Cancer Researchers Working to Combat the COVID-19 Pandemic
In this section you will learn:
- Cancer researchers were uniquely positioned to respond to the many challenges posed by COVID-19 and have played a vital role in combating the public health crisis while continuing their quest to prevent and cure cancer.
- Since the onset of the pandemic, NCI has launched many cross-cutting efforts to investigate the epidemiology of COVID-19 burden, immune responses to SARS-CoV-2 and vaccines and their impact on disease development and severity, modifiable risk factors, and genetic and immune biomarkers of severe COVID-19, among other studies.
- Scientific discoveries and technological innovations in cancer science and medicine have helped with understanding and addressing COVID-19.
- Decades of research into mRNA vaccines for cancer immunotherapy paved the way for the development of SARS-CoV-2 vaccines at an unprecedented speed.
Cancer researchers were uniquely positioned to respond to many of the challenges posed by COVID-19, and many refocused their expertise to combat the unprecedented global pandemic (172)Dolgin E. Cancer labs pivot to battle COVID-19. Cancer Discov 2020;10:634.. Extensive experience in genetics, epigenetics, immunology, and drug development, among other scientific and clinical areas, made cancer scientists well suited to investigate COVID-19 biology and therapeutics. Furthermore, cutting-edge technologies, for example, next-generation sequencing (NGS), which is routinely used in cancer research to uncover the wide range of genetic alterations within subsets of cancer cells that drive tumor growth, were repurposed effectively to characterize the genomic sequence of SARS-CoV-2 and identify novel variants of SARS-CoV-2 (see Figure 4). Genomic sequencing using NGS technology allowed public health experts and researchers to track the transmission of SARS-CoV-2 globally, detect mutations rapidly to prevent the spread of new variants, and identify mutations that can evade detection by established diagnostic assays, or that can affect vaccine potency.
The NCI lent its substantive expertise and cutting-edge resources to conduct research that continues to contribute to the global effort to address COVID-19 (173)NCI. NCI’s COVID-19 research initiatives [updated 2020 May 21; cited 2021 Nov 27]. (see Table 2). Since the onset of the pandemic, NCI has launched many cross-cutting efforts that involve all stakeholders in the medical research community. The many areas that are being investigated by cancer scientists include epidemiological analyses of trends in COVID-19 mortality, immune responses to SARS-CoV-2 and vaccines and their impact on disease development and severity, genetic and immune biomarkers of severe or fatal COVID-19, and modifiable risk factors for COVID-19 severity.
Cancer researchers are also monitoring the short- and long-term impact of COVID-19 on patients with cancer. One area in which cancer researchers have vast expertise is the integration, analysis, and sharing of large and complex datasets, also known as “big data.” This expertise is being harnessed to better understand the effects of COVID-19 on patients with cancer. The goal is to obtain clinical and other patient-related information on a large scale to answer questions about the epidemiology of COVID-19 in patients with cancer, as well as the effectiveness of COVID-19 diagnostics, vaccines, and treatments in cancer patients. Several cancer organizations as well as multi-institutional teams have already launched initiatives to catalyze data sharing (see Table 3).
