Future of Cancer Science and Medicine Beyond COVID-19
In this section you will learn:
- COVID-19 accelerated adoption of telemedicine across the cancer care continuum. Lessons learned from telehealth during the pandemic offer a blueprint for broader and permanent implementation of telemedicine, with potential to improve patient care and reduce physician burnout.
- Changes to clinical trials that ensured continuity of lifesaving clinical studies have the potential to improve and decentralize the design of clinical trials, with the potential to increase patient participation and minimize the time it takes to safely test anticancer therapeutics.
- The pandemic necessitated modifications in cancer treatment regimens, such as increased time between doses, with the potential to improve overall patient survival.
- Worldwide scientific collaborations and rapid sharing of resources and expertise among all stakeholders offer a framework for rapidly responding to future public health crises of this magnitude.
Evidence presented in the preceding chapters, including the powerful personal stories of patients dealing with challenges of cancer in the midst of the COVID-19 pandemic, underscores the many adverse effects of the pandemic on cancer research and patient care. Some of these effects, such as a sharp decline in cancer screening during the early months of 2020, have been quantified, while others, such as the long-term effect of missed cancer screenings on overall cancer morbidity and mortality, need to be continually monitored. Despite the many challenges posed by the COVID-19 pandemic, the success of some of the adaptations to mitigate the adverse effects of the pandemic highlights key lessons and provides future directions for cancer science and medicine. In this section, we highlight key adaptations that, if permanently implemented and further improved, may have far-reaching positive outcomes.
Implementing Telemedicine
According to NCI, telemedicine, also called telehealth, is the delivery of health care from a distance using electronic information and technology, such as computers, cameras, videoconferencing, satellites, wireless communications, and the Internet (see sidebar on What Is Telemedicine? (462)Gajarawala SN, Pelkowski JN. Telehealth benefits and barriers. J Nurse Pract 2021;17:218–21.. On March 20, 2020, CMS issued a regulatory flexibility that expanded coverage for telehealth services to Medicare beneficiaries (464)Zundel KM. Telemedicine: history, applications, and impact on librarianship. Bull Med Libr Assoc 1996;84:71–9.. The use of telemedicine by the elderly and patients with cancer has already had a widespread positive effect on the delivery of oncology services during the pandemic and has allowed patients, such as Federico de Armas Heinzen, to continue receiving cancer care, even when they are unable to visit a health care facility in person. The benefits of this strategy became immediately obvious and were reinforced by the rapid implementation of several additional regulatory waivers (Telehealth Policies During COVID-19 and the Impact on the Cancer Care Continuum, p. 119). However, it is imperative that the telehealth policies enacted during the pandemic are made permanent to ensure sustained adaptation and broad implementation of telemedicine for patient care (see The AACR Call to Action).
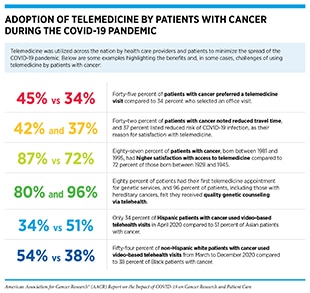
Telemedicine has a long history in health care (464)Zundel KM. Telemedicine: history, applications, and impact on librarianship. Bull Med Libr Assoc 1996;84:71–9., and in cancer care (465)Ricke J, Bartelink H. Telemedicine and its impact on cancer management. Eur J Cancer 2000;36:826–33.(466)American Society of Clinical Oncology. Telemedicine in cancer care [updated 2018 May 23; cited 2021 Nov 11].. However, the COVID-19 pandemic has accelerated adaptation of telemedicine as a key preventive measure to minimize the spread of COVID-19 and to protect patients, especially those who are immunocompromised, including patients with cancer (see sidebar on Adoption of Telemedicine by Patients with Cancer During the COVID-19 Pandemic) (467)Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med 2020;382:1679–81.. According to a July 2021 analysis, telemedicine utilization is 38 times higher than before the pandemic (468)McKinsey & Company. Telehealth: a quarter-trillion-dollar post-COVID-19 reality? [updated 2021 Jul 9; cited 2021 Dec 15].. A nationwide public opinion poll, conducted between March 26 and April 5, 2021, revealed that most Americans welcomed the expansion of telehealth. Thirty-eight percent of survey respondents had used telehealth services since the start of the pandemic, and 43 percent of the telehealth users said they want to continue using telehealth services when the COVID-19 pandemic is over (469)American Psychiatric Association. APA public opinion poll – 2021 access to care [updated 2021 Apr 30; cited 2021 Nov 27].. Other studies have reported similar findings (470)Kyle MA, Blendon RJ, Findling MG, Benson JM. Telehealth use and satisfaction among U.S. households: results of a national survey. J Patient Exp 2021;8:23743735211052737..
