Cancer in the Midst of COVID-19 and Beyond
In this chapter you will learn:
- Patients with cancer have a significantly higher risk of COVID-19 infection and dying from it compared to individuals without cancer.
- Patients with blood and lung cancers, or those on active anticancer treatments, are more vulnerable to COVID-19 infections and deaths compared to patients with other types of cancers, or those who are not on active anticancer treatments.
- COVID-19 vaccines are effective in patients with cancer, with few to no side effects. Certain patients with blood cancers, who are receiving specific types of treatments, respond to the vaccines to a lesser extent because of the nature of their cancer and the treatment.
- COVID-19 led to closures of research laboratories, resulted in a pause in clinical trials, negatively impacted career development opportunities for the science, technology, engineering and mathematics (STEM) workforce, especially women and minority early-stage investigators, and caused a burnout of health care workers.
- The pandemic disrupted patient care, with a sharp decline in cancer screening, delays or cancellation of cancer treatments, a negative impact on the mental and psychosocial health of cancer survivors, and it widened cancer health disparities.
- Some aspects of cancer research and patient care affected by the pandemic are returning to prepandemic levels, such as clinical trials testing new cancer treatments. However, the impact of disruptions on other aspects, such as missed cancer screenings during the pandemic potentially leading to an increase in advanced-stage cancer diagnoses, will only become clear in the coming years.
It is increasingly evident that cancer is an independent risk factor for adverse outcomes and mortality in patients with COVID-19 (20)Westblade LF, Brar G, Pinheiro LC, Paidoussis D, Rajan M, Martin P, et al. SARS-CoV-2 viral load predicts mortality in patients with and without cancer who are hospitalized with COVID-19. Cancer Cell 2020;38:661–71.(204)Ribas A, Sengupta R, Locke T, Zaidi SK, Campbell KM, Carethers JM, et al. Priority COVID-19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov 2021;11:233–6.(205)Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet 2020;395:1907–18.(206)Garcia-Suarez J, de la Cruz J, Cedillo A, Llamas P, Duarte R, Jimenez-Yuste V, et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: lessons from a large population-based registry study. J Hematol Oncol 202(207)Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430–6.(208)Rivera DR, Peters S, Panagiotou OA, Shah DP, Kuderer NM, Hsu CY, et al. Utilization of COVID-19 treatments and clinical outcomes among patients with cancer: a COVID-19 and Cancer Consortium (CCC19) Cohort Study. Cancer Discov 2020;10:1514–27.(209)Liu C, Wang K, Li L, Lv Q, Liu Y, Hu T, et al. Severity of COVID-19 in cancer patients versus patients without Cancer: a propensity score matching analysis. J Cancer 2021;12:3558–65.. In a study of more than 73 million patients in the U.S., the risk of COVID-19 infection was seven times higher in patients diagnosed with cancer in the past year, when compared to those with no history of cancer (210)Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol 2021;7:220–7.. The study also found that mortality in COVID-19 patients with cancer was significantly higher (almost 15 percent) than that in COVID-19 patients without cancer (five percent), or cancer patients without COVID-19 (four percent) (see also sidebar on COVID-19 in Patients with Cancer (210)Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol 2021;7:220–7.. Another recent study involving more than 195,000 COVID-19 patients found that mortality rates decreased in patients with no history of cancer between January and August of 2021, but not in patients with a history of cancer. The study also found that mortality rates were highest among patients who were being actively treated for their cancer (210)Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol 2021;7:220–7. (see Patients with Cancer on Active Anticancer Treatment). These studies, as well as others discussed in this chapter, have established patients with cancer as highly vulnerable to adverse outcomes from COVID-19.
Burden of COVID-19 in Patients with Cancer
Several factors, such as advanced age, that contribute to the development of cancer also appear to increase the risk of contracting COVID-19, as well as the overall adverse clinical outcomes from COVID-19 in patients with cancer (see Figure 5) (217)Lee LY, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 2020;395:1919–26.. These risk factors weaken the immune system, making patients with cancer more susceptible to infectious agents, including infections from SARS-CoV-2 (217)Lee LY, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 2020;395:1919–26. (see sidebar on Why Are Cancer Patients at an Increased Risk of Infection?), and increasing the likelihood of death.
Studies examining the immune system in patients with cancer who have contracted COVID-19 are providing valuable insights into the role of different types of cancer and anticancer treatments in increasing the risk of death in this population. As one example, initial findings from an ongoing study—COVID-19 antiviral response in a pan-tumor immune monitoring, or CAPTURE (219)Au L, Boos LA, Swerdlow A, Byrne F, Shepherd STC, Fendler A, et al. Cancer, COVID-19, and antiviral immunity: the CAPTURE study. Cell 2020;183:4–10.—showed that most patients with solid tumors developed a functional and durable immune response to SARS-CoV-2 infection, lasting at least 11 months. Patients with hematologic malignancies, on the other hand, had impaired immune responses that were related to the type of cancer as well as the treatment patients were receiving (220)Fendler A, Au L, Shepherd S, Byrne F, Cerrone M, Boos L, et al. Functional antibody and T-cell immunity following SARS-CoV-2 infection, including by variants of concern, in patients with cancer: the CAPTURE study. Res Sq 2021 Sep 20 [Epub ahead of print]..
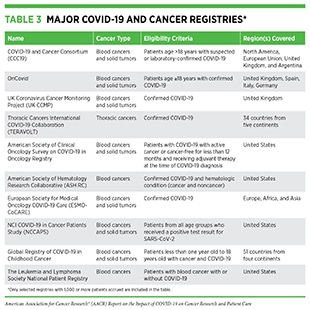
Greater risk of COVID-19 infection and death in patients with cancer has spurred the establishment of multiple collaborative registries across the globe (see Table 3) to document the interplay between COVID-19 and various aspects of cancer and anticancer treatments. Studies utilizing these resources have provided a deeper understanding of clinical features in patients with cancer that are associated with the risk of adverse COVID-19 outcomes (205)Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet 2020;395:1907–18.(217)Lee LY, Cazier JB, Angelis V, Arnold R, Bisht V, Campton NA, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: a prospective cohort study. Lancet 2020;395:1919–26.(222)Lee LYW, Cazier JB, Starkey T, Briggs SEW, Arnold R, Bisht V, et al. COVID-19 prevalence and mortality in patients with cancer and the effect of primary tumour subtype and patient demographics: a prospective cohort study. Lancet Oncol 2020;21:1309–16.(223)Pinato DJ, Lee AJX, Biello F, Segui E, Aguilar-Company J, Carbo A, et al. Presenting features and early mortality from SARS-CoV-2 infection in cancer patients during the initial stage of the COVID-19 pandemic in Europe. Cancers (Basel) 2020;12:1841.(224)Pinato DJ, Zambelli A, Aguilar-Company J, Bower M, Sng C, Salazar R, et al. Clinical portrait of the SARS-CoV-2 epidemic in European cancer patients. Cancer Discov 2020;10:1465–74.(225)Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol 2020;21:1023–34.(226)Baena Espinar J, Torri V, Whisenant J, Hirsch FR, Rogado J, de Castro Carpeño J, et al. LBA75 Defining COVID-19 outcomes in thoracic cancer patients: TERAVOLT (Thoracic cancERs international coVid 19 cOLlaboraTion). Ann Oncol 2020;31:S1204–5.(227)Wise-Draper TM, Desai A, Elkrief A, Rini BI, Flora DB, Bowles DW, et al. LBA71 Systemic cancer treatment-related outcomes in patients with SARS-CoV-2 infection: a CCC19 registry analysis. Ann Oncol 2020;31:S1201–2.(228)Grivas P, Warner J, Shyr Y, Shah D, Rubinstein SM, Kuderer NM, et al. LBA72 Assessment of clinical and laboratory prognostic factors in patients with cancer and SARS-CoV-2 infection: the COVID-19 and Cancer Consortium (CCC19). Ann Oncol 2020;31:S1202–3.. Breast and prostate cancers are the most common types of cancer diagnosed in patients with COVID-19 (five to 18 percent, and one to 14 percent, respectively) (229)Roel E, Pistillo A, Recalde M, Sena AG, Fernandez-Bertolin S, Aragon M, et al. Characteristics and outcomes of over 300,000 patients with COVID-19 and history of cancer in the United States and Spain. Cancer Epidemiol Biomarkers Prev 2021;30:1884–94.. In the following sections, we focus on the current knowledge of those cancer types and anticancer treatments that have the worst impact on the outcome of COVID-19 in patients with cancer.
Patients with Hematologic Cancers
Hematologic or blood cancers—leukemias, lymphomas, and multiple myeloma—affect cells that constitute the immune system. Patients with hematologic cancers require frequent hospital visits and/or prolonged stays for treatment, management of complications, and disease monitoring. Because of the nature of hematologic cancers and depending upon the treatment regimen, these patients are generally immunocompromised and thus are at a higher risk of SARS-CoV-2 infection (see sidebar on Why Are Cancer Patients at an Increased Risk of Infection?).
Overwhelming evidence indicates that patients with hematologic cancers not only experience significantly higher rates of COVID-19 infection and complications, but also exhibit a two- to three-fold higher mortality rate compared to patients without cancer or those with solid tumors (155)Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, et al. Case fatality rate of cancer patients with COVID-19 in a New York Hospital System. Cancer Discov 2020;10:935–41.(210)Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol 2021;7:220–7.(230)He W, Chen L, Chen L, Yuan G, Fang Y, Chen W, et al. COVID-19 in persons with haematological cancers. Leukemia 2020;34:1637–45.(231)Aries JA, Davies JK, Auer RL, Hallam SL, Montoto S, Smith M, et al. Clinical outcome of coronavirus disease 2019 in haemato-oncology patients. Br J Haematol 2020;190:e64–e7.(232)Passamonti F, Cattaneo C, Arcaini L, Bruna R, Cavo M, Merli F, et al. Clinical characteristics and risk factors associated with COVID-19 severity in patients with haematological malignancies in Italy: a retrospective, multicentre, cohort study. Lancet Hae(233)Pinana JL, Martino R, Garcia-Garcia I, Parody R, Morales MD, Benzo G, et al. Risk factors and outcome of COVID-19 in patients with hematological malignancies. Exp Hematol Oncol 2020;9:21.(234)Martin-Moro F, Marquet J, Piris M, Michael BM, Saez AJ, Corona M, et al. Survival study of hospitalised patients with concurrent COVID-19 and haematological malignancies. Br J Haematol 2020;190:e16–20.(235)Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov 2020;10:783–91.(236)Fillmore NR, La J, Szalat RE, Tuck DP, Nguyen V, Yildirim C, et al. Prevalence and outcome of COVID-19 infection in cancer patients: a National Veterans Affairs Study. J Natl Cancer Inst 2021;113:691–8.. A meta-analysis of COVID-19 outcomes in 3,377 patients with hematologic cancers across 38 studies found that the overall mortality from COVID-19 was significantly higher in adults with hematologic cancers (34 percent) compared to children with hematologic cancers (four percent) (237)Vijenthira A, Gong IY, Fox TA, Booth S, Cook G, Fattizzo B, et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood 2020;136:2881–92.. A study of 250 patients in the American Society of Hematology Research Collaborative (ASH RC) registry (see Table 3) found an overall mortality rate of 28 percent, with worse outcomes in patients who were diagnosed with hematologic cancer within the past year and whose cancer had relapsed after initial treatment (238)Wood WA, Neuberg DS, Thompson JC, Tallman MS, Sekeres MA, Sehn LH, et al. Outcomes of patients with hematologic malignancies and COVID-19: a report from the ASH Research Collaborative Data Hub. Blood Adv 2020;4:5966–75.. Another meta-analysis that included nearly 5,000 patients with cancer across eight studies found that the risk of COVID-19-related death was 47 percent greater in patients with hematologic cancers compared to those with solid tumors (239)Duffy EC, Fan K, Advani S, Bailey C, Black JRM, Strauss E, et al. Abstract S12-02: Comprehensive meta-analysis of COVID-19 mortality rates for 22 cancer subtypes from the Reboot: COVID-Cancer Project, an interactive resource with aggregated data from 21,8. These findings are further substantiated by recent results from a large study encompassing nearly 400,000 adult patients with cancer, 63,413 of whom were also COVID-19 positive, showing that patients with hematologic cancers had a higher mortality rate (17 percent) compared to patients with other types of cancer (e.g., the mortality rate in patients with breast cancer was seven percent) (240)Sharafeldin N, Bates B, Song Q, Madhira V, Yan Y, Dong S, et al. Outcomes of COVID-19 in patients with cancer: report from the National COVID Cohort Collaborative (N3C). J Clin Oncol 2021;39:2232–46. Researchers are also learning that patients with certain subtypes of blood cancers, such as acute myeloid leukemia, have a higher mortality rate compared to those with lymphomas (241)Pagano L, Salmanton-Garcia J, Marchesi F, Busca A, Corradini P, Hoenigl M, et al. COVID-19 infection in adult patients with hematological malignancies: a European Hematology Association Survey (EPICOVIDEHA). J Hematol Oncol 2021;14:168.