Studying the Immune Response to SARS-CoV-2 and COVID-19 Vaccines
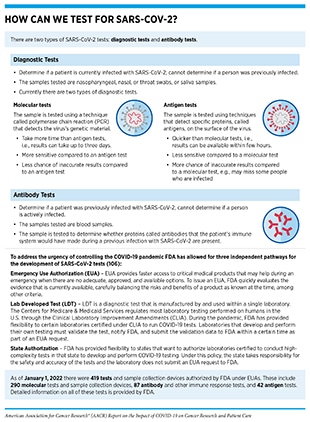
Understanding the immune response to SARS-CoV-2 as well as to the COVID-19 vaccines is key to managing community spread and developing treatments. Serology tests measure an individual’s immune response to an infection in the form of antibodies in the blood and are integral to our understanding of the prevalence of COVID-19 and SARS-CoV-2 immune response (see sidebars on How Can We Test for SARS-CoV-2?, and How Is the Immune Response to COVID-19 Vaccines Evaluated?). The NCI has the HPV Serology Laboratory, a world-class serology facility that works to standardize human papillomavirus (HPV) antibody testing. Emergency appropriations by Congress in April 2020 provided NCI with additional resources to develop, validate, improve, and implement serology testing and associated technologies to respond to the pandemic. Soon thereafter, NCI unified its national network of serology centers, including the HPV Serology Laboratory and others, and created the Serological Sciences Network (SeroNet) to support research on SARS-CoV-2 immunology and to increase the nation’s serological testing capacity. Research investigations as part of SeroNet aim to determine critical questions such as the underlying causes of differential symptoms among COVID-19 patients, prevalence of SARS-CoV-2 infection among different U.S. demographic groups, incidence of SARS-CoV-2 reinfection, cellular and molecular markers of COVID-19 immunity, and genetic and environmental factors that affect immune response, among others (174)NCI. Serological Sciences Network for COVID-19 (SeroNet) – NCI [updated 2020 May 29; cited 2021 Nov 27]..
Investigators who are part of the SeroNet initiative collaborated with FDA and CDC to validate SARS-CoV-2 antibody tests submitted to FDA (see sidebar on How Can We Test for SARS-CoV-2?). The goal was to ensure that the tests made available to the public are accurate and reliable. A key question vital for our understanding of the immunity against SARS-CoV-2 is whether a certain level of SAR-CoV-2 antibodies is needed for protection from a future infection. By following vaccinated individuals, as well as COVID-19 patients over time, SeroNet researchers aim to determine the antibody levels that correlate with protection from severe disease.
One of the challenges in quantifying antibody correlates for protection from COVID-19 across a large population is that different research groups use different methods to measure antibody levels, which makes comparison between groups difficult. Scientists at SeroNet have addressed this issue by developing a benchmark SARS-CoV-2 serology standard, which allows research teams to compare antibody levels across different studies, even if they have used different serology tests. Notably, researchers at NCI have now calibrated the serology standard to a unit of measure that is approved by WHO and can be compared across international studies. In collaboration with the National Institute of Allergy and Infectious Diseases (NIAID) and CDC, NCI has also developed and implemented an online dashboard, SeroHub, which catalogs data on the percentage of people who were infected during a certain time, called seroprevalence, so that scientists and policy makers can make evidence-based decisions on public health.
Research from NCI’s SeroNet has uncovered important insights into the mechanisms of immune response to COVID-19. These studies include comparisons between immune responses from natural infection with SARS-CoV-2 and immune responses achieved through the vaccines (175)Ebinger JE, Fert-Bober J, Printsev I, Wu M, Sun N, Prostko JC, et al. Antibody responses to the BNT162b2 mRNA vaccine in individuals previously infected with SARS-CoV-2. Nat Med 2021;27:981–4.(176)Canaday DH, Carias L, Oyebanji OA, Keresztesy D, Wilk D, Payne M, et al. Reduced BNT162b2 messenger RNA vaccine response in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-naive nursing home residents. Clin Infect Dis 2021;73:2112–5.. Others have examined the immune response achieved through COVID-19 vaccines to the newer variants of SARS-CoV-2 or among vulnerable populations, such as patients with cancer. Results from these studies indicate that the vaccines may be less effective in certain cancer patients but, encouragingly, the reduced immunity conferred by all three COVID-19 vaccines in this vulnerable population is retained against the various newer variants of the virus (177)NCI. In people with cancer, responses to COVID-19 vaccines vary [updated 2021 May 27; cited 2021 Nov 27].(178)Tarke A, Sidney J, Methot N, Yu ED, Zhang Y, Dan JM, et al. Impact of SARS-CoV-2 variants on the total CD4(+) and CD8(+) T cell reactivity in infected or vaccinated individuals. Cell Rep Med 2021;2:100355.(179)Alter G, Yu J, Liu J, Chandrashekar A, Borducchi EN, Tostanoski LH, et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature 2021;596:268–72.(180)Edara VV, Pinsky BA, Suthar MS, Lai L, Davis-Gardner ME, Floyd K, et al. Infection and vaccine-induced neutralizing-antibody responses to the SARS-CoV-2 B.1.617 variants. N Engl J Med 2021;385:664–6.. These data have been critical for public health experts at CDC for making important decisions, such as whether additional doses or boosters of the COVID-19 vaccine are needed for the general population and for vulnerable groups that include patients with cancer, and what is the optimal time frame for administering such additional doses.