Researchers are also using telemedicine to deliver palliative care interventions, such as exercise, to cancer survivors. One study found that adherence to exercise-based interventions among prostate cancer survivors and their spouses increased from 81 percent in person attendance to 91 percent participation when delivered online, while the retention increased from 84 percent in person to 92 percent online. Similar results were also evident among breast cancer survivors (477)Winters-Stone KM, Boisvert C, Li F, Lyons KS, Beer TM, Mitri Z, et al. Delivering exercise medicine to cancer survivors: has COVID-19 shifted the landscape for how and who can be reached with supervised group exercise? Support Care Cancer 2021 Nov 6 [Epub. Rapid telemedicine adaptation during the COVID-19 pandemic has allowed researchers to study the effects of reduced, changed, or eliminated aspects of clinical practice and patient care. For example, one study found that reduction in frequency of follow-up visits with health care providers had no adverse effect on quality of life of breast cancer survivors; instead, it improved cost-effectiveness (470)Kyle MA, Blendon RJ, Findling MG, Benson JM. Telehealth use and satisfaction among U.S. households: results of a national survey. J Patient Exp 2021;8:23743735211052737..
Researchers are also working to address whether a broad implementation of telemedicine will reduce or increase physician burnout, which is defined as a long-term stress reaction marked by emotional exhaustion, depersonalization, and a lack of sense of personal accomplishment (480)Agency for Healthcare Research and Quality. Physician burnout [updated 2017 Jul 1; cited 2021 Dec 15].. A 2019 National Academy of Medicine report has found that nearly half of the physicians and nurses in the U.S. have experienced symptoms of burnout (481)National Academies of Sciences, Engineering, and Medicine. Taking action against clinician burnout: a systems approach to professional well-being [updated 2019 Oct 23; cited 2021 Dec 15].. Telemedicine has the potential to reduce burnout among health care workers. In a recent survey evaluating physician perceptions and attitudes toward telemedicine during the pandemic, 42 percent of the respondents preferred using telemedicine over in-person visits when possible, and 36 percent reported improved work-life balance because of using telemedicine (472)Hasson SP, Waissengrin B, Shachar E, Hodruj M, Fayngor R, Brezis M, et al. Rapid implementation of telemedicine during the COVID-19 pandemic: perspectives and preferences of patients with cancer. Oncologist 2021;26:e679–85.. Many hospitals and health systems are applying telemedicine to improve work environment, provide health care workers access to mental health counselors, and facilitate better patients care (483)InTouch Health. Battle physician burnout with telehealth solutions [updated 2018 Aug 22; cited 2021 Dec 15].(484)mHealthIntelligence. Healthcare looks to telehealth to address physician burnout, stress [updated 2020 Aug 14; cited 2021 Dec 15]..
Telemedicine permits a decentralized approach to patient care that can be paradigm shifting in delivering quality care (see Improving Clinical Trial Design and Conduct), while reducing the physical, psychological, and financial stress for patients with cancer. However, several limitations remain in realizing the true potential of telemedicine in oncology, such as lack of infrastructure, inadequate reimbursement models, insufficient community-based resources to provide in-home interventions when needed, and concerns about the privacy and protection of patient health records. Because of these limitations, a wide-range deployment of telemedicine can potentially widen the existing cancer health disparities by causing a “digital divide,” especially for patients with limited to no access to digital communication tools. It is important to assess in depth the quantifiable impact of telemedicine on long-term clinical outcomes and patient experience, as well as to systematically identify the areas that need improving (485)Offodile AC, Aloia T. Oncology clinical transformation in response to the COVID-19 pandemic. JAMA Health Forum 2020;1:e201126.(486)Lopez AM, Lam K, Thota R. Barriers and facilitators to telemedicine: can you hear me now? Am Soc Clin Oncol Educ Book 2021;41:25–36..