As evident from presented data, the reported mortality rates among COVID-19 patients with hematologic malignancies vary across studies, likely because of the variability in patients’ demographic attributes—such as socioeconomic status and age—and clinical characteristics—such as the subtype of hematologic cancer and/or other comorbidities. It is, however, clear that patients with hematologic cancers are at a significantly higher risk of severe COVID-19 and death compared to patients with solid tumors or COVID-19 patients with no history of cancer.
Patients with Lung Cancer
Patients with lung cancer are also uniquely vulnerable to COVID-19 and carry a disproportionately higher burden of severe illness and death from the disease (243)Yang K, Sheng Y, Huang C, Jin Y, Xiong N, Jiang K, et al. Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol 2020;21:904–13.(244)Miyashita H, Mikami T, Chopra N, Yamada T, Chernyavsky S, Rizk D, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol 2020;31:1088–9.(245)Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335–7.. Reasons for these unfavorable outcomes are likely multifactorial. Lung cancer patients often have lung damage or lower lung capacity because of the cancer itself or treatments received such as radiotherapy and surgery (see sidebar on Why Are Cancer Patients at an Increased Risk of Infection?). For example, lung cancer can cause blockage of up to 30 percent of the major airways in patients with advanced-stage lung cancer (246)Verma A, Goh SK, Tai DYH, Kor AC, Soo CI, Seow DGF, et al. Outcome of advanced lung cancer with central airway obstruction versus without central airway obstruction. ERJ Open Res 2018;4:00173–2017.. Another common consequence of advanced-stage lung cancer is the buildup of fluid around lungs, which can restrict breathing (247)Skok K, Hladnik G, Grm A, Crnjac A. Malignant pleural effusion and its current management: a review. Medicina (Kaunas) 2019;55:490.. Most patients with lung cancer also exhibit multiple risk factors—such as aging, smoking, and pulmonary and cardiovascular issues—that are associated with worse outcomes from COVID-19 (see Increasing Risk for COVID-19) (216)Yang B, Choi H, Lee SK, Chung SJ, Yeo Y, Shin YM, et al. Risk of coronavirus disease 2019 occurrence, severe presentation, and mortality in patients with lung cancer. Cancer Res Treat 2021;53:678–84.. Moreover, COVID-19 is primarily a respiratory illness that can cause lasting damage to lungs (248)Rendeiro AF, Ravichandran H, Bram Y, Chandar V, Kim J, Meydan C, et al. The spatial landscape of lung pathology during COVID-19 progression. Nature 2021;593:564–9., further compounding the adverse outcomes for patients with lung cancer.
An analysis of 21 studies from across the globe, covering more than 2,000 patients with cancer, found that lung cancer was the most frequently detected type of cancer among patients with COVID-19 (250)Lemos AEG, Silva GR, Gimba ERP, Matos ADR. Susceptibility of lung cancer patients to COVID-19: a review of the pandemic data from multiple nationalities. Thorac Cancer 2021;12:2637–47.. With a few exceptions (245)Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335–7.(251)Stroppa EM, Toscani I, Citterio C, Anselmi E, Zaffignani E, Codeluppi M, et al. Coronavirus disease-2019 in cancer patients. A report of the first 25 cancer patients in a western country (Italy). Future Oncol 2020;16:1425–32.(252)Yang F, Shi S, Zhu J, Shi J, Dai K, Chen X. Clinical characteristics and outcomes of cancer patients with COVID-19. J Med Virol 2020;92:2067–73., studies have found that patients with lung cancer are at a significantly higher risk of death from COVID-19 compared to patients with other cancer types (235)Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov 2020;10:783–91.(244)Miyashita H, Mikami T, Chopra N, Yamada T, Chernyavsky S, Rizk D, et al. Do patients with cancer have a poorer prognosis of COVID-19? An experience in New York City. Ann Oncol 2020;31:1088–9.(253)de Melo AC, Thuler LCS, da Silva JL, de Albuquerque LZ, Pecego AC, Rodrigues LOR, et al. Cancer inpatients with COVID-19: a report from the Brazilian National Cancer Institute. PLoS One 2020;15:e0241261.(254)Rogado J, Pangua C, Serrano-Montero G, Obispo B, Marino AM, Perez-Perez M, et al. Covid-19 and lung cancer: a greater fatality rate? Lung Cancer 2020;146:19–22.. One review, examining data from eight independent studies covering patients with cancer and COVID-19, including more than 700 patients who had lung cancer, found that the mortality rate in patients with lung cancer was higher compared to the rate in patients who had other types of cancer and ranged from 14 percent to 55 percent (255)Kulkarni AA, Wilson G, Fujioka N, Patel MR. Mortality from COVID-19 in patients with lung cancer. J Cancer Metastasis Treat 2021;7:31.. Another analysis of 13 studies from multiple countries investigating a link between COVID-19 and lung cancer found that the mortality rate in patients with lung cancer with COVID-19 was 42 percent, which is significantly higher than 24 percent in patients with other cancer types. Interestingly, this difference in mortality rate was not significant when studies from China were also included in the analysis (256)Lei H, Yang Y, Zhou W, Zhang M, Shen Y, Tao D, et al. Higher mortality in lung cancer patients with COVID-19? A systematic review and meta-analysis. Lung Cancer 2021;157:60–5., indicating that the geographic location, ethnicity, and/or genetic makeup of the patient population may also determine mortality rates among patients with cancer and COVID-19. Such analyses also point to the need of region-specific large studies to determine the optimal course of COVID-19 management that is tailored to the specific needs and unique characteristics of patients with cancer.
Pediatric Patients with Cancer
As of December 30, 2021, almost 7.9 million of all COVID-19 cases in the U.S. were children, according to a joint report from the American Academy of Pediatrics and the Children’s Hospital Association (257)American Academy of Pediatrics and the Children’s Hospital Association. Children and COVID-19: State Data Report [updated 2021 Oct 7; cited 2021 Nov 17].. Like adults with cancer, children with cancer are frequently immunocompromised, raising concerns at the onset of the pandemic that COVID-19 will have disproportionally adverse effects on children (258)Connelly JA, Chong H, Esbenshade AJ, Frame D, Failing C, Secord E, et al. Impact of COVID-19 on pediatric immunocompromised patients. Pediatr Clin North Am 2021;68:1029–54.. However, initial evidence in early 2020 suggested that immunocompromised children and adolescents do not have increased incidence of, or severe outcomes from, COVID-19 when compared to adults with compromised immune systems. Current data suggest that asymptomatic disease or mild effects on the respiratory system, such as cough and sore throat, are the most common COVID-19-related symptoms in children with cancer (259)Moreira DC, Millen GC, Sands S, Kearns PR, Hawkins DS. The care of children with cancer during the COVID-19 pandemic. Am Soc Clin Oncol Educ Book 2021;41:1–10.. The reasons for mild impact of COVID-19 on children with cancer are currently unknown. Nonetheless, between one and six percent of pediatric cases exhibit severe disease, even though the percentage is significantly lower compared to 14 percent in adults (260)Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 among children in China. Pediatrics 2020;145:e20200702.(261)Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323:1239–42.(262)Bellino S, Punzo O, Rota MC, Del Manso M, Urdiales AM, Andrianou X, et al. COVID-19 Disease severity risk factors for pediatric patients in Italy. Pediatrics 2020;146:e2020009399..
An analysis of 16 independent studies from around the globe investigating the impact of COVID-19 on children and adolescents with cancer found that pediatric patients with cancer were at a decreased risk of death compared to adult patients with cancer, but were at a greater risk of death when compared to the general pediatric population (258)Connelly JA, Chong H, Esbenshade AJ, Frame D, Failing C, Secord E, et al. Impact of COVID-19 on pediatric immunocompromised patients. Pediatr Clin North Am 2021;68:1029–54.. Findings also indicated that the clinical outcomes in children with cancer may be worse if they belong to certain high-risk demographics, such as male or obese individuals, or have hematologic cancers (see Patients with Hematologic Cancers). A recent study of 1,500 children and adolescents with cancer and COVID-19 from 131 institutions in 45 countries indicated that 20 percent of the pediatric patients had a severe COVID-19 infection, and about four percent of all patients died, a death rate that is more than four times higher than that of the general pediatric population with COVID-19 (263)Mukkada S, Bhakta N, Chantada GL, Chen Y, Vedaraju Y, Faughnan L, et al. Global characteristics and outcomes of SARS-CoV-2 infection in children and adolescents with cancer (GRCCC): a cohort study. Lancet Oncol 2021;22:1416–26.. Children with cancer have additionally suffered from the adverse effects of the pandemic across the cancer care continuum. Studies are underway to establish the long-term impact of COVID-19 on both physical and mental health in children with cancer in the U.S. (264)ClinicalTrials.gov. Risk factors, clinical characteristics and outcomes of acute infection with coronavirus 2019 (COVID-19) in children [updated 2021 Jul 6; cited 2021 Nov 24]. and worldwide (265)Peter N, Bandyopadhyay S, Lakhoo K, Global Health Research Group on Children’s Non-Communicable Diseases Collaborative. Impact of the COVID-19 pandemic on paediatric patients with cancer in low-income, middle-income and high-income countries: protocol for.
Patients with Cancer on Active Anticancer Treatment
Certain types of cancer treatments, such as chemotherapy, can reduce the number of immune cells in the body, weakening the immune system and increasing the vulnerability of patients with cancer to infections (see sidebar on Why Are Cancer Patients at an Increased Risk of Infection?). A better understanding of the correlation between active anticancer treatment and higher mortality rates in patients with COVID-19 and cancer is emerging. A study of more than half a million COVID-19 patients, which included more than 14,000 patients who also had cancer, found that the mortality rate was the highest in patients with cancer who had received an anticancer treatment within three months before COVID-19 diagnosis (7.8 percent) compared to patients with no recent cancer treatment (5.0 percent) and patients without cancer (1.6 percent) (266)Chavez-MacGregor M, Lei X, Zhao H, Scheet P, Giordano SH. Evaluation of COVID-19 mortality and adverse outcomes in US patients with or without cancer. JAMA Oncol 2021 Oct 28 [Epub ahead of print].. Another study found that, compared to other types of anticancer treatments, such as molecularly targeted therapy, patients with cancer who received surgical treatment within 40 days of contracting COVID-19 demonstrated higher rates of death (25 percent) and higher chances of ICU admission (37.5 percent), and were likely to develop severe or critical symptoms (62.5 percent) and require invasive ventilation (25 percent) (235)Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov 2020;10:783–91..
A meta-analysis from 29 studies, including more than 5,000 patients with cancer and COVID-19, found that even though chemotherapy more than doubled mortality in patients with hematologic cancers, there was no correlation between solid tumors and increased chances of death due to anticancer treatment (267)Liu H, Yang D, Chen X, Sun Z, Zou Y, Chen C, et al. The effect of anticancer treatment on cancer patients with COVID-19: a systematic review and meta-analysis. Cancer Med 2021;10:1043–56.. Similarly, an analysis of available data on the use of immune checkpoint inhibitors suggested that use of these anticancer therapeutics did not increase COVID-19-related mortality (268)Garassino MC, Ribas A. At the crossroads: COVID-19 and immune-checkpoint blockade for cancer. Cancer Immunol Res 2021;9:261–4.. However, another analysis of 16 studies, covering more than 3,000 patients with cancer and COVID-19, found that severity of COVID-19, as well as mortality, was higher with anticancer treatment compared to no treatment (267)Liu H, Yang D, Chen X, Sun Z, Zou Y, Chen C, et al. The effect of anticancer treatment on cancer patients with COVID-19: a systematic review and meta-analysis. Cancer Med 2021;10:1043–56.. These seemingly conflicting reports point to the heterogeneity of cancer, as well as the diversity of the patient population, and indicate that additional research is needed to firmly establish the effect of anticancer treatments on COVID-19-related outcomes (269)Bersanelli M. Controversies about COVID-19 and anticancer treatment with immune checkpoint inhibitors. Immunotherapy 2020;12:269–73..
