Executive Summary
Unprecedented progress in medical research is increasing our understanding of the collection of diseases we call cancer and is driving remarkable improvements in cancer prevention, early detection, diagnosis, and treatment. These advances are made possible by investments in NIH, NCI, FDA, and CDC by the U.S. federal government. As the first and largest professional organization in the world dedicated to preventing and curing all cancers, AACR has been and continues to be a catalyst for scientific breakthroughs that save and enhance the lives of patients with cancer. AACR is also committed to increasing public understanding of cancer and advocating for increased federal funding for medical research.
The annual AACR Cancer Progress Report to Congress and the American public is a cornerstone of AACR’s educational efforts. This thirteenth edition of the report highlights how research continues to extend and improve the lives of Americans, including the lives of the eight courageous individuals featured in the report and their family members who have shared their experiences with cancer. It also underscores how unwavering, bipartisan support from Congress, in the form of robust and sustained annual increases in funding for NIH, NCI, FDA, and CDC, is urgently needed if we are to realize our vision of eradicating cancer for all populations.
Cancer in 2023
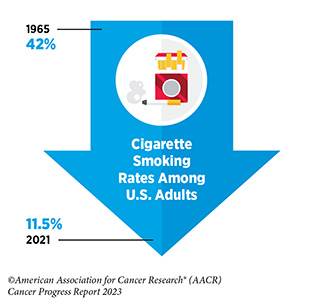
The remarkable progress being made against cancer is resulting in a steady reduction in cancer death rates, and a consistent rise in the number of people who live longer and fuller lives after a cancer diagnosis. In fact, the overall U.S. cancer death rate has fallen by 33 percent between 1991 and 2020, a reduction that translates into averting an estimated 3.8 million deaths from cancer. The reduction in overall cancer mortality is driven largely by the decline in the U.S. lung cancer death rate, the pace of which has accelerated in recent years because of reduction in smoking and advances in early detection and treatment. Additionally, the reduction in death rates for melanoma, colorectal cancer, prostate cancer, and female breast cancer has contributed to the progress against overall U.S. cancer mortality. Notably, among U.S. children (14 years or younger) and adolescents (15 to 19 years), overall cancer death rates have declined by 70 percent and 64 percent, respectively, over the past five decades, driven largely by improvements in treatment.
Despite significant advances, cancer continues to be an ongoing public health challenge in the United States and around the world. In the United States alone, it is estimated that nearly 2 million new cancer cases will be diagnosed in 2023. Among the challenges we face is the fact that the advances we have made have not been uniform for all types and stages of cancer. For example, while the death rates for many of the commonly diagnosed cancers in the United States—including breast, lung, and prostate cancer—have been declining, those for other forms of cancer—most notably pancreatic and uterine cancer—have been increasing. Moreover, the burden of cancer is shouldered disproportionately by certain segments of the population, including racial and ethnic minorities and patients from other medically underserved populations. These disparities are driven by complex and interrelated factors, called social determinants of health.
The burden of cancer and its economic toll, both on individuals and on the U.S. health care system, are expected to rise in the coming decades, underscoring the urgent need for more research to accelerate the pace of progress against cancer. The progress highlighted in this report was made as a direct result of the cumulative efforts of individuals working across the spectrum of medical research and the support from the federal government. Public sector funding from NIH and NCI directly contributes to patient benefit such as through the development of lifesaving anticancer therapeutics. Continued federal investments in NIH, NCI, FDA, and CDC will help the medical research community maintain the momentum of scientific and technological innovation, accelerate the pace of progress against cancer, and ensure that we achieve the President’s Cancer Moonshot goal of reducing U.S. cancer death rates by 50 percent by the year 2047.
Understanding the Path to Cancer Development
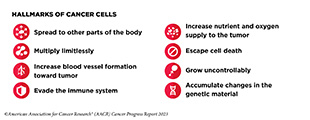
Decades-long research in basic, translational, clinical, and population sciences, and the breakthroughs stemming from it, have advanced our understanding of cancer development. Insights gleaned from this knowledge have revealed cancer as a collection of diseases that are characterized by unchecked cell multiplication. We now understand that different cancer types share many so-called hallmarks or characteristics. These hallmarks are primarily acquired through alterations in the genetic material of normal cells. The nature and the type of genetic alterations determine when cancer is initiated, how fast it progresses, and where in the body it spreads.
