal government, including NIH, NCI, FDA, and CDC, will be utilized to better prevent, detect, and treat cancer.
The Biden administration has also proposed the creation of an Advanced Research Projects Agency for Health (ARPA-H), designed to prioritize high-risk, high-reward approaches to prevent, diagnose, and cure diseases such as cancer. In the Fiscal Year (FY) 2022 funding bill, ARPA-H received $1 billion in start-up capital to begin creation of this new medical research authority, which is proposed to be housed within NIH. As Congress continues to debate the structure and location of ARPA-H, it is imperative that funding for ARPA-H supplement, and not supplant, funding for NIH’s core research functions.
The CDC’s Division of Cancer Prevention and Control (DCPC) is another important federal partner in fueling progress against cancer. DCPC brings science-driven public health interventions, including cancer screening and prevention programs, to communities across the country. DCPC works with state health agencies, territories, tribes and tribal organizations, and other key organizations to develop, implement, and promote effective cancer prevention and control practices.
President Biden’s vision of ending cancer as we know it will not be realized without robust, sustained, and predictable funding for basic research. Significant annual funding increases are essential for NIH, NCI, FDA, CDC, and other agencies to continue their vital work against cancer. Meanwhile, legislation aimed at increasing and diversifying participation in clinical trials; expanding access to quality, affordable health care; and accelerating progress against pediatric cancers will be vital to reducing cancer disparities and achieving health equity. In addition, investments in access to preventive care, reduction of tobacco-related illness through strong federal regulations, and access to healthy lifetime nutrition are some of the ways in which we can decrease cancer incidence and improve outcomes.
Investments in Research Fuel a Healthier Future
Remarkable advances in medical research have led to significant improvements in cancer prevention and reductions in cancer mortality. In the years since the enactment of the National Cancer Act in 1971, cancer mortality has dropped by 27 percent (3)Kratzer TB, et al. Progress against cancer mortality 50 years after passage of the National Cancer Act. JAMA Oncol 2022;8:156-9. [LINK NOT AVAILABLE]. This progress is a result of NCI investments in research that developed state-of-the-art anticancer therapies and more effective screening tools to detect cancers in earlier stages, as well as initiatives through CDC to raise awareness of cancer prevention and the importance of cancer screenings. As a result of these efforts, there are now more than 18 million cancer survivors living in the United States (2)Miller KD, et al. Cancer Treatment and Survivorship Statistics, 2022. CA Cancer J Clin 2022;0:1-28. [LINK NOT AVAILABLE].
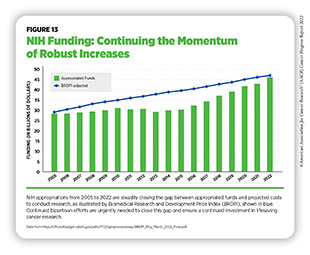
To continue progress against cancer, significant federal investments will be needed. Beginning in FY 2005, a decade of stalled funding at NIH caused budgets to be eroded by inflation. As a result, NIH’s purchasing power—the amount each dollar invested can buy—was reduced by nearly 25 percent compared to the previous decade (586)Extramural Nexus. One nation in support of biomedical research? Accessed: July 14, 2022.[cited 2020 Jul 15].. This had a devastating impact on the ability of NIH to adequately fund research. Thanks to strong bipartisan support, Congress has made investments in medical research a top priority, increasing NIH funding by $14.9 billion over the last seven fiscal years, an increase of roughly 49 percent since FY 2015 (see Figure 13).
In FY 2022 alone, congressional leaders provided an increase of $2.25 billion for NIH and an increase of $353 million for NCI. As a result, NIH’s funding for medical research has almost returned to the capacity last seen in FY 2005, as measured by the Biomedical Research and Development Price Index (BRDPI) (see Figure 13). In particular, Chair Rosa DeLauro (D-CT), Ranking Member Tom Cole (R-OK), Chair Patty Murray (D-WA), and Ranking Member Roy Blunt (R-MO) have demonstrated remarkable leadership and commitment to medical research in their roles on the Labor, Health and Human Services, Education, and Related Agencies Appropriations Subcommittees in the House and Senate, respectively.
NIH funding increases also benefited NCI, which received an increase of $1.96 billion over the last seven fiscal years, to $6.912 billion, an increase of nearly 40 percent. With these funds, NCI provides support through a competitive process to research grants that can cover anything from basic laboratory science to clinical research. Unfortunately, despite seven consecutive years of bipartisan congressional support for investments in medical research, NCI still faces significant funding pressures that limit the amount of support it can provide for meritorious investigator-initiated research.
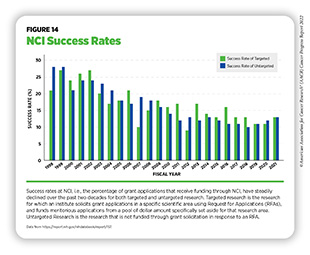
The percentage of approved research grant applications that receive funding are referred to as the success rate. In 1999, success rates reached 32 percent across NIH and 28 percent at NCI. These generous levels of funded grants fueled cancer discoveries at unprecedented rates and contributed to the advances in cancer care that we benefit from today (587)NIH Data Book. NIH Data Book – Success rates: R01-equivalent and research project grants. Accessed: July 14, 2022.[cited 2020 Jul 15].. However, NCI funding has not kept pace with the subsequent exponential growth of applications. Between 2013 and 2018, NCI received a nearly 46 percent increase in grant applications, overshadowing the increase of other institutes at NIH which only increased by 4.9 percent.