Prevention and Treatment of COVID-19 in Patients with Cancer
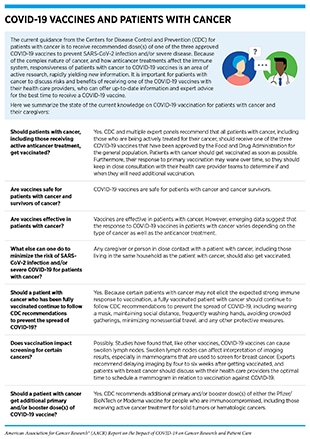
Patients with cancer constitute one of the groups most vulnerable to severe complications and death from COVID-19 (see Burden of COVID-19 in Patients with Cancer) and should take extra care to protect themselves from COVID-19. With a wider availability of highly effective vaccines against COVID-19 (see sidebar on SARS-CoV-2 Vaccination Recommendations), getting vaccinated is the first line of defense against SARS-CoV-2 infection (see sidebar on COVID-19 Vaccines and Patients with Cancer). Findings from a large study showed that patients with cancer had a 58 percent less chance of SARS-CoV-2 infection after receiving the second dose of one of the mRNA vaccines against COVID-19 (270)Wu JT, La J, Branch-Elliman W, Huhmann LB, Han SS, Parmigiani G, et al. Association of COVID-19 vaccination with SARS-CoV-2 infection in patients with cancer: a US nationwide Veterans Affairs study. JAMA Oncol 2021 Dec 2 [Epub ahead of print].. In addition, CDC, along with experts from multiple health care organizations, recommends that patients with cancer continue to exercise all preventive measures, including wearing a mask, social distancing, frequently washing hands, avoiding crowded gatherings, and minimizing non-essential travel.
Each patient with cancer has a unique risk for COVID-19 because of the type of cancer, anticancer treatment, and any other medical conditions (see Burden of COVID-19 in Patients with Cancer). Available evidence (see Varied Responses to COVID-19 Vaccines in Patients with Cancer) suggests that patients with certain types of cancer (such as those with hematologic cancers), or those receiving certain types of anticancer treatments, should exercise additional care and identify the best time to get vaccinated during their experience with cancer in consultation with their health care provider teams.
Varied Responses to COVID-19 Vaccines in Patients with Cancer
There is strong evidence that cancer patients are vulnerable to COVID-19 infection and, when infected, do not mount a strong immune response (see Burden of COVID-19 in Patients with Cancer), prompting AACR and several other cancer-focused professional organizations to recommend that patients with cancer be prioritized to receive vaccination against COVID-19 (204)Ribas A, Sengupta R, Locke T, Zaidi SK, Campbell KM, Carethers JM, et al. Priority COVID-19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov 2021;11:233–6.(280)Society for Immunotherapy of Cancer. SITC Statement on SARS-CoV-2 vaccination and cancer immunotherapy [updated 2020 Dec 23; cited 2021 Nov 27].(282)American Association for Cancer Research. AACR sends letter to CDC director urging boosters for caregivers and household members to protect patients with cancer [updated 2021 Oct 18; cited 2021 Dec 15]. and that additional doses of vaccines be given to patients with cancer who have already received their primary vaccination regimen (see sidebar on SARS-CoV-2 Vaccination Recommendations) (204)Ribas A, Sengupta R, Locke T, Zaidi SK, Campbell KM, Carethers JM, et al. Priority COVID-19 vaccination for patients with cancer while vaccine supply is limited. Cancer Discov 2021;11:233–6.(280)Society for Immunotherapy of Cancer. SITC Statement on SARS-CoV-2 vaccination and cancer immunotherapy [updated 2020 Dec 23; cited 2021 Nov 27].(281)European Society for Medical Oncology. ESMO statements for vaccination against COVID-19 in patients with cancer [updated 2021 Dec 16; cited 2021 Nov 27].. Furthermore, experts recommend that patients with cancer be included in ongoing as well as future phase III clinical trials of the vaccines, because these patients are vulnerable to COVID-19, yet were largely excluded from the initial clinical trials testing COVID-19 vaccines (283)American Society for Clinical Oncology. Inclusion of individuals with cancer on COVID-19 vaccine trials: a joint position statement from the American Society of Clinical Oncology and Friends of Cancer Research [updated 2021 May 20; cited 2021 Nov 27].(284)Corti C, Crimini E, Tarantino P, Pravettoni G, Eggermont AMM, Delaloge S, et al. SARS-CoV-2 vaccines for cancer patients: a call to action. Eur J Cancer 2021;148:316–27..
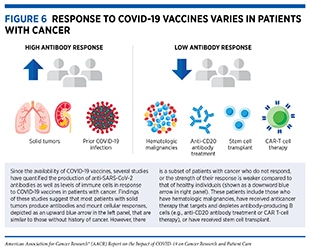
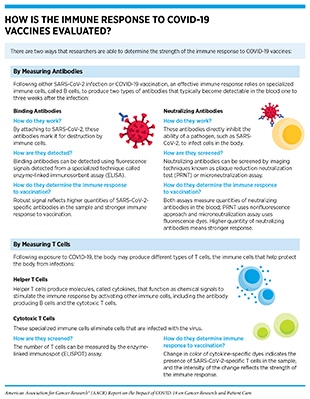
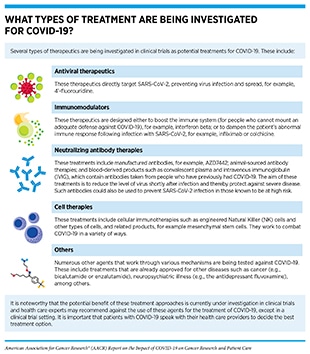
Currently available evidence suggests that the response to COVID-19 vaccines in most patients with cancer is comparable to that of the general population, with some notable exceptions (see Figure 6) (145)The Milken Institute. COVID-19 Vaccine visualization [updated 2022 Jan 5; cited 2021 Nov 27].(284)Corti C, Crimini E, Tarantino P, Pravettoni G, Eggermont AMM, Delaloge S, et al. SARS-CoV-2 vaccines for cancer patients: a call to action. Eur J Cancer 2021;148:316–27.(285)Naranbhai V, Pernat CA, Gavralidis A, St Denis KJ, Lam EC, Spring LM, et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in patients with cancer: the CANVAX cohort study. J Clin Oncol 2022;40:12–23.. Researchers are actively working to fully understand the response to COVID-19 vaccines in patients with cancer (see sidebar on How Is the Immune Response to COVID-19 Vaccines Evaluated?), and are also exploring ways to improve the responsiveness to COVID-19 vaccines in patients with cancer. An active area of research is to combine hyperimmune intravenous immunoglobulin (IVIG), which contains antibodies taken from people who have previously had COVID-19 (see sidebar on What Types of Treatment Are Being Investigated for COVID-19?), and COVID-19 vaccines to invoke a strong immune response in patients with compromised immune response.
Vaccine Response in Patients with Hematologic Cancers and Solid Tumors
In addition to the evidence that patients with blood cancers (also called hematologic malignancies) are at a higher risk of severe infection and death from COVID-19 (see Patients with Hematologic Cancers), there is also evidence that they have a less robust immune response to COVID-19 vaccines (150)Thakkar A, Gonzalez-Lugo JD, Goradia N, Gali R, Shapiro LC, Pradhan K, et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell 2021;39:1081–90.(276)Mittelman M, Magen O, Barda N, Dagan N, Oster HS, Leader A, et al. Effectiveness of the BNT162b2mRNA Covid-19 vaccine in patients with hematological neoplasms. Blood 2021 Oct 18 [Epub ahead of print].(285)Naranbhai V, Pernat CA, Gavralidis A, St Denis KJ, Lam EC, Spring LM, et al. Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in patients with cancer: the CANVAX cohort study. J Clin Oncol 2022;40:12–23.(286)Fendler A, Au L, Boos LA, Byrne F, Shepherd STC, Shum B, et al. Adaptive immunity to SARS-CoV-2 in cancer patients: the CAPTURE study. Clin Cancer Res 2020;27(287)AstraZeneca. What does immunogenicity mean in the context of COVID-19 vaccines? [updated 2020 Nov 19, cited 2021 Nov 27].(288)Addeo A, Shah PK, Bordry N, Hudson RD, Albracht B, Di Marco M, et al. Immunogenicity of SARS-CoV-2 messenger RNA vaccines in patients with cancer. Cancer Cell 2021;39:1091–8.(289)Ligumsky H, Safadi E, Etan T, Vaknin N, Waller M, Croll A, et al. Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine among actively treated cancer patients. J Natl Cancer Inst 2021 Aug 21 [Epub ahead of print].(290)Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell 2021;39:1031–3.(291)Ribas A, Dhodapkar MV, Campbell KM, Davies FE, Gore SD, Levy R, et al. How to provide the needed protection from COVID-19 to patients with hematologic malignancies. Blood Cancer Discov 2021;2:562–7.(292)Herishanu Y, Avivi I, Aharon A, Shefer G, Levi S, Bronstein Y, et al. Efficacy of the BNT162b2 mRNA COVID-19 vaccine in patients with chronic lymphocytic leukemia. Blood 2021;137:3165–73.(293)McKenzie DR, Munoz-Ruiz M, Monin L, Alaguthurai T, Lechmere T, Abdul-Jawad S, et al. Humoral and cellular immunity to delayed second dose of SARS-CoV-2 BNT162b2 mRNA vaccination in patients with cancer. Cancer Cell 2021;39:1445–7.(294)Parry H, McIlroy G, Bruton R, Ali M, Stephens C, Damery S, et al. Antibody responses after first and second Covid-19 vaccination in patients with chronic lymphocytic leukaemia. Blood Cancer J 2021;11:136.(295)Mair MJ, Berger JM, Berghoff AS, Starzer AM, Ortmayr G, Puhr HC, et al. Humoral immune response in hematooncological patients and health care workers who received SARS-CoV-2 vaccinations. JAMA Oncol 2021 Sep 30 [Epub ahead of print].. This is because not only do hematologic malignancies compromise the immune system of patients, but also certain treatments—such as chemotherapy, stem cell transplantation, and adoptive cell therapies—can further damage the immune system.
Patients who have hematologic cancers affecting B cells, which produce antibodies that are important for fighting off pathogens (e.g., SARS-CoV-2), are particularly vulnerable. In one study examining the immune response to the mRNA-1273 and BNT162b2 vaccines in patients with hematologic malignancies, almost all patients with non-Hodgkin lymphoma (NHL), the most common B-cell malignancy, were seronegative; the percent of seronegative NHL patients depended on the type of B-cell cancer and ranged from 21 percent in patients with diffuse large B-cell lymphoma to 56 percent in patients with mantle cell lymphoma (290)Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer Cell 2021;39:1031–3.. Similarly, variable rates of response to COVID-19 vaccines have been observed in patients with multiple myeloma, another type of blood cancer that affects B cells (297)Aleman A, Upadhyaya B, Tuballes K, Kappes K, Gleason CR, Beach K, et al. Variable cellular responses to SARS-CoV-2 in fully vaccinated patients with multiple myeloma. Cancer Cell 2021;39:1442–4.. Furthermore, a recent study found that patients with multiple myeloma who have completed the primary vaccination course still exhibit a significantly higher rate of breakthrough infections than the general population (298)Wang L, Berger NA, Xu R. Risks of SARS-CoV-2 breakthrough infection and hospitalization in fully vaccinated patients with multiple myeloma. JAMA Netw Open 2021;4:e2137575.. Patients with leukemia and myelodysplastic syndromes, on the other hand, appear to exhibit responses that are comparable to that of healthy individuals (291)Ribas A, Dhodapkar MV, Campbell KM, Davies FE, Gore SD, Levy R, et al. How to provide the needed protection from COVID-19 to patients with hematologic malignancies. Blood Cancer Discov 2021;2:562–7.(299)Pimpinelli F, Marchesi F, Piaggio G, Giannarelli D, Papa E, Falcucci P, et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary(300)Avivi I, Balaban R, Shragai T, Sheffer G, Morales M, Aharon A, et al. Humoral response rate and predictors of response to BNT162b2 mRNA COVID19 vaccine in patients with multiple myeloma. Br J Haematol 2021;195:186–93.(301)Chung DJ, Shah GL, Devlin SM, Ramanathan LV, Doddi S, Pessin MS, et al. Disease- and therapy-specific impact on humoral immune responses to COVID-19 vaccination in hematologic malignancies. Blood Cancer Discov 2021;2:568–76.(302)Stampfer SD, Goldwater MS, Jew S, Bujarski S, Regidor B, Daniely D, et al. Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia 2021;35:3534–41.(303)Gurion R, Rozovski U, Itchaki G, Gafter-Gvili A, Leibovitch C, Raanani P, et al. Humoral serologic response to the BNT162b2 vaccine is abrogated in lymphoma patients within the first 12 months following treatment with anti-CD2O antibodies. Haematologica 2(304)Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Anti-spike antibody response to SARS-CoV-2 booster vaccination in patients with B cell-derived hematologic malignancies. Cancer Cell 2021;39:1297–9.(305)Shapiro LC, Thakkar A, Campbell ST, Forest SK, Pradhan K, Gonzalez-Lugo JD, et al. Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell 2022;40:3–5.(306)Shroff RT, Chalasani P, Wei R, Pennington D, Quirk G, Schoenle MV, et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med 2021;27:2002–11.(307)Parvathaneni K, Torres-Rodriguez K, Meng W, Hwang WT, Frey N, Naji A, et al. SARS-CoV-2 Spike-specific T-cell responses in patients with B-cell depletion who received chimeric antigen receptor T-cell treatments. JAMA Oncol 2021 Nov 18 [Epub ahead of print.