Using Lessons Learned from Cancer Biology to Understand SARS-CoV-2 Biology
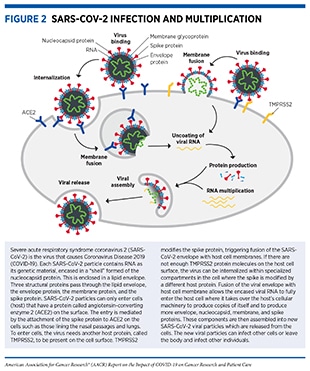
While the COVID-19 pandemic presented unprecedented challenges to the entire biomedical research community, it also emphasized the vital importance of investments in medical research including in basic research. Scientific discoveries that resulted from decades of research funding and work across many disciplines including cancer biology were rapidly harnessed to address the numerous clinical questions posed by COVID-19. In fact, critical insights into the biology of SARS-CoV-2 and the pathology of COVID-19, which led to rapid advances in the development of vaccines and potential treatments, were derived from the knowledge obtained from cancer science and medicine. Two areas where researchers have drawn knowledge from cancer biology are the mechanism by which SARS-CoV-2 enters the host cell and the immune response to COVID-19 (see Figure 4).
Coronaviruses, including SARS-CoV-2, rely on the host protein TMPRSS2 for entry into human cells (see Figure 2). Many of the insights related to TMPRSS2 protein expression and function have come from cancer research. TMPRSS2 was initially identified in prostate cancer and the gene is known to be highly upregulated in prostate cancer cells (40)Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID-19: serendipity or opportunity for intervention? Cancer Discov 2020;10:779–82.(41)Chakravarty D, Nair SS, Hammouda N, Ratnani P, Gharib Y, Wagaskar V, et al. Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer. Commun Biol 2020;3:374.. It is also known that the levels of TMPRSS2 in the healthy prostate tissue, as well as in prostate cancer, are regulated by the male hormone androgen and its receptor (13)Bakouny Z, Hawley JE, Choueiri TK, Peters S, Rini BI, Warner JL, et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell 2020;38:629–46.(40)Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID-19: serendipity or opportunity for intervention? Cancer Discov 2020;10:779–82.(41)Chakravarty D, Nair SS, Hammouda N, Ratnani P, Gharib Y, Wagaskar V, et al. Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer. Commun Biol 2020;3:374.. Studies have recently shown that TMPRSS2 is also expressed in the lungs, the main conduit for COVID-19 (13)Bakouny Z, Hawley JE, Choueiri TK, Peters S, Rini BI, Warner JL, et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell 2020;38:629–46.. Ongoing research is investigating whether TMPRSS2 levels in the lungs are also regulated by androgen, as is the case in prostate tissue, and whether males have a higher abundance of TMPRSS2 proteins compared to females (41)Chakravarty D, Nair SS, Hammouda N, Ratnani P, Gharib Y, Wagaskar V, et al. Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer. Commun Biol 2020;3:374.. Data from this line of research can provide valuable insights into the predominance of COVID-19 severity and death in men. A subsequent question is whether it is possible to reduce the susceptibility to SARS-CoV-2 infection, COVID-19 pulmonary symptoms, and severe disease by inhibiting the levels of androgen through androgen deprivation therapies (ADT), a mainstay in the treatment of prostate cancer. At the onset of the COVID-19 outbreak, a retrospective analysis from Italy found that while men generally had worse outcomes from COVID-19, prostate cancer patients treated by ADT were intriguingly less likely to be infected with SARS-CoV-2 compared to patients who were not on the therapy or patients with other types of cancers (181)Montopoli M, Zumerle S, Vettor R, Rugge M, Zorzi M, Catapano CV, et al. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532). Ann Oncol 2020;31:1040–5. Similarly, a second retrospective analysis in the United States showed that prostate cancer patients treated with ADTs were less likely to develop adverse clinical outcomes compared to those not on this regimen (182)Patel VG, Zhong X, Liaw B, Tremblay D, Tsao CK, Galsky MD, et al. Does androgen deprivation therapy protect against severe complications from COVID-19? Ann Oncol 2020;31:1419–20.. While emerging data from more recent studies do not support an association between ADT and COVID-19 mortality (183)Schmidt AL, Tucker MD, Bakouny Z, Labaki C, Hsu CY, Shyr Y, et al. Association between androgen deprivation therapy and mortality among patients with prostate cancer and COVID-19. JAMA Netw Open 2021;4:e2134330., additional ongoing clinical trials are seeking definitive answers to whether there are any potential benefits of ADTs in mitigating the symptoms of COVID-19 and are evaluating a range of TMPRSS2-targeted therapeutics that either lower its expression level or inhibit its function (23)Zong Z, Wei Y, Ren J, Zhang L, Zhou F. The intersection of COVID-19 and cancer: signaling pathways and treatment implications. Mol Cancer 2021;20:76..
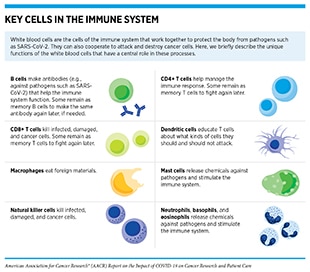
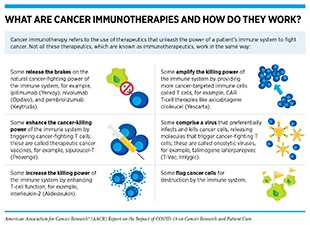
Immunotherapy is another area of cancer science and medicine that has contributed critical elements to our understanding of COVID-19 and to addressing the most severe symptoms associated with it. Immunotherapeutics work by unleashing the power of a patient’s immune system to fight cancer the way it fights pathogens such as the flu virus or the bacterium that causes strep throat. The use of immunotherapeutics in the treatment of cancer is referred to as cancer immunotherapy. In the past decade, it has revolutionized the treatment of an increasingly broad array of cancer types (38)Sengupta R, Zaidi SK. AACR Cancer Progress Report 2021: discovery science driving clinical breakthroughs. Clin Cancer Res 2021;27:5757–9.. One type of cancer immunotherapy called CAR T-cell therapy works by amplifying the killing power of the immune system by providing more cancer-targeted immune cells called T cells (see sidebars on Key Cells in the Immune System, and What Are Cancer Immunotherapies and How Do They Work?). CAR T-cell therapy was first approved by the FDA in 2017 for the treatment of children and young adults with a certain type of leukemia (186)Agarwal S, June CH. Harnessing CAR T-cell insights to develop treatments for hyperinflammatory responses in patients with COVID-19. Cancer Discov 2020;10:775–8.. Researchers who were involved with the development of CAR T-cell therapy noticed that the treatment could potentially have certain adverse effects, some of which were very severe and, in some cases, life-threatening. One of the most concerning was a phenomenon known as cytokine release syndrome (CRS). During CAR T-cell therapy, CRS occurs when the engineered T cells attack cancer cells and release chemicals called cytokines (for example, interleukin-6 or IL-6) that can help in the eradication of cancer. However, in patients affected by CRS, such as Larry Saltzman) who experienced two episodes of CRS following CAR T-cell therapy, there is an overwhelming release of cytokines into the bloodstream, which can cause high fevers, flu-like symptoms, and a dramatic drop in blood pressure. For many patients, treatment with steroids can relieve the CRS. However, others require treatment with tocilizumab (Actemra), which blocks IL-6, and was approved to treat severe or life-threatening CRS caused by CAR T-cell therapy in 2017.