Improving Clinical Trial Design and Conduct
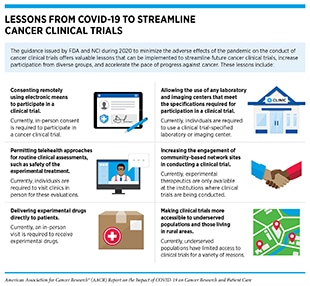
Since the onset of the pandemic, there have been concerns that clinical studies will be adversely affected, thus hampering progress against cancer. The largest impact on clinical research was between March and May 2020 (336)Lamont EB, Diamond SS, Katriel RG, Ensign LL, Liu J, Rusli E, et al. Trends in oncology clinical trials launched before and during the COVID-19 pandemic. JAMA Netw Open 2021;4:e2036353.. Trial enrollments fell at many institutions because prospective participants reduced, or altogether stopped, hospital trips, and research staff focused their efforts on responding to the public health emergency cause by the pandemic. Some trials were terminated because they were deemed too dangerous to continue (see Impact on Discovery Science and Clinical Studies). The FDA and NCI quickly issued guidance during 2020 to minimize the adverse effects of the pandemic on the conduct of cancer clinical trials, offering a roadmap to streamline future cancer clinical trials, increase participation from diverse groups, and accelerate the pace of progress against cancer (see sidebar on Lessons from COVID-19 to Streamline Oncology Clinical Trials).
The COVID-19 pandemic has had a transformational impact on how multiple aspects of clinical care are delivered to patients with cancer. For example, implementation of telemedicine has taken center stage in cancer care and has shown a high rate of adoption and satisfaction among patients and health care providers alike (see Implementing Telemedicine). According to one survey of 245 clinical trial investigators, telemedicine-based interactions between investigators of cancer clinical trials and their patients, which rose more than six times higher during the peak of the pandemic compared to the prepandemic time, remained three times higher compared to prepandemic level, even six months after the peak of the pandemic (487)Xue JZ, Smietana K, Poda P, Webster K, Yang G, Agrawal G. Clinical trial recovery from COVID-19 disruption. Nat Rev Drug Discov 2020;19:662–3.. The study also found that more than 75 percent of investigators who responded to the survey expected adaptation of telemedicine consultation and remote patient monitoring to continue once the pandemic has completely subsided; a significant number of investigators also expected many other adaptations/modifications to clinical trials to become a regular component of clinical trials postpandemic, such as in-home nurse visits (44 percent of respondents) and supply of experimental therapeutics directly from sponsor (40 percent of respondents) or site (39 percent of respondents) of clinical trial to patient (487)Xue JZ, Smietana K, Poda P, Webster K, Yang G, Agrawal G. Clinical trial recovery from COVID-19 disruption. Nat Rev Drug Discov 2020;19:662–3.. Another survey found that investigators of cancer clinical trials increasingly used, or are planning to use, telemedicine (82 percent of survey respondents) and alternative locations for patient monitoring and assessment (73 percent of survey respondents) during the pandemic (376)Upadhaya S, Yu JX, Oliva C, Hooton M, Hodge J, Hubbard-Lucey VM. Impact of COVID-19 on oncology clinical trials. Nat Rev Drug Discov 2020;19:376–7. A potential reason for such high rate of satisfaction with telemedicine among patients and optimism among clinical trial investigators is that telemedicine can help alleviate some of the uniquely high burden that cancer clinical trials place on patients, e.g., on-site follow-up surveys, which can pose a barrier for patients who live far from the study site (488)Galsky MD, Stensland KD, McBride RB, Latif A, Moshier E, Oh WK, et al. Geographic accessibility to clinical trials for advanced cancer in the United States. JAMA Intern Med 2015;175:293–5..
Clinical studies are expensive. According to a 2018 U.S. Department of Health and Human Services analysis of clinical trials conducted from 2004 to 2012, the cost of a phase III clinical study was more than 50 million U.S. dollars, and the three main reasons for the high cost included clinical procedures (15 to 22 percent), administrative staff compensation (11 to 29 percent), and site monitoring (nine to 14 percent) (489)Sertkaya A, Wong HH, Jessup A, Beleche T. Key cost drivers of pharmaceutical clinical trials in the United States. Clin Trials 2016;13:117–26.. A 2021 survey of different organizations involved in conducting clinical trials found that most respondents expected the cost of decentralized clinical trials to be lower than traditional clinical trials (490)GlobalData. Industry expects DCT costs to be lower compared with traditional trials [updated 2021 Mar 8; cited 2021 Nov 27].. Decentralized clinical trials also have the potential to increase quality of care and survival outcomes with remote monitoring tools. For example, findings from a study comparing web-based symptom monitoring in patients with lung cancer with standard follow-up show that remote symptom monitoring increased 2-year overall survival by seven months (491)Denis F, Basch E, Septans AL, Bennouna J, Urban T, Dueck AC, et al. Two-year survival comparing web-based symptom monitoring vs routine surveillance following treatment for lung cancer. JAMA 2019;321:306–7..