Mortality rate among COVID-19 patients with lung cancer is significantly higher compared to the general population (235)Dai M, Liu D, Liu M, Zhou F, Li G, Chen Z, et al. Patients with cancer appear more vulnerable to SARS-CoV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov 2020;10:783–91.(308)Garassino MC, Whisenant JG, Huang LC, Trama A, Torri V, Agustoni F, et al. COVID-19 in patients with thoracic malignancies (TERAVOLT): first results of an international, registry-based, cohort study. Lancet Oncol 2020;21:914–22.. Encouragingly, a recent study shows that COVID-19 vaccines are safe and effective in patients with thoracic cancer, including lung cancer; most patients had adequate antibody levels against SARS-CoV-2 after two doses and vaccines were safe in 90 percent of the patients (309)International Association for the Study of Lung Cancer. COVID-19 vaccine effective in patients with lung cancer; 2021..
Vaccine Response in Patients with Cancer on Active Anticancer Treatment
Evidence is accruing that the anticancer treatments which modify or deplete B cells are a significant factor in a decreased immune response (291)Ribas A, Dhodapkar MV, Campbell KM, Davies FE, Gore SD, Levy R, et al. How to provide the needed protection from COVID-19 to patients with hematologic malignancies. Blood Cancer Discov 2021;2:562–7.(299)Pimpinelli F, Marchesi F, Piaggio G, Giannarelli D, Papa E, Falcucci P, et al. Fifth-week immunogenicity and safety of anti-SARS-CoV-2 BNT162b2 vaccine in patients with multiple myeloma and myeloproliferative malignancies on active treatment: preliminary(300)Avivi I, Balaban R, Shragai T, Sheffer G, Morales M, Aharon A, et al. Humoral response rate and predictors of response to BNT162b2 mRNA COVID19 vaccine in patients with multiple myeloma. Br J Haematol 2021;195:186–93.(301)Chung DJ, Shah GL, Devlin SM, Ramanathan LV, Doddi S, Pessin MS, et al. Disease- and therapy-specific impact on humoral immune responses to COVID-19 vaccination in hematologic malignancies. Blood Cancer Discov 2021;2:568–76.(302)Stampfer SD, Goldwater MS, Jew S, Bujarski S, Regidor B, Daniely D, et al. Response to mRNA vaccination for COVID-19 among patients with multiple myeloma. Leukemia 2021;35:3534–41.. One example is the anticancer treatments – for example, rituximab or obinutuzumab – targeting the CD20 protein, which is abundantly present on the surface of B cells important for mounting an effective immune response against pathogens (e.g., SARS-CoV-2). In a recent study, 80 percent of the patients with NHL who received either rituximab or obinutuzumab more than a year ago had a seropositive response to the BNT162b2 vaccine compared to just three percent of the patients who had received their last anti-CD20 treatment within 45 days of getting vaccinated (303)Gurion R, Rozovski U, Itchaki G, Gafter-Gvili A, Leibovitch C, Raanani P, et al. Humoral serologic response to the BNT162b2 vaccine is abrogated in lymphoma patients within the first 12 months following treatment with anti-CD2O antibodies. Haematologica 2. Importantly, a third dose of either of the mRNA vaccines increased antibody levels in more than 50 percent of the patients with B-cell malignancies who had not responded to the first two doses of the vaccines (304)Greenberger LM, Saltzman LA, Senefeld JW, Johnson PW, DeGennaro LJ, Nichols GL. Anti-spike antibody response to SARS-CoV-2 booster vaccination in patients with B cell-derived hematologic malignancies. Cancer Cell 2021;39:1297–9.. However, patients with cancer who were on active or recent anticancer treatment or those receiving CAR T-cell therapies that specifically targeted proteins present on B cells, such as Larry Saltzman, MD, exhibited a poor response even to the third dose (305)Shapiro LC, Thakkar A, Campbell ST, Forest SK, Pradhan K, Gonzalez-Lugo JD, et al. Efficacy of booster doses in augmenting waning immune responses to COVID-19 vaccine in patients with cancer. Cancer Cell 2022;40:3–5.(306)Shroff RT, Chalasani P, Wei R, Pennington D, Quirk G, Schoenle MV, et al. Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med 2021;27:2002–11.(307)Parvathaneni K, Torres-Rodriguez K, Meng W, Hwang WT, Frey N, Naji A, et al. SARS-CoV-2 Spike-specific T-cell responses in patients with B-cell depletion who received chimeric antigen receptor T-cell treatments. JAMA Oncol 2021 Nov 18 [Epub ahead of print. Among these patients, those who received IVIG (see sidebar on What Types of Treatment Are Being Investigated for COVID-19?) as part of their blood cancer treatment had high levels of antibodies against the spike protein of SARS-CoV-2 virus, according to recent findings (310)Leukemia and Lymphoma Society. Largest study to date demonstrates most blood cancer patients benefit from a third primary dose of mRNA COVID-19 vaccine [updated 2021 Dec 13; cited 2021 Dec 15].. Equally interesting are the reports that the levels of anti-spike antibodies in IVIG, which is derived from blood plasma donors, have increased dramatically as more members of the U.S. population have either had COVID-19 or have been vaccinated (311)Farcet MR, Karbiener M, Schwaiger J, Ilk R, Kreil TR. Rapidly increasing SARS-CoV-2 neutralization by intravenous immunoglobulins produced from plasma collected during the 2020 Pandemic. J Infect Dis 2021 Mar 16 [Epub ahead of print].. Additional studies are needed to determine whether administration of IVIG with high levels of anti-spike antibodies can benefit a broader population of immunocompromised patients who do not produce antibodies against the virus even after the booster vaccine dose.
Apart from anticancer treatments that affect B-cell functions, other types of anticancer treatment do not appear to impact responsiveness to COVID-19 vaccines. For example, one study reported no adverse effects of COVID-19 vaccines in patients whose cancer was being actively treated with immune checkpoint inhibitors (312)Chen YW, Tucker MD, Beckermann KE, Iams WT, Rini BI, Johnson DB. COVID-19 mRNA vaccines and immune-related adverse events in cancer patients treated with immune checkpoint inhibitors. Eur J Cancer 2021;155:291–3.. Another study investigating effects of COVID-19 vaccination in patients with cancer who were being treated with immune checkpoint inhibitors found no new immune-related side effects or exacerbation of existing immune-related side effects after administration of the BNT162b2 mRNA vaccine (313)Waissengrin B, Agbarya A, Safadi E, Padova H, Wolf I. Short-term safety of the BNT162b2 mRNA COVID-19 vaccine in patients with cancer treated with immune checkpoint inhibitors. Lancet Oncol 2021;22:581–3.. Another study further corroborated that there were minimal or no side effects of COVID-19 vaccination in patients with cancer undergoing active treatment compared to healthy individuals (314)Goshen-Lago T, Waldhorn I, Holland R, Szwarcwort-Cohen M, Reiner-Benaim A, Shachor-Meyouhas Y, et al. Serologic status and toxic effects of the SARS-CoV-2 BNT162b2 vaccine in patients undergoing treatment for cancer. JAMA Oncol 2021;7:1507–13..
Because of the complex nature of cancer and its treatments, several professional organizations focused on cancer care have issued guidance for patients with cancer receiving various types of treatment regarding the need to get vaccinated against COVID-19 (272)National Comprehensive Cancer Network. Recommendations of the National Comprehensive Cancer Network (NCCN) COVID-19 Vaccination Advisory Committee [updated 2022 Jan 4; cited 2021 Nov 27].(281)European Society for Medical Oncology. ESMO statements for vaccination against COVID-19 in patients with cancer [updated 2021 Dec 16; cited 2021 Nov 27].(315)American Society for Clinical Oncology. COVID-19 Vaccines & patients with cancer [updated 2021 Mar 9; cited 2021 Nov 27].. Additionally, patients with cancer should consult their health care teams about the timing and benefits of COVID-19 vaccination. One ongoing clinical study—the Vaccination Against COVID-19 in Cancer or VOICE (316)van der Veldt AAM, Oosting SF, Dingemans AC, Fehrmann RSN, GeurtsvanKessel C, Jalving M, et al. COVID-19 vaccination: the VOICE for patients with cancer. Nat Med 2021;27:568–9.—is specifically investigating the effects of various anticancer treatments on the response to COVID-19 vaccines (see sidebar on COVID-19 Vaccines and Patients with Cancer). Recent findings from the study show that, compared to 99.6 percent of the control group, 93.1 percent of patients with solid tumors who were being treated with immunotherapy, 83.8 percent of those on chemotherapy, and 88.8 percent of the patients receiving both types of treatment achieved an adequate response to mRNA-1273 28 days after receiving the second dose of the vaccine. Of note, adequate response was defined as antibodies produced against the spike protein of SARS-CoV-2 virus by the body in response to vaccination at levels sufficient to neutralize the virus. Another study showed that, while 90 percent of patients with cancer produced antibodies in response to COVID-19 vaccination, the antibody levels were four times lower in cancer patients who were receiving a combination of chemotherapy and immunotherapy (317)Massarweh A, Eliakim-Raz N, Stemmer A, Levy-Barda A, Yust-Katz S, Zer A, et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol 2021;7:1133–40.. Future studies will continue to refine our knowledge of the effects of COVID-19 vaccines and specific cancer treatments on clinical outcomes for patients with cancer.
The overwhelming evidence presented in this section strongly advocates that patients with cancer should be offered, and encouraged to practice, additional means of protection against COVID-19 that include social distancing measures and additional doses of COVID-19 vaccines as have been approved by FDA and endorsed by CDC (279)Centers for Disease Control and Prevention. COVID-19 vaccines for moderately to severely immunocompromised people [updated 2021 Nov 23; cited 2021 Nov 27].(291)Ribas A, Dhodapkar MV, Campbell KM, Davies FE, Gore SD, Levy R, et al. How to provide the needed protection from COVID-19 to patients with hematologic malignancies. Blood Cancer Discov 2021;2:562–7.(331)U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes additional vaccine dose for certain immunocompromised individuals [updated 2021 Aug 12; cited 2021 November 27].. It is also imperative to inform patients with cancer about the misinformation surrounding COVID-19 and vaccines against SARS-CoV-2 and provide them easily accessible and evidence-based information about the risks of COVID-19 exposure and the lifesaving benefits of vaccination against SARS-CoV-2 (see sidebar on COVID-19 Vaccine Misinformation and How to Address It).
Impact of COVID-19 on Cancer Science and Medicine
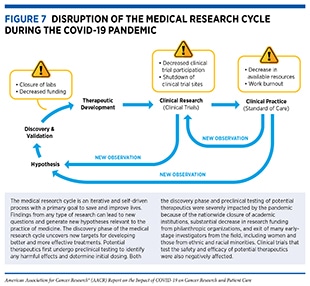
The COVID-19 pandemic has adversely affected cancer science and medicine by disrupting multiple steps of the medical research cycle (see Figure 7). The pandemic has upended the personal and professional lives of cancer researchers in the U.S. and around the world. These adverse effects include decreased productivity and lost career opportunities among cancer researchers, in particular, among early-stage, minority, and female investigators, because of the closures of and restricted access to research institutions and considerably reduced research funding by philanthropic organizations, which typically contribute up to 50 percent of all cancer research funding in the United States (332)Kamath SD, Kircher SM, Benson AB. Comparison of cancer burden and nonprofit organization funding reveals disparities in funding across cancer types. J Natl Compr Canc Netw 2019;17:849–54.; refocused expertise and resources by some cancer researchers to study SARS-CoV-2 (333)Zon L, Gomes AP, Cance WG, Ribas A, Tuveson D, Postel-Vinay S, et al. Impact of COVID-19 pandemic on cancer research. Cancer Cell 2020;38:591–3.; substantially reallocated financial resources by many health care systems away from cancer care to address the challenges imposed by the COVID-19 pandemic (334)Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood) 2020;39:2010–7.(335)Patt D, Gordan L, Diaz M, Okon T, Grady L, Harmison M, et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform 2020;4:1059–71.; and a significant decline in clinical trial enrollment and conduct (333)Zon L, Gomes AP, Cance WG, Ribas A, Tuveson D, Postel-Vinay S, et al. Impact of COVID-19 pandemic on cancer research. Cancer Cell 2020;38:591–3.(336)Lamont EB, Diamond SS, Katriel RG, Ensign LL, Liu J, Rusli E, et al. Trends in oncology clinical trials launched before and during the COVID-19 pandemic. JAMA Netw Open 2021;4:e2036353.(337)Marcum M, Kurtzweil N, Vollmer C, Schmid L, Vollmer A, Kastl A, et al. COVID-19 pandemic and impact on cancer clinical trials: an academic medical center perspective. Cancer Med 2020;9:6141–6.. It is important to note that the collective impact of these interruptions on cancer science and medicine is not yet clear, but will likely be far-reaching.