Despite the funding provided by Congress, NCI’s success rate in FY 2021 was only 13 percent, less than half the rate of two decades ago (587)NIH Data Book. NIH Data Book – Success rates: R01-equivalent and research project grants. Accessed: July 14, 2022.[cited 2020 Jul 15]. (see Figure 14). NCI’s success rate is also among the lowest of all institutes at NIH. Currently, fewer than one in seven approved grant applications is funded, leaving well-reviewed science unfunded and jeopardizing the United States’ position as a global leader in cancer research. In addition, lack of funding can potentially have far-reaching consequences for the cancer research community and the ability to recruit, train, and retain the next generation of cancer scientists. These trends can result in fewer women and underrepresented minorities (URMs) choosing careers in Science, Technology, Engineering, Mathematics, and Medicine (STEMM). By meeting the NCI Director’s Professional Judgment Budget level of $7.766 billion in FY 2023, NCI can increase the availability of research grants and accelerate the path to discoveries that will save lives.
CDC’s Division of Cancer Prevention and Control (DCPC) works with state and local governments, community organizations, and health providers to promote cancer prevention and early detection. These collaborations include funding for central cancer registries; comprehensive cancer control, which includes state, tribal, local, and territorial organization cancer planning; the National Breast and Cervical Cancer Early Detection Program; and initiatives focused on colorectal, skin, prostate, and ovarian cancer, as well as HPV-associated cancers.
Despite the importance of these public health-related programs, increased investments in CDC’s DCPC have also been minimal. Between FY 2010 and FY 2022, funding for these vital initiatives increased by a total of $8 million, or just 2.9 percent. This amounts to an estimated $100 million deficit relative to what the funding would be if adjusted for inflation from FY 2010 (588)One Voice Against Cancer. More funding needed for CDC cancer programs. Accessed: July 14, 2022.[cited 2020 Jul 15].. As more than 40 percent of cancer cases in the United States each year are linked to modifiable risk factors and can be prevented, these initiatives and collaborations are critical to reduce the cancer burden.
Congress has made a clear and decisive commitment to medical research over the last seven fiscal years, returning NIH to a trajectory of steady funding growth. However, more must be done to expand opportunities in medical research, cancer prevention, and cancer treatment. With so many scientific opportunities to make progress against cancer and other diseases, it is imperative that our elected leaders continue to provide robust, sustained, and predictable increases in funding for medical research and cancer prevention at NIH, NCI, CDC, and FDA.
Building a Diverse Cancer Research Workforce Drives Innovation
To prevent and cure all cancers, the next generation of cancer researchers will require thoughtful education, training, and support throughout their career paths. To realize the full potential of our medical research enterprise, research institutions must be proactive in recruiting, supporting, and retaining a cancer research workforce that reflects the diversity of our society. As described in the AACR Cancer Disparities Progress Report 2022, the amount of diversity within the cancer research workforce lags behind that of the general U.S. population. Also, complex, interrelated factors contribute to the low rates of URMs in STEMM. Proposed methods to overcome cancer disparities include increasing diversity early and consistently throughout the cancer research and care workforce. Furthermore, additional training in mentorship for successful senior scientists helps support the professional development of their trainees. Formal training programs, incentives, and compensation for excellence in mentorship have been shown to increase retention of URM trainees and scientists. NIH and NCI play important roles in fostering development of young researchers into becoming the scientific and clinical leaders of the future.
Encouraging early childhood interest in STEMM improves the likelihood of earning a higher degree (589)Reynolds AJ, et al. A multicomponent, preschool to third grade preventive intervention and educational attainment at 35 years of age. JAMA Pediatrics. Volume 172: American Medical Association; 2018. p 247-56. [LINK NOT AVAILABLE]. NIH sponsors the Science, Education, Partnership Awards (SEPA) Program, which facilitates partnerships between medical and clinical researchers, preK-12th grade teachers, schools, and other educational organizations (590)National Institutes of Health. Science Education Partnership Award. Accessed: July 14, 2022.[cited 2020 Jul 15].. For example, the SEPA-sponsored high school program at the University of Arizona, Q-Cubed, has been instrumental to increasing the percentage of high school students that attend college. Since the launch of Q-Cubed, 98 percent of the program participants either attended or graduated from two- to four-year colleges (591)The University of Arizone. Statistics & evaluation | Q-cubed. Accessed: July 28, 2022.[cited 2020 Jul 15].. These programs and awards provide valuable early exposures to the world of medical research and showcase the benefits of a career in research.
The NCI Center to Reduce Cancer Health Disparities provides funding support for URMs beginning in middle school and continuing to junior tenure-track faculty positions through the Continuing Umbrella of Research Experiences (CURE) program. Between 2001 and 2012, CURE supported more than 3,000 early-career researchers, who generated greater than 1,700 peer-reviewed publications (592)NCI Center to Reduce Cancer Health Disparities. National Cancer Institute’s (NCI’s) Continuing Umbrella of Research Experiences (CURE). Accessed: July 28, 2022.[cited 2020 Jul 15].. In addition, the Intramural Continuing Umbrella of Research Experiences (iCURE) program brings undergraduate students, post-baccalaureate and post-master’s degree individuals, graduate students, and postdoctoral fellows into the NCI research community and supports mentored research experiences. iCURE particularly encourages the participation of individuals from underrepresented populations and aims to further NCI’s interest in increasing diversity in cancer research workforce.