Notably, severe COVID-19 is associated with an increase in proinflammatory cytokines and an overactive immune response that is similar to that observed in CRS (185)Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science 2020;368:473–4.(186)Agarwal S, June CH. Harnessing CAR T-cell insights to develop treatments for hyperinflammatory responses in patients with COVID-19. Cancer Discov 2020;10:775–8.. Inflammation in the lungs can progress to acute respiratory distress syndrome (ARDS), causing difficulty breathing and low blood oxygen levels. If unchecked, ARDS can progress to respiratory failure, which is the cause of death in many fatal COVID-19 cases. In addition, uncontrolled CRS may lead to failure of other organs, most notably the heart, liver, and kidneys. Although there may be certain differences in the underlying biology between inflammatory responses in cancer and in COVID-19, many of the same cytokines, such as IL-6, are involved in both phenomena. Researchers have utilized the knowledge learned in cancer to identify cytokine biomarkers that are indicative of severe COVID-19 and to investigate therapeutic options to mitigate CRS among severely ill COVID-19 patients (23)Zong Z, Wei Y, Ren J, Zhang L, Zhou F. The intersection of COVID-19 and cancer: signaling pathways and treatment implications. Mol Cancer 2021;20:76.(168)Aldea M, Michot JM, Danlos FX, Ribas A, Soria JC. Repurposing of anticancer drugs expands possibilities for antiviral and anti-inflammatory discovery in COVID-19. Cancer Discov 2021;11:1336–44.(186)Agarwal S, June CH. Harnessing CAR T-cell insights to develop treatments for hyperinflammatory responses in patients with COVID-19. Cancer Discov 2020;10:775–8.(187)Del Valle DM, Kim-Schulze S, Huang HH, Beckmann ND, Nirenberg S, Wang B, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 2020;26:1636–43.. These approaches include clinical studies on “repurposing” tocilizumab and other IL-6 inhibitors to slow and reduce inflammation in COVID-19 patients with immune system hyperactivation (see Repurposing Anticancer Agents to Treat COVID-19) (168)Aldea M, Michot JM, Danlos FX, Ribas A, Soria JC. Repurposing of anticancer drugs expands possibilities for antiviral and anti-inflammatory discovery in COVID-19. Cancer Discov 2021;11:1336–44..
Beyond the management of CRS, cancer researchers have lent their scientific and technological expertise from immune profiling in cancer research to characterize key aspects of the immune response to SARS-CoV-2 (188)NCI. Cancer researchers working on COVID-19 studies [updated 2021 Feb 12; cited 2021 Nov 27].. As one example, cancer researchers adapted tests that they routinely use to monitor T-cell responses to tumor proteins for studies of the coronavirus to determine how T cells are activated in response to certain viral proteins, such as the SARS-CoV-2 spike protein. These tests can determine whether COVID-19 vaccines can elicit effective immune responses especially in cancer patients undergoing treatment.
Developing COVID-19 Vaccines
The successful development of several safe and effective COVID-19 vaccines in an unprecedentedly short time was made possible due to the rapid mobilization of the global medical research community and concerted efforts from all stakeholders including the government and private sectors. It should be noted that the path to successful mRNA vaccines drew on extensive research in cancer science and medicine (131)Wherry EJ, Jaffee EM, Warren N, D’Souza G, Ribas A, AACR COVID-19 and Cancer Task Force. How did we get a COVID-19 vaccine in less than 1 year? Clin Cancer Res 2021;27:2136–8.(189)Xu S, Yang K, Li R, Zhang L. mRNA vaccine era—mechanisms, drug platform and clinical prospection. Int J Mol Sci 2020;21:6582.. For decades, scientists have studied how to use mRNA to help the immune system fight cancer. While the development of effective mRNA-based cancer vaccines has proven challenging because of the complexities of cancer—a collection of more than 200 diseases (190)Kwok M, Fritsch EF, Wu CJ. Cancer and COVID-19: on the quest for effective vaccines. Blood Cancer Discov 2021;2:13–8., the incremental progress in understanding the science and refining the technology has contributed tremendously to the rapid development of the COVID-19 vaccines. Beyond mRNA vaccines, many other COVID-19 vaccine candidates built around inactivated viruses, viral vectors, or engineered viral proteins that are currently being evaluated, feature design elements informed by prior cancer therapy efforts (189)Xu S, Yang K, Li R, Zhang L. mRNA vaccine era—mechanisms, drug platform and clinical prospection. Int J Mol Sci 2020;21:6582.(191)Xu X, Chen W, Zhu W, Chen J, Ma B, Ding J, et al. Adeno-associated virus (AAV)-based gene therapy for glioblastoma. Cancer Cell Int 2021;21:76.(192)Dolgin E. How cancer vaccine tech shaped COVID response. Cancer Discov 2021;11:218..