Impact on Research Funding and Workforce
In response to the COVID-19 pandemic, and to mitigate its effect on medical research in the U.S., both NIH and NCI extended deadlines of grant applications, relaxed reporting requirements, and provided flexibility on how the grant money is spent (341)NCI. Impact of COVID-19 on NCI grants; 2020. (see Steps That Helped Offset Pandemic Impact on Cancer Research). Although the federal government supported ongoing research and offered cancer researchers the flexibility to apply their skill set and knowledge to studying SARS-CoV-2 (13)Bakouny Z, Hawley JE, Choueiri TK, Peters S, Rini BI, Warner JL, et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell 2020;38:629–46.(342)Singer DS. NCI’s work to advance cancer research while responding to the COVID-19 pandemic. Cancer Cell 2020;37:746–8., COVID-19 drastically decreased fundraising opportunities for cancer-focused philanthropic research organizations (345)Cancer Research UK. Beating cancer, now and in the future: annual report and accounts 2020/21 [updated 2021 Apr 30; cited 2021 Nov 27]., which provide up to half of all cancer research funding in the U.S. (334)Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood) 2020;39:2010–7. and often fund high-risk, high-reward projects.
Progress against cancer stems from global collaborative efforts. Thus, examining the impact of the pandemic on cancer-focused research organizations around the world is instructive. For example, Cancer Research UK, a leading United Kingdom charity, which funds roughly 50 percent of all publicly funded cancer research in the country and collaborates extensively with organizations and institutions in the U.S., reduced its research budget by almost seven percent, amounting to 12 fewer fellowships, 24 fewer 5-year research programs, and 68 fewer projects (334)Birkmeyer JD, Barnato A, Birkmeyer N, Bessler R, Skinner J. The impact of the COVID-19 pandemic on hospital admissions in the United States. Health Aff (Millwood) 2020;39:2010–7.. In its annual report published in July 2021, the organization reported a more than 11 percent drop in its fundraising income (335)Patt D, Gordan L, Diaz M, Okon T, Grady L, Harmison M, et al. Impact of COVID-19 on cancer care: how the pandemic is delaying cancer diagnosis and treatment for American seniors. JCO Clin Cancer Inform 2020;4:1059–71.. Similar trends have been observed in the U.S. and Canada (344)Burki TK. Cuts in cancer research funding due to COVID-19. Lancet Oncol 2021;22:e6.(346)Cahan E. COVID-19 cancels charity galas and walks. Science is paying the price. Science. 2021 Jun 24.(347)The Cancer Letter. ACS faces precipitous drop in fundraising [updated 2020 Jun 19; cited 2021 Nov 27]., underscoring the challenges faced by philanthropic organizations that fund collaborative cancer research worldwide.
Over the past two decades, rapid advances in cancer science and medicine have transformed the cancer research community into an expansive and thriving collaborative workforce that includes researchers across the spectrum of cancer science and medicine, as well as additional disciplines across the broader fields of science, technology, engineering, mathematics, and medicine (STEMM). Impact on any one STEMM workforce ultimately impacts innovations and advances in cancer research and patient care. Data specifically evaluating the impact of the pandemic on the cancer research workforce are still emerging, but there is available information on how the COVID-19 pandemic has impacted the broader STEMM workforce, which includes the cancer research community. A survey of more than 45,000 NIH-funded researchers shows the impact of the pandemic on the medical research community; 55 percent of survey respondents said that the pandemic will have a negative impact on their career trajectory, while 76 percent reported lower levels of productivity (348)NIH. 2020 NIH Extramural Surveys: the impact of COVID-19 on the research community [updated 2020 Nov 1; cited 2021 Nov 30]..
Of the many adverse effects of COVID-19 on the STEMM workforce, perhaps the most far-reaching is its impact on early-career investigators (ECI)—students (undergraduate, graduate, medical), postdoctoral fellows or medical residents, junior faculty—especially women and those belonging to underrepresented population groups. Eighty percent of early-career and 81 percent of mid-career investigators who responded to an NIH survey indicated a lower level of productivity (348)NIH. 2020 NIH Extramural Surveys: the impact of COVID-19 on the research community [updated 2020 Nov 1; cited 2021 Nov 30].. The negative impact of the COVID-19 pandemic was significantly more pronounced on female oncologists compared to their male peers. Eighty-nine percent of female oncologists indicated that the pandemic has negatively affected their personal life compared to 78 percent of male oncologists. During lockdowns, women reported increased time spent on hospital and laboratory tasks compared to men (53 versus 46 percent and 33 versus 26 percent, respectively), and a significantly higher proportion of women than men spent less time on science and personal care (39 versus 25 percent and 58 versus 39 percent, respectively) (349)Garrido P, Adjei AA, Bajpai J, Banerjee S, Berghoff AS, Choo SP, et al. Has COVID-19 had a greater impact on female than male oncologists? Results of the ESMO Women for Oncology (W4O) Survey. ESMO Open 2021;6:100131.. The pandemic also exacerbated already existing disparities and created additional challenges for women with children (350)Langin K. Pandemic hits scientist parents hard. Science 2020;369:609–10.. For example, mothers suffered a 33 percent larger decrease in research hours compared to fathers (351)Deryugina T, Shurchkov O, Stearns J. COVID-19 Disruptions disproportionately affect female academics; 2021. National Bureau of Economic Research..
In March 2021, the National Academies of Science, Engineering, and Medicine reported—based on a survey of nearly 800 women in the academic STEMM fields—the disproportionately adverse impact of the pandemic on their research careers. According to the survey’s findings, 28 percent of women reported an increase in workload or hours worked, and 25 percent stated a decrease in productivity (352)National Academies of Sciences, Engineering, Medicine. The impact of COVID-19 on the careers of women in academic sciences, engineering, and medicine [updated 2021 Mar 1; cited 2021 Nov 27].. Researchers worry that the impact of the COVID-19 pandemic will likely exacerbate these disparities if left unaddressed (353)Jeffers AE, Leung MA, Jeffers A. The COVID-19 pandemic is widening the gap for women in STEM. Comput Sci Eng 2021;23:96–8.(354)Carr RM, Lane-Fall MB, South E, Brady D, Momplaisir F, Guerra CE, et al. Academic careers and the COVID-19 pandemic: reversing the tide. Sci Transl Med 2021;13:eabe7189..
Researchers belonging to underrepresented minorities (URM) are uniquely vulnerable to the disruptions caused by the pandemic. Even before the pandemic, URM researchers working in academia were less likely to receive NIH funding compared to their nonminority counterparts and thus had smaller research programs (356)Lewellen-Williams C, Johnson VA, Deloney LA, Thomas BR, Goyol A, Henry-Tillman R. The POD: a new model for mentoring underrepresented minority faculty. Acad Med 2006;81:275–9., increasing their vulnerability to the pandemic-related shutdown of research operations. URM researchers also share a disproportionally high burden of participating in services to their departments and institutions (357)Rodriguez JE, Campbell KM, Pololi LH. Addressing disparities in academic medicine: what of the minority tax? BMC Med Educ 2015;15:6.(358)Armijo PR, Silver JK, Larson AR, Asante P, Shillcutt S. Citizenship tasks and women physicians: additional woman tax in academic medicine? J Womens Health 2021;30:935–43.(359)Obasi AI. Equity in excellence or just another tax on Black skin? Lancet 2020;396:651–3.(360)Gewin V. The time tax put on scientists of colour. Nature 2020;583:479–81.. There are growing concerns that URM researchers may be called upon to take on additional service burden during a period of recovery from shutdowns, thus worsening inequities and hampering their ability to focus on research (356)Lewellen-Williams C, Johnson VA, Deloney LA, Thomas BR, Goyol A, Henry-Tillman R. The POD: a new model for mentoring underrepresented minority faculty. Acad Med 2006;81:275–9.. Because many URM researchers are more likely to engage in research pertaining to underserved populations (360)Gewin V. The time tax put on scientists of colour. Nature 2020;583:479–81.(361)Komaromy M, Grumbach K, Drake M, Vranizan K, Lurie N, Keane D, et al. The role of Black and Hispanic physicians in providing health care for underserved populations. N Engl J Med 1996;334:1305–10., the non-research-related burden on their time can have a long-term effect on research of minority communities, further widening health disparities. It is critical for policy makers and the medical research community alike to develop effective approaches and accessible resources to mitigate the adverse effects of the pandemic on URM researchers.
Another concerning aspect is the limited availability or lack of job opportunities for ECIs because of the pandemic. At the onset of the outbreak, academic institutions faced unprecedented financial challenges that resulted from a precipitous decline in clinical revenue and philanthropic funding. Consequently, many institutions in the U.S. and Europe implemented hiring freezes, slowdowns in hiring and/or salary reductions (363)Woolston C. Junior researchers hit by coronavirus-triggered hiring freezes. Nature 2020;582:449–50.(364)The Scientist. Universities issue hiring freezes in response to COVID-19 [updated 2020 Mar 26; cited 2021 Nov 27].. According to an analysis of advertisements on a STEMM-focused job board, faculty job openings at U.S. institutions were down by 70 percent in July-October 2020 compared to similar time frames in the previous three years (365)Langin K. U.S. faculty job market tanks. Science. 2020 Oct 16.. The ripple effect of these measures will likely persist for years and may force many ECIs to either stay longer in current positions than intended or prematurely exit the cancer research workforce entirely (366)Levine RL, Rathmell WK. COVID-19 impact on early career investigators: a call for action. Nat Rev Cancer 2020;20:357–8.. These data point to an urgent need to provide ECIs, especially women and URM researchers, the necessary funding, mentorship, and support to prevent talented scientists from exiting the cancer workforce and to maintain the momentum of the impressive progress against cancer (38)Sengupta R, Zaidi SK. AACR Cancer Progress Report 2021: discovery science driving clinical breakthroughs. Clin Cancer Res 2021;27:5757–9..
Impact on Discovery Science and Clinical Studies
On March 13, 2020, the United States declared a national emergency in response to the COVID-19 pandemic (367)Executive Office of the President of the United States of America. Declaring a national emergency concerning the novel coronavirus disease (COVID-19) outbreak [updated 2020 Mar 18; cited 2021 Nov 27]., which led to most state and local governments issuing “shelter-in-place” orders (368)New York Times. See which states and cities have told residents to stay at home [updated 2020 Mar 24; cited 2021 Nov 27].. In response to the shutdown, academic institutions, including cancer centers, only allowed restricted access to the facilities to maintain the most irreplaceable cell lines, genetically modified model organisms, or other time-sensitive experiments; many opted to stop all experiments completely (369)Mulholland EJ. Impact of COVID-19 on in vivo work and patient sample availability for cancer research. Nat Rev Cancer 2021;21:139–40.(370)Omary MB, Eswaraka J, Kimball SD, Moghe PV, Panettieri RA Jr, Scotto KW. The COVID-19 pandemic and research shutdown: staying safe and productive. J Clin Invest 2020;130:2745–8.. The shutdown of laboratories forced turnover of staff, reduction of patient biospecimens, and delays and/or shortage of laboratory materials due to national lockdown restrictions and reprioritization of health systems resources, resulting in a halt of most ongoing experiments (369)Mulholland EJ. Impact of COVID-19 on in vivo work and patient sample availability for cancer research. Nat Rev Cancer 2021;21:139–40.(371)Colbert LE, Kouzy R, Abi Jaoude J, Ludmir EB, Taniguchi CM. Cancer research after COVID-19: where do we go from here? Cancer Cell 2020;37:637–8.. Furthermore, the pandemic disrupted academic networking and in-person conferences, the longstanding models of productivity, innovation, and collaborations, respectively (372)Sohrabi C, Mathew G, Franchi T, Kerwan A, Griffin M, Soleil CDMJ, et al. Impact of the coronavirus (COVID-19) pandemic on scientific research and implications for clinical academic training – a review. Int J Surg 2021;86:57–63.. Because of the interwoven nature of basic research and clinical studies (see Figure 7), the shutdown also adversely affected clinical trials. The collective impact of the numerous effects of the pandemic on cancer science and medicine was captured in a survey of more than 200 cancer researchers in November 2020. The surveyed researchers estimated that their work has been set back by an average of six months and that research breakthroughs would be delayed by almost 18 months (373)Institute of Cancer Research. Pandemic to delay cancer advances by nearly 18 months, researchers fear [updated 2020 Nov 30; cited 2021 Nov 27]..