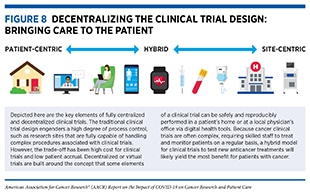
Key lessons learned from the pandemic, together with the guidance that NCI and FDA issued during the COVID-19 pandemic, highlight the opportunity to decentralize clinical trial design, so that lifesaving anticancer therapeutics can be brought quickly to as many patients as possible, without compromising the safety and efficacy of the treatments (Figure 8).
The concept of decentralized or virtual clinical trials is not new (493)Pfizer. Pfizer conducts first “virtual” clinical trial allowing patients to participate regardless of geography [updated 2011 Jun 7; cited 2021 Nov 27].. In fact, 33 percent of respondents to a survey of institutions which perform clinical trials indicated that they were conducting virtual trials prior to the COVID-19 pandemic (494)Alacrita. Could COVID-19 be a catalyst for virtualizing clinical trials? [updated 2020 Aug 30; cited 2021 Nov 27].. As we look forward to using the lessons learned from the COVID-19 pandemic to modernize cancer clinical trials, some challenges remain. A key consideration for virtual clinical trials will be to ensure secure and safe sharing and exchange of electronic health records containing sensitive patient data. Educating both patients and the clinical research staff to use the digital tools necessary to implement a decentralized clinical trial, as well as to accurately report data in a timely manner, will also require financial and technical resources as well as time and training. It will be equally important to ensure that a decentralized trial for a specific anticancer drug is feasible and safe for the participants (for example, it may be easier to conduct a decentralized clinical trial for a drug that can be taken orally and thus can be delivered to patients at home compared to a therapeutic that requires intravenous infusion at the clinic).
It is well established that the participation of racial and ethnic minorities and other medically underserved populations remains low; the FDA 2021 summary report on the diversity of clinical trial participants showed only eight percent Black and 11 percent Hispanic participation in clinical trials for the drugs approved in 2020 (495)U.S. Food and Drug Administration. 2020 Drug trial snapshots summary report.. Therefore, it is concerning, albeit unsurprising, that the use of telemedicine, a major conduit for decentralized trials, during the pandemic has been significantly lower among patients from racial and ethnic minorities and other medically underserved populations (496)Pierce RP, Stevermer JJ. Disparities in use of telehealth at the onset of the COVID-19 public health emergency. J Telemed Telecare 2020 Oct 21 [Epub ahead of print].(497)Weber E, Miller SJ, Astha V, Janevic T, Benn E. Characteristics of telehealth users in NYC for COVID-related care during the coronavirus pandemic. J Am Med Inform Assoc 2020;27:1949–54.(498)Campos-Castillo C, Anthony D. Racial and ethnic differences in self-reported telehealth use during the COVID-19 pandemic: a secondary analysis of a US survey of internet users from late March. J Am Med Inform Assoc 2021;28:119–25..
The potential of decentralized trials to reduce time and cost of traveling to clinical trial centers offers an opportunity to increase participation of racial and ethnic minorities and other medically underserved populations in clinical trials. However, realizing an equitable decentralized clinical trial design for all patients, regardless of age, gender, race, ethnicity, socioeconomic status, and/or geographic location, will require modernizing infrastructure that includes universal access to high-speed Internet through broadband connection and/or Wi-Fi hotspots, and the development of digital tools that are easy for all patients to use. Such approaches will help minimize the emerging impact of virtual cancer clinical trials on widening cancer health disparities, especially those based on age and socioeconomic status (see Impact on Cancer Health Disparities) (492)McKinsey & Company. No place like home? Stepping up the decentralization of clinical trials [updated 2021 Jun 10; cited 2021 Nov 17]..