The mRNA vaccine platform was developed and tested for cancer, albeit as an experimental therapeutic rather than preventive agent. Genetic mutations that lead to cancer leave their signature in various altered proteins (see sidebar on Genetic Mutations). Therefore, the aim was to create mRNAs that encode the altered proteins—called tumor antigens or neoantigens—that are expressed in cancer cells and contribute to their malfunction. When injected into a patient, these mRNAs generate the tumor antigens or neoantigens, which flag the cancer cell for destruction by the immune system. It should be noted that the tumor antigens may look very different in different patients, which necessitates sampling of each patient’s tumor and characterizing the mutations to develop personalized vaccines that are specific to individual patients.
For more than 25 years, cancer researchers have been refining the science and technology of mRNA vaccination by evaluating potential routes of mRNA administration as well as various delivery methods to identify the best way to elicit optimal immune responses (193)Dolgin E. The tangled history of mRNA vaccines. Nature 2021;597:318–24.. Among the delivery approaches that have been tested include isolating immune cells known as dendritic cells from the blood (see sidebar on Key Cells in the Immune System), modifying them by introducing an mRNA that encodes tumor antigen, and injecting them back into the cancer patient where these cells would trigger cancer-fighting T cells to attack and eliminate tumors (189)Xu S, Yang K, Li R, Zhang L. mRNA vaccine era—mechanisms, drug platform and clinical prospection. Int J Mol Sci 2020;21:6582.. Additionally, extensive research has been done to identify ways to increase the efficiency of mRNA delivery, e.g., the use of lipid-based delivery systems, much like the lipid nanoparticle formulation that was used for the COVID-19 vaccines. Furthermore, while researchers are monitoring the long-term health of individuals who have received the new SARS-CoV-2 mRNA vaccines, it should be noted that preclinical and clinical studies in cancer have thus far established the feasibility and safety of this approach (194)Sahin U, Kariko K, Tureci O. mRNA-based therapeutics–developing a new class of drugs. Nat Rev Drug Discov 2014;13:759–80..
Notably, a key consideration for the development of clinically useful personalized mRNA-based cancer vaccines is the time it takes to develop these agents. For cancer patients in clinical trials, time is of the essence. The expertise to generate a personalized cancer vaccine rapidly for each new patient has served as an advantage for developing the COVID-19 vaccine. It took less than two months from the time the SARS-CoV-2 genome sequence was determined to begin human testing of the mRNA vaccines (131)Wherry EJ, Jaffee EM, Warren N, D’Souza G, Ribas A, AACR COVID-19 and Cancer Task Force. How did we get a COVID-19 vaccine in less than 1 year? Clin Cancer Res 2021;27:2136–8.. Research tools that were previously used to predict which parts of mutated tumor proteins make the best targets for immune activation were repurposed to identify specific parts of SARS-CoV-2 proteins that could be encoded in mRNA vaccines for optimal immune responses (192)Dolgin E. How cancer vaccine tech shaped COVID response. Cancer Discov 2021;11:218..