Clinical trials evaluate the safety and efficacy of lifesaving anticancer therapeutics and play a pivotal role in realizing the potential of discovery science as a driver of clinical breakthroughs (374)Umscheid CA, Margolis DJ, Grossman CE. Key concepts of clinical trials: a narrative review. Postgrad Med 2011;123:194–204.. One way to measure how COVID-19 has altered cancer science and medicine is to examine the impact of the pandemic on clinical trials. In the first half of 2020, the pandemic-necessitated lockdown measures, combined with severely strained health care systems, had an immediate adverse effect on clinical trials. In the United States, the number of patients enrolled each week in NCI-sponsored clinical trials was more than halved between early March and early April 2020 (375)Unger JM, Blanke CD, LeBlanc M, Hershman DL. Association of the coronavirus disease 2019 (COVID-19) outbreak with enrollment in cancer clinical trials. JAMA Netw Open 2020;3:e2010651.. Another report found that only 20 percent of the surveyed U.S. institutions were continuing to enroll patients in cancer clinical trials at pre-COVID-19 rates (376)Upadhaya S, Yu JX, Oliva C, Hooton M, Hodge J, Hubbard-Lucey VM. Impact of COVID-19 on oncology clinical trials. Nat Rev Drug Discov 2020;19:376–7. According to one study that examined clinical trial registration on a global commercial platform, there was a 60 percent decrease in the number of new cancer clinical trials launched during the five-month period from January 2020 to May 2020, when compared to the prepandemic period (336)Lamont EB, Diamond SS, Katriel RG, Ensign LL, Liu J, Rusli E, et al. Trends in oncology clinical trials launched before and during the COVID-19 pandemic. JAMA Netw Open 2021;4:e2036353..
The negative impact of the pandemic on clinical studies went beyond patient recruitment. One study found that 70 percent of survey respondents who were offered the opportunity to enroll in a clinical trial declined to do so because of the fear of increased COVID-19 exposure (377)Fleury ME, Farner AM, Unger JM. Association of the COVID-19 outbreak with patient willingness to enroll in cancer clinical trials. JAMA Oncol 2021;7:131–2.. Findings from a survey carried out between March and April 2020 show that 49 percent of institutions conducting early phase clinical trials for childhood cancers in Spain interrupted recruitment in ongoing trials. Furthermore, all institutions suffered personnel shortages (a 59 percent decrease in staff availability) and difficulties in enrolling patients (a 75 percent decline in patient recruitment) or monitoring activity (73 percent of trials were postponed) (378)Rubio-San-Simon A, Verdu-Amoros J, Hladun R, Juan-Ribelles A, Molero M, Guerra-Garcia P, et al. Challenges in early phase clinical trials for childhood cancer during the COVID-19 pandemic: a report from the new agents group of the Spanish Society of Paedi. Clinical research investigators considered concern for patient care to be a key factor in the decline in trial recruitment, closely followed by the concern for the type of cancer therapy, including route of administration (for example, whether patients needed to visit the facility for treatment) (376)Upadhaya S, Yu JX, Oliva C, Hooton M, Hodge J, Hubbard-Lucey VM. Impact of COVID-19 on oncology clinical trials. Nat Rev Drug Discov 2020;19:376–7. In a one-year follow-up analysis (March 2021), the authors examined the collective impact of COVID-19 on cancer clinical trials around the world. Encouragingly, the total number of cancer clinical trials that had to be stopped due to the pandemic started to recover in May 2020, and the trials that were reactivated after initial suspension did so quickly, in a matter of months, regardless of the cancer type being studied (379)Upadhaya S, Yu JX, Hodge J, Campbell J. COVID-19 impact on oncology clinical trials: a 1-year analysis. Nat Rev Drug Discov 2021;20:415.. This recovery was, in part, because of adaptations in enrolling and treating clinical trial participants that were made in response to the pandemic (380)Unger JM, Xiao H, LeBlanc M, Hershman DL, Blanke CD. Cancer clinical trial participation at the 1-year anniversary of the outbreak of the COVID-19 pandemic. JAMA Netw Open 2021;4:e2118433..
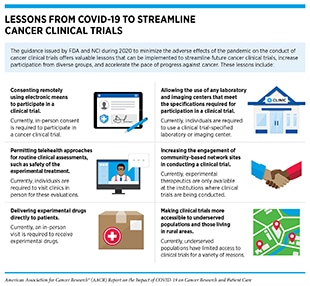
The quick rebound of cancer clinical trials after the first peak of the pandemic is, in part, the result of a collaborative and concerted effort among key stakeholders, including investigators, research funding agencies, and regulatory bodies, in quickly adapting to a revised workflow to minimize the adverse effects of the pandemic on this critical aspect of medical research (see sidebar on Lessons from COVID-19 to Streamline Oncology Clinical Trials, and see section on Pandemic-Related Flexibilities for Cancer Clinical Trials).
Impact of COVID-19 on the Cancer Continuum
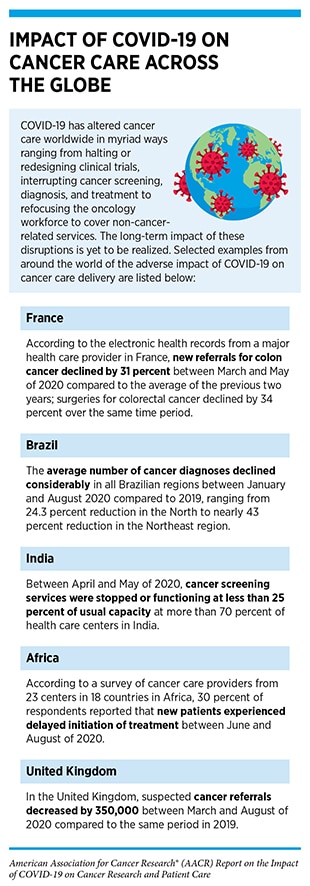
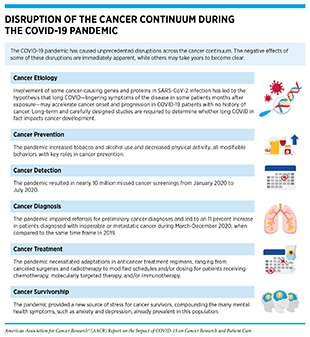
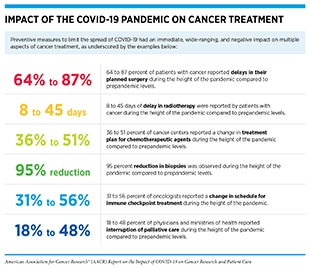
The COVID-19 pandemic has impacted every aspect of the cancer care continuum worldwide for patients with cancer, cancer survivors, and their caregivers, with some effects that are immediate and many that are long term, but currently unclear (13)Bakouny Z, Hawley JE, Choueiri TK, Peters S, Rini BI, Warner JL, et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell 2020;38:629–46. (see sidebars on Impact of COVID-19 on Cancer Care Across the Globe, and Disruption of the Cancer Continuum During the COVID-19 Pandemic). In this section, we will discuss pronounced delays in cancer screening during the early months of the pandemic that may increase late-stage cancer diagnoses in coming years; interruptions in treatment of some patients with cancer that may have potentially life-threatening outcomes; adjustments in lifestyle that may affect the long-term physical and mental health of cancer survivors; and widened cancer health disparities that disproportionally and adversely affect racial and ethnic minorities and other medically underserved populations.
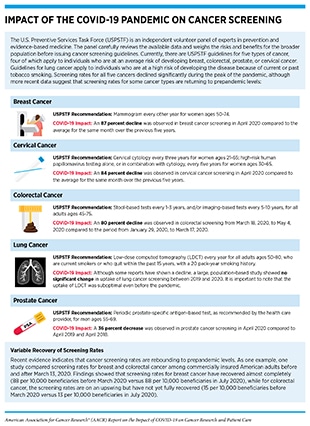
Impact on Cancer Screening
Cancer screening is an evidence-based practice of identifying precancerous lesions or cancer in an individual before any of its signs or symptoms appear (398)uspreventiveservicestaskforce.org [homepage on the internet]. Maryland: United States Preventive Services Taskforce; c2021–22 [updated 2022 Jan 5; cited 2021 Dec 15].. If an aberration is detected at the earliest possible time during cancer development, health care providers can make an informed decision on whether to monitor, treat, or surgically remove the aberration before it progresses to a more advanced stage.
Not only has the COVID-19 pandemic increased the risk of morbidity and mortality in patients with cancer (see Burden of COVID-19 in Patients with Cancer) (399)Saini KS, Tagliamento M, Lambertini M, McNally R, Romano M, Leone M, et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer 2020;139:43–50., but the full magnitude of its adverse effects on cancer care will become evident in coming years (400)Guven DC, Aktas BY, Aksun MS, Ucgul E, Sahin TK, Yildirim HC, et al. COVID-19 pandemic: changes in cancer admissions. BMJ Support Palliat Care 2020 Jul 14 [Epub ahead of print].. In April 2020, the Centers for Medicare and Medicaid Services (CMS) recommended that individuals consider postponing non-urgent services, including preventive care visits and cancer screening examinations for colorectal, breast, cervical, and lung cancers (401)Center for Medicare and Medicaid Services. Non-emergent, elective medical services, and treatment recommendations [updated 2020 Apr 7; cited 2021 Nov 29].. Many institutions halted cancer screening procedures, postponed elective surgeries, and allocated hospital resources to COVID-19 care (402)Dinmohamed AG, Visser O, Verhoeven RHA, Louwman MWJ, van Nederveen FH, Willems SM, et al. Fewer cancer diagnoses during the COVID-19 epidemic in the Netherlands. Lancet Oncol 2020;21:750–1.(403)Sud A, Jones ME, Broggio J, Loveday C, Torr B, Garrett A, et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol 2020;31:1065–74.. Additionally, the referral cascade from the primary care for the preliminary cancer diagnosis was impaired (404)Sud A, Torr B, Jones ME, Broggio J, Scott S, Loveday C, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol 2020;21:1035–44.. The collective outcome of these necessary measures to prevent the spread of COVID-19 was a precipitous decline in recommended cancer screening across five cancer types (see sidebar on Impact of the COVID-19 Pandemic on Screening) (38)Sengupta R, Zaidi SK. AACR Cancer Progress Report 2021: discovery science driving clinical breakthroughs. Clin Cancer Res 2021;27:5757–9.(65)Sengupta R, Honey K. AACR Cancer Disparities Progress Report 2020: achieving the bold vision of health equity for racial and ethnic minorities and other underserved populations. Cancer Epidemiol Biomarkers Prev 2020;29:1843.(399)Saini KS, Tagliamento M, Lambertini M, McNally R, Romano M, Leone M, et al. Mortality in patients with cancer and coronavirus disease 2019: a systematic review and pooled analysis of 52 studies. Eur J Cancer 2020;139:43–50.(405)Fedorenko CR, Kreizenbeck KL, Li L, Panattoni LE, Shankaran V, Ramsey SD. Stage at cancer diagnosis during the COVID-19 pandemic in western Washington state. J Clin Oncol 2021;39:145.(406)American Society for Radiation Oncology. COVID-19 & radiation oncology [updated 2021 Feb 25; cited 2021 Nov 27].(407)Issaka RB, Taylor P, Baxi A, Inadomi JM, Ramsey SD, Roth J. Model-based estimation of colorectal cancer screening and outcomes during the COVID-19 pandemic. JAMA Netw Open 2021;4:e216454.(408)Fedewa SA, Bandi P, Smith RA, Silvestri GA, Jemal A. Lung cancer screening rates during the COVID-19 pandemic. Chest 2021 Jul 21 [Epub ahead of print].(409)Ward ZJ, Walbaum M, Walbaum B, Guzman MJ, Jimenez de la Jara J, Nervi B, et al. Estimating the impact of the COVID-19 pandemic on diagnosis and survival of five cancers in Chile from 2020 to 2030: a simulation-based analysis. Lancet Oncol 2021;22:1427–37.(410)Velazquez AI, Hayward JH, Gregory B, Dixit N. Trends in breast cancer screening in a safety-net hospital during the COVID-19 pandemic. JAMA Netw Open 2021;4:e2119929.(411)Carethers JM, Sengupta R, Blakey R, Ribas A, D’Souza G. Disparities in cancer prevention in the COVID-19 era. Cancer Prev Res 2020;13:893–6.(412)Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh QD. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol 2021;7:458–60.(413)McBain RK, Cantor JH, Jena AB, Pera MF, Bravata DM, Whaley CM. Decline and rebound in routine cancer screening rates during the COVID-19 pandemic. J Gen Intern Med 2021;36:1829–31..