Within the cancer research and care workforce, early-career researchers are instrumental in making advances against cancers as they bring innovative ideas and highly original perspectives to their research projects. Graduate students and postdoctoral fellows are the largest share of the academic research workforce. Trainees can be supported under their advisors’ grants, competitive institutional “T” awards, or individual “F” and “K” awards, as well as competitive philanthropic awards. These awards cover stipend and research costs of promising pre- and postdoctoral scientists, which enables them to take on more ambitious research. Some of these awards are focused on trainees that are URM in STEMM, while others, like the K99/R00 award, are designed to transition postdoctoral researchers into independent investigator positions. NCI and NIH have created several funding mechanisms to directly support URM early-stage investigators (ESIs). For example, the K01, K99/R00, and R21 grant mechanisms support the transition of postdoctoral early-career scientists into becoming independent researchers and some K01 and R21 grants are focused on supporting URM scientists (593)National Cancer Institute. NCI mentored research scientist development award to promote diversity (K01). Accessed: July 14, 2022.[cited 2020 Jul 15].(594)National Cancer Institute. Exploratory grant award to promote workforce diversity in basic cancer research (R21). Accessed: July 14, 2022. [LINK NOT AVAILABLE]. Additionally, NIH Institutes and Centers issued 171 student loan repayment awards in FY 2020 totaling almost $13 million for investigators involved in health disparities research (595)NIH Extramural Nexus. What’s new with the NIH loan repayment programs: FY 2022 applications, anniversaries, and a new program. Accessed: July 14, 2022.[cited 2020 Jul 15].. Focused approaches to funding ESIs, and women researchers identifying as URMs, should be a priority, as this could improve recruitment and retention within the cancer research workforce (see sidebar on NIH and NCI Initiatives to Promote Workforce Diversity and Outreach).
NCI has also taken steps to support junior tenure-track research faculty. For example, NCI has helped ESI applicants establish independent laboratories by extending R01 paylines to the 16th percentile, instead of the standard 11th percentile (596)National Cancer Institute. NCI full year funding policy for RPG awards FY 2022. Accessed: July 14, 2022.[cited 2020 Jul 15].. Additionally, ESI R01 applications within the 11th percentile are eligible for the R37 Method to Extend Research in Time (MERIT) award, which provides funding for up to seven years instead of the traditional five years (597)National Cancer Institute. MERIT Award (R37). Accessed: July 14, 2022.[cited 2020 Jul 15].. The additional time provided by R37 MERIT awards enables further data collection for a second grant application and also supports the awardee through the tenure process, which lasts approximately seven years.
The influx of innovative ideas from young scientists continues to be critical for future breakthroughs against cancer and other deadly diseases. As Congress considers appropriations for NIH and NCI, it will be vital to invest in additional resources to support early-career researchers. Robust, sustained, and predictable funding increases for NIH and NCI are critical to ensure that these programs continue.
Improving Regulatory Science to Ensure Safety and Efficacy of Cancer Therapies
Regulatory review by FDA ensures medical research delivers safe and effective anticancer therapies for patients. To provide efficient oversight, FDA’s processes, staff, and technology must keep pace with the rapid advances in new target discovery and drug development to treat cancer. User fees paid by the industry when submitting applications and congressionally appropriated funds are both essential sources of support to FDA’s mission. Investments from Congress support critical regulatory science programs that help improve the regulatory process and shorten the time it takes for new advances in medicine to reach patients in need.
As one example, FDA OCE was established in 2017 by the 21st Century Cures Act to support development of anticancer therapies and improve regulatory efficiency in oncology. OCE facilitates collaborations between staff members with oncology expertise from other FDA centers, including the Center for Drug Evaluation and Research, Center for Biologics Evaluation, and Center for Devices and Radiological Health.
Diverisfying and Decentralizing Clinical Trials
The types of cancer included in clinical trials see the greatest advances in treatment and survival (598)Bleyer A, et al. Role of clinical trials in survival progress of American adolescents and young adults with cancer—and lack thereof. Pediatric Blood and Cancer 2018;65. [LINK NOT AVAILABLE](599)Hunger SP, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children’s oncology group. Journal of Clinical Oncology 2012;30. [LINK NOT AVAILABLE]. Clinical trial participants often experience better clinical outcomes compared to nonparticipants (600). On average, 55 percent of adult patients with cancer join a trial when asked (601)Unger JM, et al. “When offered to participate”: A systematic review and meta-analysis of patient agreement to participate in cancer clinical trials. Journal of the National Cancer Institute. Volume 1132021. [LINK NOT AVAILABLE]. Unfortunately, overall participation in clinical trials is very low; only 8 percent of adult patients and 19.9 percent of pediatric and adolescent patients with cancer participate in clinical trials in the United States (602)Unger JM, et al. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. Journal of the National Cancer Institute. Volume 1112019. [LINK NOT AVAILABLE](603)Faulk KE, et al. Assessment of enrollment characteristics for Children’s Oncology Group (COG) upfront therapeutic clinical trials 2004-2015. PLoS ONE 2020;15. [LINK NOT AVAILABLE]. While academic medical centers tend to have above average trial participation rates (602)Unger JM, et al. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. Journal of the National Cancer Institute. Volume 1112019. [LINK NOT AVAILABLE], most patients with cancer are seen at community clinics or hospitals where trials are less prevalent. Another key reason for low trial participation is that more than 75 percent of patients with cancer either do not have a trial available for their specific disease or the strict eligibility criteria exclude them because of comorbidities or prior treatments (602)Unger JM, et al. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. Journal of the National Cancer Institute. Volume 1112019. [LINK NOT AVAILABLE]. Additional challenges for clinical trials include communities without any health care facilities, patients not being asked to join a trial, lack of trust in medical research, dependent care needs, and costs and time related to participation (604)Nipp RD, et al. Addressing the financial burden of cancer clinical trial participation: Longitudinal effects of an equity intervention. The Oncologist. Volume 24: Wiley; 2019. p 1048-55. [LINK NOT AVAILABLE](605)Valecha G, et al. Clinical trial awareness in oncology patients of diverse ethnic background: A single-institution analysis. Journal of Clinical Oncology. Volume 382020. [LINK NOT AVAILABLE](606)Institute of Medicine. Barriers to patient recruitment and physician participation. Accessed: June 30, 2022.[cited 2020 Jul 15].. These challenges disproportionately impact racial and ethnic minorities, contributing to disparities in clinical trial participation rates.