Rethinking Cancer Treatments
The COVID-19 pandemic has affected how clinical oncologists and cancer researchers approach cancer treatments. For example, preventive measures to contain the spread of COVID-19 resulted in interruptions or delays in certain cancer treatments, such as surgery or chemotherapeutic infusions, that require visiting a health care facility because of the complexities associated with their delivery. Moreover, certain treatments, such as chemotherapy, which have become a part of routine cancer care, result in weakened immune systems, making those being actively treated for their cancer more vulnerable to COVID-19 infection (see sidebar on Why Are Cancer Patients at an Increased Risk of Infection?).
During the early months of the pandemic, FDA used data from initial phase III cancer clinical trials to approve a revised dosing schedule for pembrolizumab (Keytruda), a commonly used immune checkpoint inhibitor to treat advanced lung cancer and many other cancer types (38)Sengupta R, Zaidi SK. AACR Cancer Progress Report 2021: discovery science driving clinical breakthroughs. Clin Cancer Res 2021;27:5757–9., from 200 mg every three weeks to 400 mg every six weeks (499)Sehgal K, Costa DB, Rangachari D. Extended-interval dosing strategy of immune checkpoint inhibitors in lung cancer: will it outlast the COVID-19 pandemic? Front Oncol 2020;10:1193.(500)U.S. Food and Drug Administration. FDA approves new dosing regimen for pembrolizumab [updated 2020 Apr 29; cited 2021 Nov 27].. These measures helped reduce recurrent hospital visits, which have been identified as potential risk factors for infection with SARS-CoV-2 (501)Zhang L, Zhu F, Xie L, Wang C, Wang J, Chen R, et al. Clinical characteristics of COVID-19-infected cancer patients: a retrospective case study in three hospitals within Wuhan, China. Ann Oncol 2020;31:894–901.(502)Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol 2020;6:1108–10.. Other potential advantages of redosing strategies for immune checkpoint inhibitors may include reduced side effects as well as less financial burden for patients. However, long-term studies are needed to identify any adverse effects of the revised dosing of these lifesaving anticancer treatments on clinical outcomes for patients with cancer.
Patients with cancer expressed anxiety and concern about missing cancer treatment early during the pandemic and its potential impact on their overall health (see Impact on Cancer Treatment). To alleviate these concerns and to develop scientific evidence on whether a delay in cancer treatment during the pandemic will have an adverse impact on patients with cancer, researchers analyzed data from patients with cancer from the National Cancer Data Base that encompasses more than 70 percent of new cancer diagnoses in the U.S. (503)American College of Surgeons. National Cancer Database [updated 2022 Jan 12; cited 2021 Nov 28].. One study examined data between 2010 and 2016 and investigated whether patients with certain types of noninvasive and invasive early-stage breast cancer experienced any negative impacts, such as cancer diagnosis at a more advanced stage or a decrease in overall survival, because of delays in surgery. The usual treatment for this type of cancer is surgery soon after diagnosis. Researchers found that greater than 98 percent of the patients underwent surgical treatment within 120 days of diagnosis, and that this longer wait was not associated with diagnosis of a more advanced-stage cancer later. Even in patients who had a slightly greater risk of developing advanced-stage cancer because of the delay in surgery, there was no negative impact on overall survival (504)Minami CA, Kantor O, Weiss A, Nakhlis F, King TA, Mittendorf EA. Association between time to operation and pathologic stage in ductal carcinoma in situ and early-stage hormone receptor-positive breast cancer. J Am Coll Surg 2020;231:434–47.. The second study analyzed data from 2004 to 2014 and found that up to a 6-month delay in treatment of patients with intermediate-, high-, or very high-risk prostate cancer with radiation was not associated with worse overall survival (505)Dee EC, Mahal BA, Arega MA, D’Amico AV, Mouw KW, Nguyen PL, et al. Relative timing of radiotherapy and androgen deprivation for prostate cancer and implications for treatment during the COVID-19 pandemic. JAMA Oncol 2020;6:1630–2.. Consistent with these findings, studies have found little to no evidence that delayed or modified cancer treatments because of the pandemic have adverse outcomes for patients with cancer (506)Lee MC, Erickson TR, Stock S, Howard LE, De Hoedt AM, Amling CL, et al. Association between delay to radical prostatectomy and clinically meaningful outcomes among patients with intermediate and high-risk localized prostate cancer. J Urol 2021 Oct 25 [Epu.