Research has shown that there are two types of genetic mutations associated with cancer: inherited and somatic. Inherited mutations are passed on from parents to their progeny and contribute to about 10 percent of all cancer cases. Most cancers are caused by somatic mutations. Somatic mutations are acquired throughout a person’s lifetime and can arise in multiple ways, including from errors made during cell division, smoking, certain viral infections, exposure to UV radiation, and/or exposure to mutagens or other cancer-causing chemicals.
Although cancer is a genetic disease at a fundamental level, transformation of normal cells into cancer cells, accumulation of cancer cells to form tumors, and spread of tumors to distant sites are all complex, multistep processes that are further influenced by changes outside the cell. As the disease progresses, cancer cells acquire additional characteristics that give them the ability to manipulate their cellular and molecular environment. The resultant tumor microenvironment can affect how the tumor grows and spreads, and cancer cells can reciprocally influence the tumor microenvironment to promote their survival.
A technological revolution in our ability to study cancer at the levels of single cells and molecules has led to important insights, one of which is that each patient’s cancer is unique, thus providing the basis for precision medicine. Also called personalized medicine, precision medicine is broadly defined as treating patients based on molecular characteristics that distinguish them from other individuals with the same disease. As ongoing research continues to unravel the mechanisms of cancer onset and progression, researchers are already leveraging existing knowledge to develop more effective, personalized anticancer therapeutics and improve health outcomes for patients with cancer.
Reducing the Risk of Cancer Development
Research in basic, translational, and population sciences has broadened our understanding of the factors that increase an individual’s risk of developing cancer. Many of these risk factors are modifiable, such as reducing tobacco use, avoiding an unhealthy diet, lowering physical inactivity, lowering exposure to UV radiation, limiting alcohol consumption, and preventing pathogenic infections. It is estimated that 40 percent of all cancer cases in the United States are attributable to preventable causes.
The development and implementation of public education and policy initiatives designed to eliminate or reduce exposure to preventable causes have reduced cancer incidence, morbidity, and mortality in the United States. Unfortunately, while the prevalence of some risk factors like tobacco use is on the decline, the rise in prevalence of other risk factors such as obesity threatens to reverse the significant progress against cancer that has been made in the last five decades. Therefore, it is essential that all stakeholders work together to enhance the dissemination of our current knowledge to reduce cancer risk and implement evidence-based policies and programs that minimize the incidence, morbidity, and mortality of cancer attributable to preventable causes.
Certain cancer risk factors are not always easy to avoid. These include carcinogens and pollutants encountered in the environment. Hormonal factors that result from normal physiology can also increase or decrease the risk of developing certain types of cancers. Furthermore, occupational or life stressors, such as chronic stress, lack of sleep, and night shift work, increase a person’s risk of developing certain types of cancers.
As we learn more about environmental and occupational cancer risk factors and identify segments of the U.S. population who are exposed to these factors, new and equitable policies need to be developed and implemented to reduce cancer risk and improve the health of all populations.
Screening for Early Detection
Cancer screening means checking for the disease, or for abnormal cells that may become cancerous, in people who have no signs or symptoms of cancer. Cancer screening can help detect aberrations at the earliest possible stage during cancer development when they are successfully treatable, with a higher likelihood of being cured. The overarching goal of recommended screening is to reduce the burden of cancer at the population level.
Cancer screening recommendations are developed for individuals who are at an average or higher-than-average risk of being diagnosed with cancer. Key considerations that determine who should receive screening and for which cancer include gender and age, as well as genetic, environmental, behavioral, and social influences.
In the United States, the U.S. Preventive Services Task Force (USPSTF)—an independent, volunteer panel of national experts in disease prevention and evidence-based medicine— has recommendations for individuals who are at an average risk of being diagnosed with breast, cervical, colorectal, or prostate cancer. The USPSTF recommends that people who currently smoke or have a history of smoking, i.e., individuals who are at a high risk of being diagnosed with the disease, receive lung cancer screening.
Despite the evidence that cancer screening saves lives, systemic and structural barriers disproportionately limit the access of medically underserved populations to routine cancer screening. Researchers are using evidence-based interventions—such as comprehensive public health campaigns and culturally tailored strategies—to reduce these barriers, but more work is needed to ensure that all eligible individuals have equitable access to routine cancer screening and follow-up testing if findings of the screening test are abnormal.