Improving representation of racial and ethnic minorities in oncology clinical trials is a key priority of OCE. In April 2022, OCE released draft voluntary guidance on creating prospective diversity action plans when submitting Investigational New Drug or marketing applications (607)U.S. Food and Drug Administration. Diversity plans to improve enrollment of participants from underrepresented racial and ethnic populations in clinical trials guidance for industry. Accessed: July 14, 2022.[cited 2020 Jul 15]..
Voluntary FDA guidance is an important first step to improving clinical trial participation and representation. Additional authority to issue and enforce requirements in clinical trials could greatly enhance positive changes to the drug development process. The Diverse and Equitable Participation in Clinical Trials (DEPICT, H.R. 6584) Act would help accomplish these goals by allowing FDA to require diverse representation (608)Congress.Gov. H.R.6584 – 117th Congress (2021-2022): DEPICT Act. Accessed: June 30, 2022.[cited 2020 Jul 15].. The Diversifying Investigations Via Equitable Research Studies for Everyone (DIVERSE) Trials Act (H.R. 5030/S. 2706) would also support greater trial participation by allowing trial sponsors to reimburse patients for transportation costs and increasing the use of telemedicine and remote data collection (609)Congress.Gov. S.2706 – 117th Congress (2021-2022): DIVERSE Trials Act. Accessed: June 30, 2022.[cited 2020 Jul 15].. Furthermore, engaging patients and other stakeholders is critical to identify creative solutions in the policy making process and build trust in medical research.
Improving Anticancer Therapy Access for Older Adults
FDA is also working to improve trial access and outcomes for patients with cancer older than 65 years old. These patients represent more than half of all patients with cancer (610)National Cancer Institute. Risk Factors: Age. Accessed: August 12, 2022.[cited 2020 Jul 15]., but are often excluded from clinical trials due to explicit age eligibility criteria or exclusion criteria for having other medical conditions or taking medications. As a result, oncology clinical trials include patients that are on average 6.49 years younger than the patient population affected (611)Ludmir EB, et al. Factors Associated With Age Disparities Among Cancer Clinical Trial Participants. JAMA Oncol 2019;5:1769-73. [LINK NOT AVAILABLE].
FDA’s Project Silver is a global regulatory effort to highlight drug development programs with indications particularly impacting patients 65 years old and older. This public health initiative promotes increased enrollment of geriatric patients in clinical trials for anticancer therapeutics (612)U.S. Food and Drug Administration. Project Silver. Accessed: August 12, 2022.[cited 2020 Jul 15].. As part of Project Silver, FDA issued final voluntary guidance in March 2022 to encourage trial sponsors to broaden the age range to increase the number of participants over the age of 65 in oncology clinical trials (613)U.S. Food and Drug Administration. Inclusion of Older Adults in Cancer Clinical Trials. Accessed: August 12, 2022.[cited 2020 Jul 15].. The guidance emphasizes the importance of including older adult patients in early phase trials to analyze safety with co-morbid conditions and other medications. Another key recommendation was to add older adult patients to standard randomized clinical trials as an additional trial arm. This would allow trial sponsors to keep primary endpoints focused on outcomes of younger adult patients, and secondary endpoints could include data from older adult patients while expanding access to investigational therapies.
Congress recently enacted legislation intended to benefit older adults, including those with cancer, who receive health coverage under Medicare. This new law limits the out-of-pocket amount that a Medicare beneficiary would pay for prescription drugs to $2,000 per year beginning in 2025. It also allows the federal government to negotiate the price of some high-cost prescription drugs with manufacturers. Together, these policies are intended to reduce the cost of prescription drugs and make lifesaving treatments and more accessible and affordable.
Advancing Policy to Strengthen Cancer Prevention and Screening Programs
Preventable risk factors, including tobacco use, infections, and UV exposure, account for approximately 40 percent of cancer cases in the United States (see Preventing Cancer: Identifying Risk Factors). Detecting cancer early through routine screenings for common cancers also greatly improves treatment options and outcomes (see Screening for Early Detection). Inequities in access to cancer screenings and follow-up treatment are major contributors to late-stage diagnoses among underinsured and uninsured patients. CDC’s National Breast and Cervical Cancer Early Detection Program and Colorectal Cancer Control Program help provide underserved patients with routine cancer screenings (see sidebar on CDC and NCI Cancer Screening Programs). Unfortunately, limited funds result in continuing gaps in access to cancer screenings (614)Tangka F, et al. The eligibility and reach of the national breast and cervical cancer early detection program after implementation of the affordable care act. Cancer Causes Control 2020;31:473-89. [LINK NOT AVAILABLE]. Additional federal investment for these programs would improve equity in cancer screening and follow-up care. Growing evidence suggests expanding Medicaid has resulted in early detection of breast cancers (615)LeBlanc JM, et al. Association of medicaid expansion under the affordable care act with breast cancer stage at diagnosis. JAMA Surg 2020;155:752-8. [LINK NOT AVAILABLE]; thus, Medicaid expansion is another substantive approach to achieving health equity.
HPV infections can lead to six types of cancer, including nearly every case of cervical cancer (see Prevent and Eliminate Infection with Cancer-causing Pathogens) (616)Centers for Disease Control and Prevention. How many cancers are linked with HPV each year? Accessed: July 14, 2022.[cited 2020 Jul 15].. Guideline-concordant HPV vaccination, cervical cancer screenings, and timely follow-up care are effective strategies to prevent cancer and potentially eliminate all cases of cervical cancer. However, uptake of HPV vaccination has been suboptimal; among eligible U.S. teens in 2020, less than 60 percent were fully vaccinated against HPV (194)Pingali C, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13-17 years – United States, 2020. MMWR Morb Mortal Wkly Rep 2021;70:1183-90. [LINK NOT AVAILABLE]. State-level policies requiring vaccines for other diseases, such as measles, have been particularly effective at nearly eradicating the viruses that cause them. However, only Hawaii, Rhode Island, Virginia, Puerto Rico, and Washington, DC, require HPV vaccination for attending public school (617)National Conference of State Legislatures. HPV vaccine: State legislation and regulation. Accessed: July 14, 2022.[cited 2020 Jul 15].. Eliminating HPV-related cancers will only be achieved by coordinated strategies among all stakeholders to build confidence in vaccination and improve screening and treatment for HPV-related lesions.