It is, however, important to note that the outcome of delayed treatment likely depends on the type of cancer. For example, one study found that patients with colon cancer had an increased 5-year predicted mortality if time-to-treatment was delayed from 61-120 days (39 percent) to 181-365 days (48 percent) (507)Cone EB, Marchese M, Paciotti M, Nguyen DD, Nabi J, Cole AP, et al. Assessment of time-to-treatment initiation and survival in a cohort of patients with common cancers. JAMA Netw Open 2020;3:e2030072.. Delayed anticancer treatment can also increase the likelihood of disease recurrence, as has been reported for patients with stage I non–small cell lung cancer; for each month of surgical delay beyond 12 weeks, the risk of disease recurrence for these patients increased by 1.6 percent (508)Heiden BT, Eaton DB, Jr., Engelhardt KE, Chang SH, Yan Y, Patel MR, et al. Analysis of delayed surgical treatment and oncologic outcomes in clinical stage I non-small cell lung cancer. JAMA Netw Open 2021;4:e2111613.. These findings point to the importance of patients with cancer consulting with their health care providers about the risks and benefits of delaying treatment of cancer. Furthermore, these findings also underscore the opportunity to develop scientific evidence and consensus for re-evaluating current treatment regimens in a manner that leads to better clinical outcomes for patients with cancer.
Accelerating Collaborations, Resource Sharing, and Team Science
Progress against cancer is driven by collaborative and team science approach to medical research that also including cancer research. Years-long interdisciplinary collaborations among key stakeholders have resulted in major discovery science milestones, leading to lifesaving clinical breakthroughs. But the speed and scale of achievement during the COVID-19 pandemic — globally, 25 vaccines have been approved so far, with many additional in various III clinical trials — are rare, if not unprecedented (509)Regulatory Affairs Professionals Society. COVID-19 vaccine tracker [updated 2021 Dec 20; cited 2022 Jan 1].. According to a report from the Organization of Economic Co-operation and Development (OCED), an international organization with 38 member countries, including the U.S., around 75,000 scientific papers on COVID-19 were published from January to November 2020, with the highest level of collaboration between scientists in the U.S. and China; three quarters of the papers were open access and freely available to other researchers (510)Organisation for Economic Co-operation and Development. OECD Science, Technology and Innovation Outlook; 2021.. Moving forward, it will be pivotal for stakeholders in academia and the biopharmaceutical industry to devise open communication channels for sharing critical data necessary for developing effective therapeutics while preserving the intellectual property rights of all involved.
Nowhere has the collaborative and team science spirit been more apparent than in the unprecedented speed with which vaccines against COVID-19 were developed. The traditional pathway to developing a vaccine or an anticancer drug, from target discovery and validation to bringing it to the clinic, can take up to 10-15 years (511)Dhingra K. Oncology 2020: a drug development and approval paradigm. Ann Oncol 2015;26:2347–50.(512)Heaton PM. The Covid-19 vaccine-development multiverse. N Engl J Med 2020;383:1986–8.. On April 17, 2020, the NIH announced a public-private partnership—Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV)—to develop a coordinated research strategy for prioritizing and accelerating the development of the most promising vaccines and therapeutics against COVID-19. ACTIV brought together the NIH and other U.S. government agencies; the European Medicines Agency (EMA); and representatives from academia, philanthropic organizations, and numerous biopharmaceutical companies (164)NIH. Accelrating COVID-19 therapeutic interventions and vaccines (ACTIV) [updated 2020 Apr 17; cited 2021 Nov 27].. Similarly, the National Institute of Allergy & Infectious Diseases (NIAID) founded a new clinical trial network, the COVID-19 Prevention Trials Network (COVPN), to leverage existing infrastructure and engage communities to facilitate the enrollment of the thousands of volunteers needed for late-stage clinical trials of promising vaccines against SARS-CoV-2.
Vaccine development for COVID-19 provides a blueprint for the rapid development of vaccines and other therapeutics against deadly diseases, including cancer types for which there are not many treatment options available (514)Bloomberg Law. Pandemic cost NIH $16 billion in delayed, lost medical research [updated 2021 Mar 19; cited 2021 Dec 17]..
Next Section: Policies to Combat the Impact of the Pandemic on Cancer Science and Medicine Previous Section: Cancer in the Midst of COVID-19 and Beyond