Use of artificial intelligence (AI) to aid clinicians in cancer detection, and of liquid biopsy to detect multiple types of cancer from a single test, is an exciting new frontier that holds enormous potential for improving early detection of cancer. In recent years, FDA has approved several AI-assisted medical devices to aid clinicians in cancer diagnosis. However, a cautious approach to using AI in cancer care is warranted to avoid exacerbating inequities that can result from the fact that much of the data used to train current AI-driven models lacks diverse and proportional representation of the patient population. Similarly, although liquid biopsy-based tests using the existing specimens from patients with cancer have shown the great potential of this approach for early detection, large prospective studies must demonstrate that screening using these tests can extend lives before these tests can be used in the clinic for simultaneous detection of multiple cancers.
Advancing the Frontiers of Cancer Science and Medicine
The dedicated efforts of individuals working in medical research fuel advances across the clinical cancer care continuum that are improving survival and quality of life for people around the world. Surgery, radiotherapy, and cytotoxic chemotherapy are three of the five main pillars of cancer treatment. However, these therapies can have long- term adverse effects on patients. Through ongoing clinical trials, researchers are evaluating whether less aggressive surgery, radiotherapy, and cytotoxic chemotherapy can be appropriate for some patients, allowing them to experience improved quality of life without adverse effect on their long-term survival.
Among the advances made from August 1, 2022, to July 31, 2023, are the 14 new anticancer therapeutics approved for use by FDA. During this period, FDA also approved two new optical imaging agents to help visualize cancerous tissue and new uses for 12 previously approved anticancer therapeutics.
Seven of the new anticancer therapeutics approved by FDA target specific molecules involved in cancer development and are referred to as molecularly targeted therapeutics. They are part of the precision medicine revolution in cancer care that is improving the lives of numerous patients. Among these treatments is the first folate receptor alpha (FRα) targeted therapeutic, mirvetuximab soravtansine-gynx (Elahere) that was approved for patients with ovarian cancer, such as Jaclyn (Jackie) VanRaaphorst. Among the FDA expansions of previously approved therapy is the combination of HER2- targeted therapeutics, tucatinib (Tukysa) and trastuzumab (Herceptin), a new and first of a kind treatment option for certain patients with colorectal cancer, such as Brian Beck.
In the 12 months from August 1, 2022, to July 31, 2023, FDA also approved several molecularly targeted therapeutics for patients with rare forms of cancer, including certain bile duct cancers and hematologic cancers.
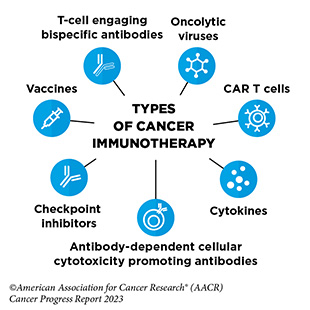
Spotlight: Immunotherapy— Pushing the Frontier of Cancer Medicine
Cancer immunotherapeutics work by unleashing the power of a patient’s immune system to fight cancer the way it fights pathogens, like the virus that causes flu and the bacterium that causes strep throat. Not all immunotherapeutics work in the same way.
Over the past decade, cancer immunotherapy has emerged as one of the most exciting new approaches to cancer treatment. This is, in part, because some patients with metastatic disease who have been treated with these revolutionary anticancer treatments have had remarkable and durable responses, raising the possibility that they might be cured. It is also because some of the immunotherapeutics have been shown to work against an increasingly broad array of cancer types.
Immune checkpoint inhibitors (ICIs) are a class of immunotherapeutics that can release the brakes on T cells and trigger T cells to destroy cancer cells. The first ICI, ipilimumab (Yervoy), was approved by FDA in 2011 for the treatment of patients with metastatic melanoma. Since then, the use of ICIs has expanded rapidly, and these revolutionary new therapeutics have transformed the landscape of cancer treatment.
As of July 31, 2023, the FDA has approved 11 ICIs, and there is at least one ICI approved for treating 20 cancer types and for treating any type of solid tumors that share certain molecular characteristics. Just in the 12 months covered in this report, between August 1, 2022, and July 31, 2023, FDA approved two new ICIs, tremelimumab (Imjudo) and retifanlimab-dlwr (Zynyz), for the treatment of patients with a certain type of liver cancer and a rare form of skin cancer. During the same period, FDA also expanded the use of a previously approved ICI, atezolizumab (Tecentriq), for the treatment of patients including children and young adults with alveolar soft part sarcoma, such as Isabella (Bella) Snow Fraser and Alexis Browning.