With a return of cancer screening rates to prepandemic levels, there are also reports of an increase in cancer diagnoses at an advanced stage of the disease (405)Fedorenko CR, Kreizenbeck KL, Li L, Panattoni LE, Shankaran V, Ramsey SD. Stage at cancer diagnosis during the COVID-19 pandemic in western Washington state. J Clin Oncol 2021;39:145.(406)American Society for Radiation Oncology. COVID-19 & radiation oncology [updated 2021 Feb 25; cited 2021 Nov 27].. Important lessons are emerging from these studies that can be instructive in improving future uptake of cancer screening. As one example, recovery of screening rates for colorectal cancer using noninvasive, in-home tests, such as stool-based tests, has been quicker compared to imaging-based, more invasive approaches, such as colonoscopy (407)Issaka RB, Taylor P, Baxi A, Inadomi JM, Ramsey SD, Roth J. Model-based estimation of colorectal cancer screening and outcomes during the COVID-19 pandemic. JAMA Netw Open 2021;4:e216454.. Development of non-invasive, at-home screening tests for different cancer types may increase broader uptake of cancer screening. Similarly, there was a substantive uptake of lung cancer screening in 19 states, even though the overall lung cancer screening did not change between 2019 and 2020 (408)Fedewa SA, Bandi P, Smith RA, Silvestri GA, Jemal A. Lung cancer screening rates during the COVID-19 pandemic. Chest 2021 Jul 21 [Epub ahead of print].. A closer and careful examination of the underlying reasons that led to an increase in lung cancer screening in these states may help in devising plans to encourage routine screening in individuals at risk of developing lung cancer.
There is suboptimal uptake of cancer screening among those individuals who should get screened (38)Sengupta R, Zaidi SK. AACR Cancer Progress Report 2021: discovery science driving clinical breakthroughs. Clin Cancer Res 2021;27:5757–9.(65)Sengupta R, Honey K. AACR Cancer Disparities Progress Report 2020: achieving the bold vision of health equity for racial and ethnic minorities and other underserved populations. Cancer Epidemiol Biomarkers Prev 2020;29:1843.. Furthermore, there are well documented disparities in the uptake of cancer screening among the racial and ethnic minorities and other medically underserved segments of the U.S. population. These challenges have been exacerbated in the past two years due to the ongoing COVID-19 pandemic (410)Velazquez AI, Hayward JH, Gregory B, Dixit N. Trends in breast cancer screening in a safety-net hospital during the COVID-19 pandemic. JAMA Netw Open 2021;4:e2119929.(411)Carethers JM, Sengupta R, Blakey R, Ribas A, D’Souza G. Disparities in cancer prevention in the COVID-19 era. Cancer Prev Res 2020;13:893–6., which led to a sharp decline in cancer screening during its initial peak. Additionally, distrust in health care professionals may be more prevalent in racial and ethnic minority and other medically underserved populations with suboptimal uptake of cancer screening (418)Lei F, Lee E. Barriers to lung cancer screening with low-dose computed tomography. Oncol Nurs Forum 2019;46:E60–71.(419)Mouslim MC, Johnson RM, Dean LT. Healthcare system distrust and the breast cancer continuum of care. Breast Cancer Res Treat 2020;180:33–44.(420)Adams LB, Richmond J, Corbie-Smith G, Powell W. Medical mistrust and colorectal cancer screening among African Americans. J Community Health 2017;42:1044–61., especially against the backdrop of the pandemic. It will be important to implement strategies, such as culturally appropriate, clear, and simple messaging and community outreach, to restore trust in health care professionals. Equally important will be coordinated and concerted efforts to raise awareness of the importance of cancer screening in individuals who should routinely receive the USPSTF-recommended cancer screening.
Cancer screenings that were delayed or missed because of halted preventive services during the first half of 2020 have already resulted in diagnoses of early-stage cancers in patients like Wenora Johnson and Senator Amy Klobuchar. Researchers are increasingly concerned that missed cancer screening will lead to an increase in cancer diagnoses at an advanced stage and decreased cancer survival in the coming years (225)Maringe C, Spicer J, Morris M, Purushotham A, Nolte E, Sullivan R, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol 2020;21:1023–34.(421)Kaufman HW, Chen Z, Niles JK, Fesko YA. Changes in newly identified cancer among US patients from before COVID-19 through the first full year of the pandemic. JAMA Netw Open 2021;4:e2125681.. Two recent models from the UK reported a more than five percent increase in cancer mortality risk with the late diagnoses and the delayed referrals during the pandemic (404)Sud A, Torr B, Jones ME, Broggio J, Scott S, Loveday C, et al. Effect of delays in the 2-week-wait cancer referral pathway during the COVID-19 pandemic on cancer survival in the UK: a modelling study. Lancet Oncol 2020;21:1035–44.. Other modeling studies further predict increased rates of advanced cancer diagnoses, as well as higher morbidity and mortality due to decreased and delayed cancer screening in 2020 (422)Dinmohamed AG, Cellamare M, Visser O, de Munck L, Elferink MAG, Westenend PJ, et al. The impact of the temporary suspension of national cancer screening programmes due to the COVID-19 epidemic on the diagnosis of breast and colorectal cancer in the Nether(423)Ricciardiello L, Ferrari C, Cameletti M, Gaianill F, Buttitta F, Bazzoli F, et al. Impact of SARS-CoV-2 pandemic on colorectal cancer screening delay: effect on stage shift and increased mortality. Clin Gastroenterol Hepatol 2021;19:1410–7.. Concerns of cancer diagnoses at advanced stages are reinforced by findings of a survey—conducted between January 15 and February 7, 2021—of radiation oncologists, medical professionals who utilize radiation-based methods to treat cancer. Two-thirds of the respondents said that new patients are presenting with more advanced-stage cancers and 73 percent of physicians noted that patients are not receiving recommended cancer screenings (406)American Society for Radiation Oncology. COVID-19 & radiation oncology [updated 2021 Feb 25; cited 2021 Nov 27].. One study found that circulating tumor DNA, a biomarker for assessing tumor burden, was much higher in patients who were diagnosed with colorectal cancer after the pandemic-associated lockdown (424)Thierry AR, Pastor B, Pisareva E, Ghiringhelli F, Bouche O, De La Fouchardiere C, et al. Association of COVID-19 lockdown with the tumor burden in patients with newly diagnosed metastatic colorectal cancer. JAMA Netw Open 2021;4:e2124483.. The global nature of this concern is underscored by a study from Japan that found that patients with gastrointestinal cancer were diagnosed at an advanced stage during the COVID-19 pandemic (425)Kuzuu K, Misawa N, Ashikari K, Kessoku T, Kato S, Hosono K, et al. Gastrointestinal cancer stage at diagnosis before and during the COVID-19 pandemic in Japan. JAMA Netw Open 2021;4:e2126334.. An increase in cancer diagnoses at an advanced stage may result in increased cancer morbidity and mortality in the coming years (409)Ward ZJ, Walbaum M, Walbaum B, Guzman MJ, Jimenez de la Jara J, Nervi B, et al. Estimating the impact of the COVID-19 pandemic on diagnosis and survival of five cancers in Chile from 2020 to 2030: a simulation-based analysis. Lancet Oncol 2021;22:1427–37.. It is critical to continue monitoring whether the substantial decrease in cancer screening leads to any long-term changes in U.S. cancer mortality (412)Bakouny Z, Paciotti M, Schmidt AL, Lipsitz SR, Choueiri TK, Trinh QD. Cancer screening tests and cancer diagnoses during the COVID-19 pandemic. JAMA Oncol 2021;7:458–60.(413)McBain RK, Cantor JH, Jena AB, Pera MF, Bravata DM, Whaley CM. Decline and rebound in routine cancer screening rates during the COVID-19 pandemic. J Gen Intern Med 2021;36:1829–31..
Impact on Cancer Treatment
The cancer treatment paradigm is built on five “pillars,” namely, surgery, radiotherapy, chemotherapy, molecularly targeted therapy, and immunotherapy (38)Sengupta R, Zaidi SK. AACR Cancer Progress Report 2021: discovery science driving clinical breakthroughs. Clin Cancer Res 2021;27:5757–9.. Most respondents to surveys of patients with cancer—conducted early during the pandemic—indicated that they were at least moderately worried about the impact of delays in their cancer care (426)Smith KL, Caston NE, Lawhon V, Gallagher KD, Angove R, Anderson E, et al. Association of fear of COVID-19 with delays in care or treatment interruptions in patients with cancer. J Clin Oncol 2021;39:98.(427)Leach CR, Kirkland EG, Masters M, Sloan K, Rees-Punia E, Patel AV, et al. Cancer survivor worries about treatment disruption and detrimental health outcomes due to the COVID-19 pandemic. J Psychosoc Oncol 2021;39:347–65.. Later evidence suggested that preventive measures to contain the pandemic resulted in interruptions across all pillars of the cancer treatment paradigm (see sidebar on Impact of the COVID-19 Pandemic on Cancer Treatment).
Pandemic-related interruptions in treatment of patients with cancer, such as Federico de Armas Heinzen, were reported worldwide, and across different types of cancer and treatments. One study from Canada found that 57 percent of patients with lung cancer experienced changes in their treatment plans between March 2 and May 30, 2020, as a direct result of the pandemic. Most changes encompassed either a delay (nearly 40 percent) or cessation (almost 15 percent) of palliative chemotherapy, while 26 percent of changes occurred in dosing and schedule of anticancer treatment (429)Elkrief A, Kazandjian S, Bouganim N. Changes in lung cancer treatment as a result of the coronavirus disease 2019 pandemic. JAMA Oncol 2020;6:1805–6.. According to a prediction model based on data from the United Kingdom, a six-month delay in surgery because of the pandemic could cause more than 30 percent reduction in five-year survival of patients who had an advanced cancer of the lung, pancreas, or ovary (403)Sud A, Jones ME, Broggio J, Loveday C, Torr B, Garrett A, et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol 2020;31:1065–74.. These concerning predictions are compounded by findings of a September 2021 report that clearing the backlog of cancer treatment and referrals can take over a decade (430)Institute of Public Policy Research. Building back cancer services in England [updated 2021 Sep 1; cited 2021 Nov 29].. Similarly troubling patterns are emerging in the U.S. For example, researchers predict that there will be nearly 2,500 additional deaths related to breast cancer because of the delays in cancer care caused by the pandemic, and if these delays are extended for 12 months, breast cancer-related deaths will likely double (431)Alagoz O, Lowry KP, Kurian AW, Mandelblatt JS, Ergun MA, Huang H, et al. Impact of the COVID-19 pandemic on breast cancer mortality in the US: estimates from collaborative simulation modeling. J Natl Cancer Inst 2021;113:1484–94..
It is important to note that the full extent to which the modified cancer care, necessitated by the pandemic, including changes in treatment regimen, will affect long-term survival and quality of life of patients with cancer remains unclear. A few recent studies analyzing effects of delayed or revised cancer treatments on clinical outcomes for patients with cancer indicate that some of the modified treatment schedules have not had an adverse effect on clinical outcomes for patients with cancer and may, in fact, result in positive effects on their physical, psychological, and financial health (432)Fillon M. Cancer treatment delays caused by the COVID-19 pandemic may not hinder outcomes. CA Cancer J Clin 2021;71:3–6. (see Rethinking Cancer Treatments). Carefully designed and executed long-term studies, such as the NCI COVID-19 in Cancer Patients Study (NCCAPS) (434)Cordova MJ, Riba MB, Spiegel D. Post-traumatic stress disorder and cancer. Lancet Psychiatry 2017;4:330–8., will provide valuable insights into any lasting impact of the pandemic on cancer patients and survivors.
Impact on Cancer Survivorship
Clinical breakthroughs across the continuum of cancer care are saving lives and helping individuals live longer and fuller lives after a cancer diagnosis. The number of cancer survivors has increased from 1.4 percent of the U.S. population in 1971 to more than five percent in 2019 and is projected to account for nearly seven percent of the U.S. population by 2040.