As SARS-CoV-2 variants with novel mutations are continuing to emerge across the globe, the versatility and speed of the mRNA vaccine development ensure that these vaccines can be quickly modified to target the newer viral strains. In fact, researchers believe that, in the same manner in which cancer vaccines can be generated against novel tumor-specific proteins within months, new mutations in SARS-CoV-2 proteins could be incorporated into future COVID-19 vaccines in a short time. Beyond the scientific and technological expertise generated in cancer research, the unprecedented pace of COVID-19 vaccine development was also made possible because of a robust manufacturing infrastructure that was already in place to develop anticancer products. Taken together this evidence emphasizes how decades of ongoing efforts in cancer medicine have contributed strongly to the key innovations that led to the design and implementation of the mRNA-based COVID-19 vaccines. Notably, given the global spotlight on the tremendous success of the COVID-19 vaccines, there has been increased enthusiasm for mRNA-based cancer immunotherapies, and many cancer scientists are refocusing on developing mRNA vaccines to fight cancer. Researchers believe that the next wave of breakthroughs in mRNA technologies will revolutionize the landscape of cancer vaccines.
Repurposing Anticancer Agents to Treat COVID-19
Over the last decade, oncology has emerged as one the most exciting areas of drug development. A wide range of agents with diverse mechanisms of action is being evaluated, reviewed, and approved by the FDA each year, many of which have been chronicled in the annual AACR Cancer Progress Report over last 11 years (195)American Association for Cancer Research. AACR Cancer Progress Report [updated 2021 Oct 18; cited 2021 Dec 19].. Building on the deep scientific knowledge gained from experience in cancer, researchers around the world have worked together to accelerate the SARS-CoV-2 therapeutic development. One area of particular interest is to repurpose treatments used in cancer care for the benefit of patients with COVID-19. Ongoing investigations in cancer drug repurposing are focused mainly on two areas: identifying existing drugs that may target the mechanisms of SARS-CoV-2 entry into the host cell and viral replication; and identifying those that mitigate the overactive immune response seen in patients with severe disease (167)Poh A. Repurposing cancer drugs for COVID-19. Cancer Discov 2020;10:OF2.(168)Aldea M, Michot JM, Danlos FX, Ribas A, Soria JC. Repurposing of anticancer drugs expands possibilities for antiviral and anti-inflammatory discovery in COVID-19. Cancer Discov 2021;11:1336–44.(196)Costa B, Vale N. A review of repurposed cancer drugs in clinical trials for potential treatment of COVID-19. Pharmaceutics 2021;13:185.. Although definitive evidence of clinical benefit is still lacking, data from these studies suggest that clinical trials for hypothesis testing of anticancer therapeutics are an encouraging strategy for discovering potential new treatments for COVID-19.
An effective immune response against SARS-CoV-2 depends on the proper activation of T cells, which can eradicate virus-infected cells. It has been shown that patients with severe COVID-19 have reduced numbers of T cells and that the existing T cells exhibit an exhausted characteristic. Cancer immunotherapeutics such as immune checkpoint inhibitors release certain brakes on exhausted T cells, thereby unleashing their ability to eradicate tumors or virus-infected cells. Researchers are therefore interested in examining the potential of treatment with checkpoint inhibitors early in the disease (prior to any signs of CRS) in mitigating COVID-19 (197)El Bairi K, Trapani D, Petrillo A, Le Page C, Zbakh H, Daniele B, et al. Repurposing anticancer drugs for the management of COVID-19. Eur J Cancer 2020;141:40–61.. Ongoing studies are carefully examining in which patient populations and at what stage of the disease these treatments could be used to achieve the best outcomes for patients with COVID-19 (168)Aldea M, Michot JM, Danlos FX, Ribas A, Soria JC. Repurposing of anticancer drugs expands possibilities for antiviral and anti-inflammatory discovery in COVID-19. Cancer Discov 2021;11:1336–44.(197)El Bairi K, Trapani D, Petrillo A, Le Page C, Zbakh H, Daniele B, et al. Repurposing anticancer drugs for the management of COVID-19. Eur J Cancer 2020;141:40–61..