Leveraging Policy to Reduce Tobacco-related Illness
Smoking rates among U.S. adults are at a historic low following decades of awareness campaigns and effective tobacco control policies. Adult smoking rates peaked in the 1960s when nearly half of adults smoked. In 2020, 19 percent of U.S. adults regularly used any tobacco product (87)Cornelius ME, et al. Tobacco Product Use Among Adults – United States, 2020. MMWR Morb Mortal Wkly Rep 2022;71:397-405. [LINK NOT AVAILABLE], and only 12.5 percent of adults regularly smoked cigarettes. Concerningly, tobacco use remained higher among U.S. youth in 2020, including 23.6 percent of high school students who used tobacco products, primarily flavored e-cigarettes (618)Truth Initiative. E-cigarettes drive overall youth tobacco use to highest rate in decades. Accessed: July 15, 2022.[cited 2020 Jul 15].(619)U.S. Food and Drug Administration. Results from the annual national youth tobacco survey. Accessed: July 15, 2022.[cited 2020 Jul 15].. The ongoing epidemic of youth nicotine addiction threatens to reverse progress made against tobacco-related disease. Additional tobacco control policies across all levels of government remain important to continue reducing tobacco-related cancers as smoking remains the number one preventable cause of cancer.
In April 2022, FDA unveiled two proposals that would prohibit menthol cigarettes, as well as all flavored cigars (620)Federal Register. Tobacco product standard for menthol in cigarettes. Accessed: July 15, 2022.[cited 2020 Jul 15].(621)Federal Register. Tobacco product standard for characterizing flavors in cigars. Accessed: July 15, 2022.[cited 2020 Jul 15].. These proposals were welcomed by public health organizations, including AACR, that have advocated for a menthol cigarette ban for nearly 10 years. A large body of evidence, including studies from the tobacco industry, demonstrates that menthol increases smoking initiation, nicotine exposure, and the difficulty of tobacco use cessation (622)Villanti AC, et al. Menthol cigarettes and the public health standard: a systematic review. BMC Public Health 2017 17:1 2017;17:1-13. [LINK NOT AVAILABLE] (see Figure 15). Decades of predatory advertising practices for menthol cigarettes in predominantly racial and ethnic minority communities are responsible for large tobacco-related health disparities (623)Campaign for Tobacco-free Kids. Stopping menthol, saving lives. Accessed: July 15, 2022.[cited 2020 Jul 15]..
Additionally, FDA also announced in June 2022 that it would pursue a new proposal to limit the amount of nicotine in combustible tobacco products (625)U.S. Food and Drug Administration. FDA announces plans for proposed rule to reduce addictiveness of cigarettes and other combusted tobacco products. Accessed: July 15, 2022.[cited 2020 Jul 15].. This rule is estimated to prevent eight million tobacco-related deaths during the next 80 years (626)Apelberg BJ, et al. Potential public health effects of reducing nicotine levels in cigarettes in the united states. https://doiorg/101056/NEJMsr1714617. Volume 378: Massachusetts Medical Society; 2018. p 1725-33. [LINK NOT AVAILABLE]. If finalized, this could be one of the most powerful regulations ever implemented by FDA to protect public health.
In an effort to address the negative public health impacts of e-cigarettes, especially among youth, FDA deemed e-cigarettes to be classified as tobacco products and therefore under FDA’s authority. Following this classification, manufacturers were required to submit premarket tobacco product applications (PMTAs) for e-cigarettes to FDA for regulatory review. The Family Smoking Prevention and Tobacco Control Act places the responsibility on the manufacturers to provide scientific evidence within PMTAs proving that their products are appropriate for the protection of public health. More than 6.6 million PMTAs were submitted to FDA by the September 2020 deadline (627)U.S. Food and Drug Administration. FDA issues decisions on additional e-Cigarette products. Accessed: July 15, 2022.[cited 2020 Jul 15].. FDA has since reached decisions on 99 percent of the submitted products, and almost all were denied marketing orders. In 2022, FDA reached decisions on several e-cigarette brands with large market shares. While FDA authorized several VUSE and NJOY branded e-cigarettes, they decided to remove JUUL-branded e-cigarettes from the market, pending an appeal (628)U.S. Food and Drug Administration. FDA denies authorization to market JUUL products. Accessed: July 15, 2022.[cited 2020 Jul 15].. JUUL e-cigarettes comprised approximately 75 percent of the e-cigarette market in 2019 and were a major contributor to a doubling of the youth e-cigarette use between 2017 and 2019 (629)Wang TW, et al. Tobacco product use and associated factors among middle and high school students – United States, 2019. MMWR Surveill Summ 2019;68:1-22. [LINK NOT AVAILABLE](630)technavio Blog. JUUL market share in 2019: Dominating the US e-cigarette market. Accessed: July 15, 2022.[cited 2020 Jul 15].. JUUL’s intentional marketing to youth and addicting millions to nicotine demonstrate that its products are not appropriate for public health.
Further policies that could reduce tobacco-related illness include expanding flavor prohibitions to all tobacco products; increasing restrictions on tobacco product advertising and promotions; and increasing funding for awareness and cessation programs within FDA, NCI, and CDC’s Office on Smoking and Health.