Cancer survivorship is a broad term to capture a wide range of unique experiences of living with, through, and beyond cancer. These experiences are defined by the type and stage of cancer, the age at which an individual is diagnosed, and the treatment a patient with cancer is receiving. Experience with cancer can cause a range of strains on an individual that include physical, psychosocial, and financial stresses and can often also disrupt the lives of family members, friends, and other caregivers (434)Cordova MJ, Riba MB, Spiegel D. Post-traumatic stress disorder and cancer. Lancet Psychiatry 2017;4:330–8.(435)Weber D, O’Brien K. Cancer and cancer-related fatigue and the interrelationships with depression, stress, and inflammation. J Evid Based Complementary Altern Med 2017;22:502–12.(436)Bandinelli L, Ornell F, von Diemen L, Kessler FHP. The sum of fears in cancer patients inside the context of the COVID-19. Front Psychiatry 2021;12:557834.. The COVID-19 pandemic and associated preventive measures have caused additional stress in the lives of many cancer survivors, their family members, friends, and other caregivers.
Evidence is accruing of the negative impact of the pandemic on the mental and physical health of cancer patients, survivors, and caregivers arising from social isolation, financial stress, food insecurity, concerns about timely access to cancer treatments, and disease recurrence (205)Kuderer NM, Choueiri TK, Shah DP, Shyr Y, Rubinstein SM, Rivera DR, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet 2020;395:1907–18.(245)Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol 2020;21:335–7.(437)Fung M, Babik JM. COVID-19 in immunocompromised hosts: what we know so far. Clin Infect Dis 2021;72:340–50.(438)van de Haar J, Hoes LR, Coles CE, Seamon K, Frohling S, Jager D, et al. Caring for patients with cancer in the COVID-19 era. Nat Med 2020;26:665–71.(439)Al-Shamsi HO, Alhazzani W, Alhuraiji A, Coomes EA, Chemaly RF, Almuhanna M, et al. A practical approach to the management of cancer patients during the novel coronavirus disease 2019 (COVID-19) pandemic: an International Collaborative Group. Oncologist 20(440)Slivjak ET, Fishbein JN, Nealis M, Schmiege SJ, Arch JJ. Cancer survivors’ perceived vulnerability to COVID-19 and impacts on cognitive, affective, and behavioral responses to the pandemic. J Psychosoc Oncol 2021;39:366–84.(441)Zhao F, Henderson TO, Cipriano TM, Copley BL, Liu M, Burra R, et al. The impact of coronavirus disease 2019 on the quality of life and treatment disruption of patients with breast cancer in a multiethnic cohort. Cancer 2021;127:4072–80.. As an example, most participants in an Internet-based survey reported social isolation (76%) and worsening mental health impact (70%) since the beginning of the COVID-19 pandemic, with food insecurity and financial hardship correlating significantly with adverse mental health impact (442)Amaniera I, Bach C, Vachani C, Hampshire M, Arnold-Korzeniowski K, Healy M, et al. Psychosocial impact of the COVID-19 pandemic on cancer patients, survivors and caregivers. J Psychosoc Oncol 2021;39:485–92.. In a survey administered in late May 2020, 53 percent of patients with a cancer diagnosis who completed the survey reported experiencing loneliness, which is an independent risk factor for early mortality (443)Holt-Lunstad J, Smith TB, Baker M, Harris T, Stephenson D. Loneliness and social isolation as risk factors for mortality: a meta-analytic review. Perspect Psychol Sci 2015;10:227–37., as well as more severe symptoms of anxiety, depression, fatigue, sleep disturbance, cognitive dysfunction, and pain (444)Miaskowski C, Paul SM, Snowberg K, Abbott M, Borno HT, Chang SM, et al. Loneliness and symptom burden in oncology patients during the COVID-19 pandemic. Cancer 2021;127:3246–53.. Another study found that the alarmingly high rates of stress and stress symptoms in cancer patients and survivors are comparable to those of noncancer patients who have post-traumatic stress disorder, for example, war veterans (445)Miaskowski C, Paul SM, Snowberg K, Abbott M, Borno H, Chang S, et al. Stress and symptom burden in oncology patients during the COVID-19 pandemic. J Pain Symptom Manage 2020;60:e25–34.. Long-term effects of COVID-19, such as respiratory symptoms and fatigue, affect more than 15 percent of cancer survivors, who have contracted COVID-19, and adversely affect their survival and clinical outcomes after recovery (446)Pinato DJ, Tabernero J, Bower M, Scotti L, Patel M, Colomba E, et al. Prevalence and impact of COVID-19 sequelae on treatment and survival of patients with cancer who recovered from SARS-CoV-2 infection: evidence from the OnCovid retrospective, multicentr.
The pandemic-related challenges are also apparent, and in some cases worse, in pediatric and adolescent and young adult (AYA) patients with cancer, such as Rachel Orth and Allyson Pile, who face a unique set of challenges from cancer diagnosis and treatment, including long-term cancer care, with a prolonged monitoring for recurrent disease and long-term complications that can arise from cancer treatments (38)Sengupta R, Zaidi SK. AACR Cancer Progress Report 2021: discovery science driving clinical breakthroughs. Clin Cancer Res 2021;27:5757–9.(448)Fidler MM, Frobisher C, Hawkins MM, Nathan PC. Challenges and opportunities in the care of survivors of adolescent and young adult cancers. Pediatr Blood Cancer 2019;66:e27668.(449)Robison LL, Hudson MM. Survivors of childhood and adolescent cancer: life-long risks and responsibilities. Nat Rev Cancer 2014;14:61–70.. For example, younger patients with breast cancer experienced more delays in their cancer care during the pandemic compared to older patients (450)Papautsky EL, Hamlish T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res Treat 2020;184:249–54.. Long-term cancer care in this population is a key contributing factor to financial toxicity; i.e., the financial burden cancer survivors face from out-of-pocket payments, uncovered treatment-related expenses, and lost income and productivity due to treatment-related employment disruptions and symptoms such as fatigue (451)Desai A, Gyawali B. Financial toxicity of cancer treatment: moving the discussion from acknowledgement of the problem to identifying solutions. EClinicalMedicine 2020;20:100269.. Across cancer types, financial toxicity may result in poorer quality of life (452)Lentz R, Benson AB III, Kircher S. Financial toxicity in cancer care: prevalence, causes, consequences, and reduction strategies. J Surg Oncol 2019;120:85–92.. Cancer survivors, especially those belonging to the AYA age group, may have higher vulnerability as they face financial hardships from both the pandemic and their cancer care (453)Baddour K, Kudrick LD, Neopaney A, Sabik LM, Peddada SD, Nilsen ML, et al. Potential impact of the COVID-19 pandemic on financial toxicity in cancer survivors. Head Neck 2020;42:1332–8.. In one survey of AYA cancer survivors, two thirds of the respondents reported experiencing at least one negative economic impact of COVID-19, such as increased credit card debt, and 71 percent reported engaging in at least one medical cost-coping behavior, such as not filling a prescription (454)Thom B, Benedict C, Friedman DN, Watson SE, Zeitler MS, Chino F. Economic distress, financial toxicity, and medical cost-coping in young adult cancer survivors during the COVID-19 pandemic: findings from an online sample. Cancer 2021;127:4481–91..
Impact on Cancer Health Disparities
Cancer health disparities are adverse differences in cancer experienced by certain segments of the U.S. population, such as the number of new cancer cases and deaths, cancer-related health complications, decreased quality of life after cancer treatment, financial burden, screening rates, and stage at diagnosis (455)NCI. Cancer health disparities definitions and examples; 2015.. The underlying causes of cancer health disparities are multifactorial and include limited access to health care, structural and social determinants of health, racism, and discrimination. The COVID-19 pandemic has exacerbated these baseline inequities (65)Sengupta R, Honey K. AACR Cancer Disparities Progress Report 2020: achieving the bold vision of health equity for racial and ethnic minorities and other underserved populations. Cancer Epidemiol Biomarkers Prev 2020;29:1843..
An examination of electronic health records of more than 73 million patients across the 50 states of the U.S. revealed that Black patients with a recent cancer diagnosis were at significantly higher risk of COVID-19 infection (see section on Disparities in the Burden of COVID-19 Among Certain U.S. Populations). Compared to whites, Black patients with breast or prostate cancer were at more than five times higher risk of COVID-19 infection. Furthermore, compared to whites, the risk of COVID-19 infection was more than three times higher for Black patients with colorectal cancer, and more than two times higher for Black patients with lung cancer or non-Hodgkin lymphoma (210)Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol 2021;7:220–7.. Because both cancer (38)Sengupta R, Zaidi SK. AACR Cancer Progress Report 2021: discovery science driving clinical breakthroughs. Clin Cancer Res 2021;27:5757–9.(65)Sengupta R, Honey K. AACR Cancer Disparities Progress Report 2020: achieving the bold vision of health equity for racial and ethnic minorities and other underserved populations. Cancer Epidemiol Biomarkers Prev 2020;29:1843. and COVID-19 are risk factors disproportionately affecting racial and ethnic minorities and other medically underserved populations, additional studies are needed to establish whether both risk factors are additive in increasing pre-existing health disparities.
Another study of patients with gynecologic cancer and COVID-19 in a New York City area hospital system found that Black patients were more likely to require hospitalization compared to non-Black patients (71.6 percent versus 46 percent, respectively) (456)Lara OD, Smith MJ, Wang Y, O’Cearbhaill R, Blank SV, Kolev V, et al. Racial disparities in patients with coronavirus disease 2019 infection and gynecologic malignancy. Cancer 2021;127:1057–67.. Cancer screening declined significantly during the first half of 2020 (see Impact on Cancer Screening). The reduction in cancer screening was even more noticeable for racial and ethnic minorities and those belonging to medically underserved populations of the U.S. For example, researchers found that the breast cancer screening decline in Washington state during April-December 2020 compared to the same time frame in 2019, was significantly more pronounced for women who were Hispanic (a 64.2 percent decrease), American Indian/Alaska Native (a 60.9 percent decline), Native Hawaiian or Pacific Islander (a 54.5 percent reduction), or Black (a 53.9 percent drop) compared to women who were white (a 49.2 percent decrease) (457)Amram O, Robison J, Amiri S, Pflugeisen B, Roll J, Monsivais P. Socioeconomic and racial inequities in breast cancer screening during the COVID-19 pandemic in Washington State. JAMA Netw Open 2021;4:e2110946..
In a large multi-institutional study, a 90.9 percent lower rate of prostatectomies—surgery to remove the prostate gland completely or partially—was observed among Black patients with prostate cancer compared to a 17.4 percent lower rate of prostatectomies among white patients during the initial wave of the COVID-19 pandemic (458)Bernstein AN, Talwar R, Handorf E, Syed K, Danella J, Ginzburg S, et al. Assessment of prostate cancer treatment among Black and White patients during the COVID-19 pandemic. JAMA Oncol 2021;7:1467–73..
Cancer health disparities impact medically underserved populations of the U.S. beyond cancer diagnosis and treatment and extend well into cancer survivorship. More than 40 percent of Black cancer survivors enrolled in the Detroit Research on Cancer Survivors study, who responded to a survey in the first half of 2020, reported feeling depressed, anxious, and/or isolated during the COVID-19 pandemic (459)Beebe-Dimmer JL, Lusk CM, Ruterbusch JJ, Baird TE, Pandolfi SS, Wenzlaff AS, et al. The impact of the COVID-19 pandemic on African American cancer survivors. Cancer 2021 Oct 27 [Epub ahead of print].. Socioeconomic status is another major contributor to financial toxicity, i.e., financial distress directly related to cancer diagnosis, treatment, and management (460)Abbott DE, Voils CL, Fisher DA, Greenberg CC, Safdar N. Socioeconomic disparities, financial toxicity, and opportunities for enhanced system efficiencies for patients with cancer. J Surg Oncol 2017;115:250–6.. In a telephone-based survey of 100 women with gynecologic cancer between March 15, 2020, and April 15, 2020, researchers found that patients who had an annual income of less than $40,000 were significantly more worried about future finances, and the pandemic-related delay in medical care resulted in a four-fold increased rate of anxiety (461)Chen YS, Zhou ZN, Glynn SM, Frey MK, Balogun OD, Kanis M, et al. Financial toxicity, mental health, and gynecologic cancer treatment: the effect of the COVID-19 pandemic among low-income women in New York City. Cancer 2021;127:2399–408..
All stakeholders must build upon the concerted efforts to learn from and address disparities exposed by the COVID-19 pandemic and use this knowledge to eliminate all health disparities, including disparities caused by cancer. As the cancer research community recovers from the COVID-19 pandemic, it is more important than ever to invest in cancer health disparities research, including community outreach, education, and engagement efforts.
Next Section: Future of Cancer Science and Medicine Beyond COVID-19 Previous Section: Cancer Researchers Working to Combat the Pandemic