CAR T-cell therapy is designed to dramatically increase the number of cancer-killing T cells a patient has, thereby boosting the immune system’s ability to seek and destroy cancer cells. The first CAR-T cell therapy was approved in
2017 for the treatment of children and young adults with acute lymphoblastic lymphoma, such as Cayden Addison. As of July 31, 2023, the FDA has approved six distinct CAR T-cell therapies for the treatment of a range of hematologic cancers.
T-cell engaging bispecific antibodies are another type of immunotherapy that works by bringing cancer-killing T cell in close proximity to the cancer cells. Between August 1, 2022, and July 31, 2023, FDA approved four new T-cell engaging bispecific antibodies for the treatment of patients with multiple myeloma, such as Cindy Brown, and several additional hematologic cancers.
Cytokines, such as interferon-alpha (IFNα), are molecules that are released by immune cells, and can boost the cancer-killing function of the immune system. The December 2022 FDA approval of nadofaragene firadenovec-vncg (Adstiladrin), an interferon-based cancer immunotherapy, was a major advance in the treatment of patients with bladder cancer such as Lesa Kirkman.
Despite the significant advances that have been made, only a small number of patients who are treated with an FDA- approved immunotherapeutic respond to the treatment. There are disparities in the access to these cutting-edge treatments. In addition, the current FDA-approved immunotherapeutics are not effective against all types of cancer. Identifying ways to increase the number of patients for whom treatment with an immunotherapeutic yields a remarkable and durable response is an area of intensive basic and clinical research investigation.
The new frontier of cancer immunotherapy, which includes preventive and therapeutic vaccines, innovative cell therapies, novel checkpoint inhibitors, and a new age of treatment combinations, is poised to transform the future of clinical cancer care.
Suppporting Cancer Patients and Survivors
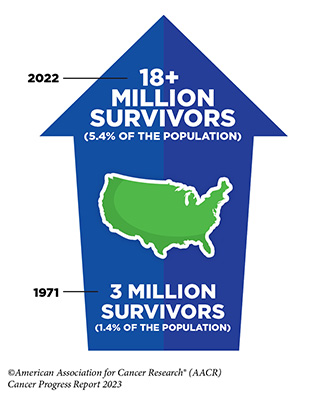
Unprecedented advances in cancer treatments over the past decade have led to more patients living longer and fuller lives after a cancer diagnosis. As of January 2022, the most recent year for which such data are available, there are more than 18 million people living with a history of a cancer diagnosis, which equates to about five percent of the U.S. population. This is a significant improvement from 50 years ago when cancer survivors constituted only 1.4 percent of the U.S. population. The number of survivors is expected to grow to 26 million by 2040. Understanding and addressing the short- and long-term challenges faced by cancer survivors, supporting their quality of life, and ensuring that care is accessible and equitable are important priorities in cancer survivorship research.
Cancer survivors are individuals who receive a diagnosis of cancer, beginning from when they are diagnosed through the balance of their lives. Survivors often face physical, psychosocial, and financial challenges, both during and after the conclusion of treatment; some continue to receive treatment indefinitely to manage their cancer. These challenges also extend to friends and family members who often act as informal caregivers. Health-related quality of life is increasingly being assessed by researchers in the development of new therapies and in clinical trials using patient reported outcomes. Understanding these challenges as well as how to reduce or eliminate them is an active area of research and continues to evolve as new therapies are discovered and used in the clinic.
Researchers are exploring ways to utilize palliative care, psycho- oncology, and other evidence-based strategies to improve quality of life for survivors of cancer. Engaging in physical activity, eliminating tobacco use, and eating a healthy diet have all been shown to improve the survivorship experience and improve cancer outcomes. Ongoing research is investigating the potential of new technologies, such as wearable devices, and innovative interventions, such as coordinated care, that may improve quality of life and meet the personalized needs of cancer survivors and their caregivers.
Envisioning the Future of Cancer Science and Medicine
Advances against cancer are driven by research, which provides the foundational knowledge of cancer onset and progression. This knowledge is essential to develop better ways to predict, prevent, diagnose, and treat cancer, as well as to enact evidence- based policies that improve public health.