Accelerating Progress Against Pediatric Cancers
Pediatric cancers are the leading cause of disease-related deaths in children up to the age of 14 years (1). Advances in cancer treatments over the last few decades have resulted in an increase in survival rates for pediatric cancer to 85 percent (1)Siegel RL, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [LINK NOT AVAILABLE]. However, there are many types of pediatric cancers with significantly poorer outcomes and for which there are no effective treatments. Additionally, children who survive cancer face long-term side effects from their treatment, as well as life-threatening late effects of childhood cancer (see Challenges Faced by Cancer Survivors). It is imperative to develop policies that support identifying new treatments for pediatric cancers and advocate for survivors of childhood cancers. This is critical to ensuring the best outcome for every child impacted by cancer. The most comprehensive childhood cancer legislation to date was passed by Congress in 2018, the Childhood Cancer Survivorship, Treatment, Access, and Research (STAR) Act. Congress has consistently appropriated $30 million per year to fund programs created by the STAR Act. Numerous provisions within the STAR Act have been implemented to improve data collection, tracking, and survivorship support related to childhood cancers, such as:
- Awarding NCI grants to support and expand the collection of biospecimens from children, adolescents, and young adults diagnosed with cancer;
- Expanding childhood cancer surveillance programs at CDC by developing a new cloud-based data reporting system;
- Supporting research that will investigate the late effects of pediatric cancer treatments, improve collaboration among health care providers, and identify novel methods of care for pediatric cancer survivors; and
- Mandating the inclusion of at least one pediatric oncologist on the National Cancer Advisory Board.
Continued full appropriations will be essential to realizing the potential of the STAR Act. The Childhood STAR Reauthorization Act (H.R. 7630/S. 4120) was introduced in the House and the Senate in April 2022. Congress will need to reauthorize the STAR Act before its expiration at the end of FY 2023 to continue NCI-supported research and further development of biorepositories, identify and train pediatric cancer researchers, and strengthen infrastructure to capture pediatric cancer incidences.
The Childhood Cancer Data Initiative (CCDI) is another NCI program designed to improve data collection and research sharing related to pediatric cancers. The goals are to better understand cancer biology specific to children and to improve prevention, treatment, quality of life, and survivorship. CCDI funding is proposed for 10 years, from FY 2020 to FY 2029, with $50 million to be allocated each year. Congress fully funded the initiative in both FY 2020 and FY 2021. NCI has granted CCDI funds for pediatric cancers and research activities and has also engaged the entire childhood cancer community in the implementation of the initiative. In March 2022, the CCDI Molecular Characterization Initiative was launched to characterize tumors and develop biomarker testing in children (631)National Cancer Institute. Molecular characterization initiative for childhood cancers. Accessed: July 15, 2022.[cited 2020 Jul 15].. These data will allow researchers to develop better clinical trials, identify the drivers of pediatric cancers, and support development of novel treatments for some pediatric cancers that currently lack effective treatments.
Molecularly targeted therapies have shown remarkable success for the treatment of adults with specific mutations that fuel cancer development. Many pediatric cancers exhibit the same mutations as adult cancers. However, designing clinical trials only for pediatric cancers with specific mutations is difficult because all pediatric cancers are rare. The low availability of molecularly targeted trials for pediatric patients means that targeted drugs approved to treat adult forms of cancer often do not get approved for children even when there is a strong potential of benefit. To address this issue, Congress passed key provisions for the Research to Accelerate Cures and Equity (RACE) for Children Act as part of the FDA Reauthorization Act of 2017 to amend the Pediatric Research Equity Act (PREA). In August 2021, the RACE Act came into full effect. It requires drug manufacturers to study molecularly targeted therapeutics developed for adult patients with cancer in pediatric populations with the same mutations. In response to these provisions, FDA developed a Pediatric Molecular Target List to provide guidance to companies as they plan for new drug and biologic submissions (632)U.S. Food and Drug Administration. Pediatric oncology. Accessed: July 15, 2022.[cited 2020 Jul 15].. Additionally, applications submitted to FDA for therapies that meet the RACE Act criteria must have agency-approved pediatric study plans (633)U.S. Food and Drug Administration. Pediatric study plans: content of and process for submitting initial pediatric study plans and amended initial pediatric study plans. Accessed: July 15, 2022.[cited 2020 Jul 15]..
New discoveries in understanding the biology of pediatric cancers and the connection to birth defects are also being supported by The Gabriella Miller Kids First Pediatric Research Program (Kids First) at NIH. Funding for this program was established in the Gabriella Miller Kids First Research Act, passed by Congress in 2014. As of 2021, the program had completed genome sequencing of more than 20,000 participants within 44 childhood cancer and structural birth defect cohorts for whole genome sequencing and is in the process of selecting additional cohorts for 2022. More than $75 million has been invested in pediatric research through this initiative. The bipartisan Gabriella Miller Kids First Research Act 2.0 was introduced in the House in January 2021 and would redirect penalties against pharmaceutical, cosmetic, supplement, and medical device companies for specified violations to the Kids First program, which is part of the NIH Common Fund. NIH would make allocations from this fund to support lifesaving pediatric research that does not duplicate existing activities.
The central sources of health insurance coverage for more than half of the children in the United States are Medicaid and the Children’s Health Insurance Program (CHIP) (634)Medicaid.Gov. Federal Fiscal Year (FFY) 2020 Statistical Enrollment Data System (SEDS) reporting. Accessed: July 15, 2022.[cited 2020 Jul 15].(635)ChildStats.Gov. POP1 Child population: Number of children (in millions) ages 0-17 in the United States by age, 1950-2020 and projected 2021-2050. Accessed: July 15, 2022.[cited 2020 Jul 15].. Coverage is limited to providers in the child’s home state. If out-of-state care is necessary for treatment, the health care provider and his or her team are required to undergo screening and enrollment within the Medicaid program in the child’s home state. In addition to funding research to identify novel treatments for pediatric cancers, it is imperative for Congress to reduce the regulatory hurdles for eligible health care providers to treat children enrolled in Medicaid or CHIP across state lines. The Accelerating Kids’ Access to Care Act would improve access to time-sensitive care by allowing eligible out-of-state providers to enroll in multiple state Medicaid programs without undergoing additional state-by-state screening.