One of the most severe symptoms for patients with COVID-19 is an overactive immune system leading to CRS. This can lead to potentially life-threatening complications in the lungs, heart, and other major organs. Scientists are exploring whether anticancer treatments that are known to target inflammation-inducing pathways can be used to slow this overactive immune response in COVID-19 patients. The different classes of therapeutics that are being explored for repurposing include corticosteroids, such as dexamethasone, Bruton tyrosine kinase (BTK) inhibitors such as acalabrutinib and ibrutinib, and JAK/STAT inhibitors such as ruxolitinib, all of which are common treatments for hematologic cancers (168)Aldea M, Michot JM, Danlos FX, Ribas A, Soria JC. Repurposing of anticancer drugs expands possibilities for antiviral and anti-inflammatory discovery in COVID-19. Cancer Discov 2021;11:1336–44.(198)Rada M, Qusairy Z, Massip-Salcedo M, Macip S. Relevance of the Bruton tyrosine kinase as a target for COVID-19 therapy. Mol Cancer Res 2021;19:549–54.. In addition, many research teams are evaluating inhibitors of the cytokine IL-6, such as tocilizumab, currently approved by FDA to mitigate cytokine release in cancer patients treated with CAR T-cell therapy, for patients with COVID-19 (199)Lo CKL, Chagla Z. In patients hospitalized for COVID-19, tocilizumab reduces mortality at 28 d. Ann Intern Med 2021;174:JC125.(200)Gupta S, Leaf DE. Tocilizumab in COVID-19: some clarity amid controversy. Lancet 2021;397:1599–601.(201)The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Shankar-Hari M, Vale CL, Godolphin PJ, Fisher D, Higgins JPT, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-.
Based on the observation that COVID-19 is associated with abnormal blood vessels which may lead to low blood oxygen, morbidity, and mortality, cancer researchers are repurposing antiangiogenic therapeutics used in cancer to treat the vascular defects in COVID-19 (202)Munn LL, Stylianopoulos T, Jain NK, Hardin CC, Khandekar MJ, Jain RK. Vascular normalization to improve treatment of COVID-19: lessons from treatment of cancer. Clin Cancer Res 2021;27:2706–11.. It should be noted that abnormal vasculature and poor oxygenation are also hallmarks of solid tumors.
There are efforts to identify approved anticancer agents that can block SARS-CoV-2 entry and its multiplication in human cells. As described earlier in the report, proteins ACE2 and TMPRSS2, expressed on the cell surface in the human respiratory tract among other organs, are involved in SARS-CoV-2 entry and infection. TMPRSS2 levels are elevated in prostate cancer cells and regulated by the male hormone androgen, which led to clinical trials testing antiandrogenic therapeutics that are commonly used in prostate cancer in patients with COVID-19 (40)Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID-19: serendipity or opportunity for intervention? Cancer Discov 2020;10:779–82.(203)Karimi A, Nowroozi A, Alilou S, Amini E. Effects of androgen deprivation therapy on COVID-19 in patients with prostate cancer: a systematic review and meta-analysis. Urol J 2021;18:577–84.. While currently available data from these trials remain inconclusive, there are continued efforts to evaluate the clinical benefits of targeting TMPRSS2 levels and/or function in patients with COVID-19 (168)Aldea M, Michot JM, Danlos FX, Ribas A, Soria JC. Repurposing of anticancer drugs expands possibilities for antiviral and anti-inflammatory discovery in COVID-19. Cancer Discov 2021;11:1336–44.. Beyond TMPRSS2, molecularly targeted anticancer therapeutics against several other host proteins that are involved in cellular multiplication and/or survival are being investigated (167)Poh A. Repurposing cancer drugs for COVID-19. Cancer Discov 2020;10:OF2.(168)Aldea M, Michot JM, Danlos FX, Ribas A, Soria JC. Repurposing of anticancer drugs expands possibilities for antiviral and anti-inflammatory discovery in COVID-19. Cancer Discov 2021;11:1336–44.(197)El Bairi K, Trapani D, Petrillo A, Le Page C, Zbakh H, Daniele B, et al. Repurposing anticancer drugs for the management of COVID-19. Eur J Cancer 2020;141:40–61..
Next Section: Cancer in the Midst of COVID-19 and Beyond Previous Section: Understanding the COVID-19 Pandemic