As we envision a future where the success of treating all cancers is higher, and where the likelihood of a cure is possible, researchers, including AACR President, 2023-2024, Philip D. Greenberg, MD, FAACR, are excited that the advances in discovery science, and the technologies that enable it, are opening exciting new frontiers in cancer science and medicine that will continue to benefit patients with cancer.
Researchers are using applications of powerful new technologies—such as the ability to study molecular changes in every cell of the tumor, the ability to visualize single molecules inside the cancer cell through simple chemical reactions, the ability of AI to analyze large datasets, or the ability of wearables to aid patient reported outcomes—to classify tumors at a molecular level, understand tumor heterogeneity, diagnose primary and metastatic cancers, develop better and more specific drugs, characterize treatment responses, track how cancer evolves over time, and predict overall survival.
Another frontier in cancer research is developing successful treatment for currently intractable cancers, such as pancreatic cancer and glioblastoma. Clinical studies are evaluating the potential of innovative anticancer therapeutics in treating cancers that have been difficult to tackle. Similarly, contributions of the human gut microbiome in modulating the response to cancer treatment, as well as findings that the human gut microbiome may help reduce the risk of developing cancer, are being investigated in multiple clinical studies, findings of which have the potential to revolutionize future cancer treatment and care.
Advancing the Future of Cancer Research and Patient Care Through the Adoption of Evidence-based Policies
Continued investment in medical research through NIH and NCI is essential for making progress against all aspects of cancer, including prevention, early detection, and treatment. These investments are not possible without the support of key members of Congress, whose efforts have ensured eight consecutive years of funding increases for NIH. While these additional investments have led to great strides in cancer research, many unmet needs remain.
For the medical research enterprise to reach its full potential, additional resources for NIH and NCI are necessary to support early-career researchers and to include patients from diverse backgrounds in clinical trials, which could help address cancer disparities. Further federal investments are necessary for cancer screening and prevention programs because approximately 40 percent of cancer cases in the U.S. can be attributed to preventable risk factors, such as tobacco use and exposure to UV radiation. In particular, the increasing use of e-cigarettes among adolescents demands greater understanding of the tobacco marketplace and stronger restrictions on tobacco products. Additionally, because patients are living longer due to advances in treatment, it is also taking longer to determine if these new drugs are safe and effective; FDA has recently published new guidance to improve the quality of clinical trials and timely drug development. FDA is also responding to record levels of drug shortages to ensure that approved drugs remain available to patients.
AACR Call to Action
Eight consecutive years of funding increases for medical research have contributed to the development of breakthrough therapies, as well as improvements in cancer prevention and screening, leading to a steady decline in U.S. cancer death rates for both men and women. With the number of cancer survivors continuing to grow, Congress cannot afford to reduce investments in cancer research and support for patients with cancer. Lawmakers must enact legislation to improve the quality of life for survivors by supporting patient navigation services and aiding in survivors’ transition back to primary care. Furthermore, an increase in investments in medical research will expand access to a new generation of therapies that can transform cancer treatment and help patients with cancer live longer, healthier lives. Policymakers must continue to provide robust, sustained, and predictable funding increases for NIH to ensure greater availability of promising cancer treatments and to amplify cancer prevention and screening measures.
Therefore, AACR urges Congress to:
- Increase the FY 2024 base budgets of the NIH and NCI by at least $3.465 billion and $2.6 billion, respectively, for total funding levels of $50.924 billion for NIH and $9.988 billion for NCI.
- Provide $1.7 billion in dedicated funding for Cancer Moonshot activities in FY 2024 across NCI, FDA, and CDC with the assurance that Moonshot funding will supplement rather than supplant NIH funding in FY 2024
- Appropriate at least $472.4 million in FY 2024 appropriations for the CDC Division of Cancer Prevention to support comprehensive cancer control, central cancer registries, and screening and awareness programs for specific cancers.
- Allocate $50 million in funding for the Oncology Center of Excellence at FDA in FY 2024 to provide regulators with the capable staff and necessary tools to conduct expedited review of cancer-related medical products.
In summary, if we are to achieve the Cancer Moonshot goal of ending cancer as we know it, it will require robust funding for NIH and NCI biomedical research programs, as well as significant budget increases for FDA and CDC. If Congress
follows through on these recommendations, we will improve our nation’s health, including the lives of patients with cancer, and sustain our leadership in cancer research and medical science.