Building Health Equity by Addressing Cancer Disparities
As described in the AACR Cancer Disparities Progress Report 2022 and discussed by Congresswoman Nikema Williams), systemic disadvantages greatly contribute to poorer health outcomes for medically underserved populations. Centuries of policies that restrict housing, educational, and employment opportunities for racial and ethnic minorities have led to lower health insurance coverage rates, lower utilization of preventive health services, poor nutrition, and inadequate access to quality health care. Additionally, the underrepresentation of high-quality health care facilities in low-income neighborhoods and rural communities results in a lower quality of care even for those who can afford it. Reducing cancer health disparities will require a long-term, multipronged approach that supports individuals, communities, health care centers, and federal agencies, as well as local, tribal, and state governments. Recent policy developments related to cancer screening, clinical trial participation, nutrition, and health insurance have demonstrated that progress in addressing cancer health disparities is occurring.
Routine cancer screenings are necessary to detect precancerous lesions as early as possible in cancer development; however, variability along the cancer screening continuum contributes to cancer health disparities. In 2021, USPSTF broadened lung cancer screening requirements and eligibility, reducing previously identified disparities (636)Pu CY, et al. Comparison between the 2021 USPSTF lung cancer screening criteria and other lung cancer screening criteria for racial disparity in eligibility. JAMA Oncol 2022;8:374-82. [LINK NOT AVAILABLE]. Unfortunately, follow-up care is less likely to occur in minority populations for many reasons, including being uninsured or underinsured, decreased access to care, health care system bias, and miscommunication with health care providers (270)Kaiser Family Foundation. Racial disparities in cancer outcomes, screening, and treatment. Accessed: July 15, 2022.[cited 2020 Jul 15].. To address the health care needs of medically underserved populations, the Affordable Care Act provided states the option to expand Medicaid coverage to families earning 138 percent of the federal poverty line or less. In June 2022, the North Carolina Senate passed House Bill 149 that would expand Medicaid no later than July 2023 (637)General Assembly of North Carolina. House Bill 149. Accessed: July 15, 2022.[cited 2020 Jul 15].. If House Bill 149 passes the North Carolina House of Representatives and is signed into law, North Carolina will join 38 other states (and Washington, DC) in having expanded Medicaid coverage (638)Kaiser Family Foundation. Status of state medicaid expansion decisions: Interactive map. Accessed: July 15, 2022.[cited 2020 Jul 15].. Uninsured rates in those states have decreased by nearly half in states that have expanded Medicaid compared to those that have not (638)Kaiser Family Foundation. Status of state medicaid expansion decisions: Interactive map. Accessed: July 15, 2022.[cited 2020 Jul 15].. Medicaid expansion has been particularly beneficial for young adult survivors of cancer (639)Su CT, et al. Affordable care act and cancer survivors’ financial barriers to care: Analysis of the national health interview survey, 2009-2018. JCO Oncology Practice2021. [LINK NOT AVAILABLE](640)Nathan NH, et al. Evaluating Medicaid expansion benefits for patients with cancer: National Cancer Database analysis and systematic review. J Cancer Policy 2021;29:100292. [LINK NOT AVAILABLE], who have seen dramatic increases in the ability to afford health care and are therefore less likely to skip medications or delay refills.
Food security—having reliable access to affordable and nutritious food—is instrumental to cancer treatment adherence and survival (641)Gany F, et al. Do our patients have enough to eat?: Food insecurity among urban low-income cancer patients. J Health Care Poor Underserved 2014;25:1153-68. [LINK NOT AVAILABLE]. The United States Department of Agriculture has two categories for food insecurity: low and very low. Low food security is reported reduced quality, variety, or desirability of diet without any indication of decreased food intake. Very low food security is a disruption of eating patterns with reduced food intake (642)HealthyPeople.Gov. Food insecurity. Accessed: July 15, 2022.[cited 2020 Jul 15].(643)USDA Economic Research Services. Definitions of food security. Accessed: July 15, 2022.[cited 2020 Jul 15].. Low food security can contribute to obesity, a known risk factor for many different cancers (644)Centers for Disease Control and Prevention. Obesity and cancer. Accessed: July 15, 2022.[cited 2020 Jul 15].. Very low food security is a gap in navigating cancer management as patients with cancer and survivors of cancer may be without reliable access to a sufficient quantity of affordable, nutritious food (645)Patel KG, et al. Food insecurity screening: A missing piece in cancer management. Cancer 2019;125:3494-501. [LINK NOT AVAILABLE].
Addressing the nutritional needs of patients with cancer and survivors has the potential to decrease cancer disparities and promote healthy outcomes. One of the encouraging efforts from CDC is the Racial and Ethnic Approaches to Community Health initiative (646)Centers for Disease Control and Prevention. Racial and ethnic approaches to community health. Accessed: July 15, 2022.[cited 2020 Jul 15].. This program funds local, culturally appropriate public health efforts that promote reaching one’s full health potential. That includes promoting exercise and ensuring underserved individuals have options for good nutrition across their lifespan.
Several additional initiatives organized by NIH, NCI, the National Institute on Minority Health and Health Disparities (NIMHD), and CDC are designed to address cancer disparities. For example, NIH’s All of Us program aims to improve precision medicine research by building one of the largest and most diverse health databases. To date, over 400,000 people have joined the research program. The NCI Community Oncology Research Program is a national network that brings cancer clinical trials and care delivery studies to people in their own communities (647)National Cancer Institute. The NCI Community Oncology Research Program (NCORP). Accessed: July 28, 2022.[cited 2020 Jul 15].. Additionally, the NCI Center to Reduce Cancer Health Disparities supports disparities research within NCI and reinforces training a diverse cancer research workforce. NIMHD is NIH’s core institute to support research on the many factors that contribute to disparate health outcomes, including socioeconomics, politics, discrimination, culture, and environment. Several NIMHD-promoted funding opportunities will support the investigation of underlying factors contributing to disparities in liver and lung cancer in medically underserved populations (648)National Institute of Minority Health and Health Disparities. Solicited and investigator-initiated research. Accessed: July 15, 2022.[cited 2020 Jul 15].. CDC’s National Program of Cancer Registries is essential for understanding the scope of cancer disparities by tracking cancer rates and incidence across the United States.
Learning from COVID-19 to Strengthen Digital Health Infrastructure for Cancer Care
A robust public health infrastructure is vital for building capacity to prevent chronic diseases, such as cancer, promote healthy living, and prepare for and respond to emergencies. Every public health service relies on basic infrastructure and staffing to understand and respond to the needs of a community. However, chronic underfunding of public health efforts has left federal, state, and local public health agencies with limited staff and obsolete technology (649)Maani N, et al. COVID-19 and underinvestment in the public health infrastructure of the United States. Milbank Q 2020;98:250-9. [LINK NOT AVAILABLE](650)Kaiser Family Foundation. Hollowed-out public health system faces more cuts amid virus. Accessed: July 15, 2022.[cited 2020 Jul 15].. Federal funds cover roughly one quarter of public health spending in the United States, while the remaining three quarters comes from state and local governments (650)Kaiser Family Foundation. Hollowed-out public health system faces more cuts amid virus. Accessed: July 15, 2022.[cited 2020 Jul 15].. Unfortunately, per capita state public health funding decreased 16 percent between 2010 and 2020 and local funding decreased 18 percent. Rural communities are especially affected by public health divestment (650)Kaiser Family Foundation. Hollowed-out public health system faces more cuts amid virus. Accessed: July 15, 2022.[cited 2020 Jul 15].(651)New York Times. ‘Small town, no hospital’: Covid-19 is overwhelming rural west Texas. Accessed: July 15, 2022.[cited 2020 Jul 15].. Public health departments have struggled to quickly hire staff and replace outdated technology (652)Kaiser Family Foundation. States have yet to spend hundreds of millions of federal dollars to tackle covid health disparities. Accessed: July 15, 2022.[cited 2020 Jul 15].. Challenges during the COVID-19 pandemic for cancer screening and prevention programs have clearly demonstrated that robust and sustainable investments are needed to strengthen public health infrastructure to eliminate disparities in access to cancer services (653)Trust for America’s Health. The impact of chronic underfunding on America’s public health system: Trends, risks, and recommendations, 2021. Accessed: Dec 17, 2021.[cited 2020 Jul 15].(654)Fedewa SA, et al. Changes in cancer screening in the US during the COVID-19 pandemic. JAMA Netw Open 2022;5:e2215490. [LINK NOT AVAILABLE].
Effective public health programs for cancer prevention depend on high-quality data to identify which communities and population groups are most impacted. However, public health data-reporting systems and quality of data collected vary greatly across geography and facility type (655)Politico. Bad state data hides coronavirus threat as Trump pushes reopening. Accessed: July 15, 2022.[cited 2020 Jul 15].(656)MedPage Today. Nursing homes shocked at ‘insanely wrong’ CMS data on COVID-19. Accessed: Nov 12, 2021.[cited 2020 Jul 15].. It is concerning that many states continue to rely on outdated fax machines to report public health data (657)Kaiser Family Foundation. Faxes and snail mail: Will pandemic-era flaws unleash improved health technology? Accessed: July 15, 2022.[cited 2020 Jul 15].. Fortunately, Congress appropriated an initial $50 million for CDC Data Modernization activities in FY 2020 (658)Centers for Disease Control and Prevention. Surveillance and data strategy: Notable milestones. Accessed: July 15, 2022.[cited 2020 Jul 15].; an additional $1 billion was included in the CARES Act and the American Rescue Plan as well as $50 million in FY 2021 and $100 million in FY22 appropriations (659)Centers for Disease Control and Prevention. FY 2022 operating plan. Accessed: July 15, 2022.[cited 2020 Jul 15].. These funds represent a down payment on the first ever national automated public health reporting system. This system could greatly improve the efficiency of monitoring public health issues, such as cancer incidence and risk factors like obesity, as well as support real-world evidence studies to analyze population-level efficacy of cancer treatments, screenings, and prevention programs.
The growing use of telehealth during the COVID-19 pandemic by patients with cancer has demonstrated the importance of reliable and fast Internet connections for cancer care (see sidebar on What Is Telemedicine?). Unfortunately, approximately 42 million Americans lack access to Internet fast enough to stream video (660)Broadband Now Research. Broadbandnow estimates availability for all 50 states; confirms that more than 42 million americans do not have access to broadband. Accessed: July 15, 2022.[cited 2020 Jul 15].. Historically marginalized urban and rural communities disproportionately experience limited access to Internet services. In FY 2020, Congress appropriated $8 billion for efforts to expand Internet and telehealth infrastructure (661)Universal Services Administrative Co. 2020 annual report. Accessed: July 15, 2022.[cited 2020 Jul 15].. Furthermore, the 2021 Bipartisan Infrastructure law included an additional $65 billion for Internet access and to subsidize subscription costs for low-income families(662)U.S. Department of Commerce. Fact sheet: Department of Commerce’s use of bipartisan infrastructure deal funding to help close the digital divide. Accessed: July 15, 2022.[cited 2020 Jul 15].. Continued support for increased Internet access and digital public health infrastructure at the federal and local levels will be vital for addressing public health challenges.
Next Section: Conclusion Previous Section: Looking to the Future of Cancer Science and Medicine