Understanding the Path to Cancer Development
In this section, you will learn:
- Cancer is a collection of diseases in which some of the body’s cells acquire changes that allow them to grow uncontrollably and spread to other parts of the body.
- Basic research has played a pivotal role in understanding how cancer develops and spreads.
- Changes inside the cell and influences from outside the cell drive cancer initiation and progression.
- Technological advances have accelerated the identification of molecular and cellular mechanisms that drive cancer.
- Integrating the knowledge gained from decades of research into cancer development has fueled the field of precision medicine.
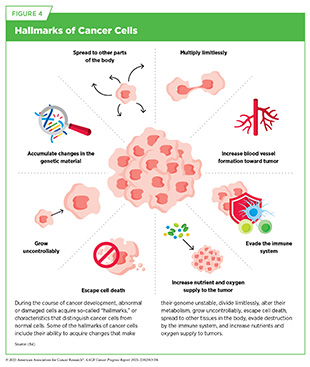
Precise molecular mechanisms control normal cell growth and division, which are required for development, regeneration, and overall healthy tissue and organ functions. In cancer, these mechanisms go awry, allowing cells to multiply uncontrollably and spread to other parts of the body. Cancer is not one disease, but a collection of related diseases that can affect nearly any part of the body. Throughout the course of cancer development, abnormal or damaged cells acquire distinct traits—known as the “hallmarks of cancer”—that set them apart from normal cells.
These hallmarks include the ability of cancer cells to multiply unchecked; acquire changes that make their genetic code unstable; ignore signals that stop normal cells from dividing; utilize different metabolic strategies to sustain rapid growth; leave the tissue of origin and spread to other organs; evade the immune system, which typically eliminates abnormal or damaged cells; and increase the supply of nutrients and oxygen to tumors (see Figure 4) (84)Hanahan D (2022) Cancer Discov, 12: 31.(85)Hanahan D, et al. (2011) Cell, 144: 646..
Cancer Development: Generating Knowledge Through Basic Research
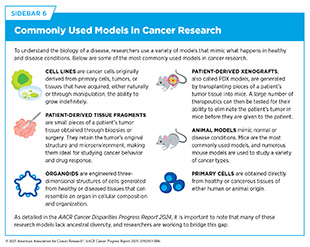
Basic research focuses on gaining new knowledge or a deeper understanding of observable events. For example, basic research plays an essential role in characterizing normal cell behavior and identifying the changes that drive cancer initiation and progression. The knowledge gained from basic research deepens our understanding of cancer biology and provides a foundation for discovering new ways to target cancer cells, developing more effective treatments, and improving strategies for early detection and prevention. Researchers explore these biological mechanisms using a wide array of experimental models that mimic healthy and disease conditions (see Sidebar 6). In recognition of the critical role of basic research in improving overall health for all individuals, more than 50 percent of the National Institutes of Health (NIH) budget has been allocated to basic research every year since 2003 (86)National Institutes of Health. The Office of Budget. National Institutes of Health FY 2003 – FY 2024 Distribution of Budget Authority Percentages for Basic and Applied Research. Accessed: June 11, 2025,
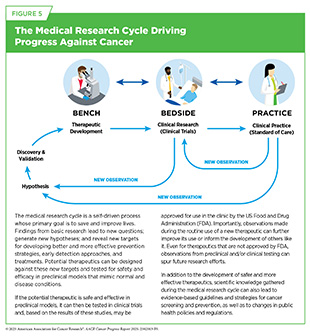
Findings and hypotheses stemming from basic research are fundamental to understanding what triggers cancer development, how cancer evades the body’s defenses, and how cancer spreads within the body. Basic research plays a central role in the medical research cycle (see Figure 5). Collectively, knowledge gained from medical research has advanced cancer risk reduction, enabled innovative early detection and diagnosis, and improved personalized treatments. These advances are contributing to progress against cancer that is saving lives and improving health outcomes for countless patients.
Basic Research Decoding Cancer’s Complexities
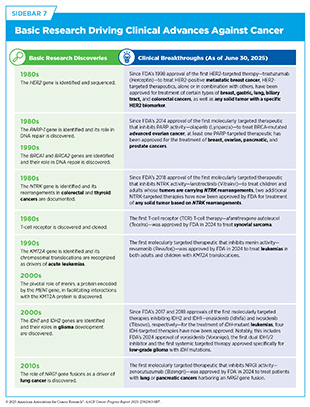
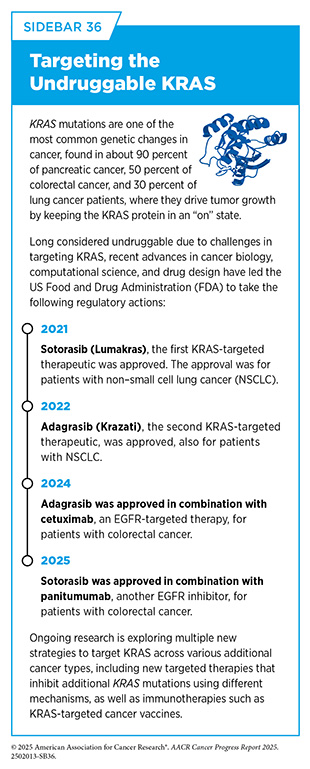
Decades of basic research, and discoveries stemming from it, have provided the foundation for progress against cancer (see Sidebar 7). One notable example of this is the development of small molecule inhibitors of Kirsten rat sarcoma viral oncogene homologue (KRAS) protein, which is encoded by the KRAS gene, one of the most commonly mutated genes in human cancers. Mutations in the KRAS gene drive roughly 30 percent of all cancers, including some of the deadliest forms, such as pancreatic, lung, and colorectal cancers. For decades KRAS remained one of the most elusive targets in cancer because of the complex structure and function of the protein, often representing the prime example of an “undruggable” target.
First discovered in 1982, the KRAS gene plays an important role in controlling how cells grow and survive. Researchers found that mutations in this gene can cause KRAS protein to be permanently turned “on,” which can lead to uncontrolled cell growth and cancer development. Even though researchers understood early on through basic research discoveries that KRAS mutations could drive cancer, the protein’s unique structure made it extremely difficult to target with drugs, since there were no obvious pockets for therapeutic molecules to bind.
Progress toward overcoming this challenge was eventually made possible through a series of fundamental research breakthroughs. Advances in understanding the three-dimensional (3D) shape of proteins uncovered a hidden “pocket” on a mutant form of KRAS, called KRAS G12C, and new chemistry tools helped researchers design drugs that could bind to it and inhibit its cancer-driving activity (87)Weinberg RA (2024) Cell, 187: 6123.. These breakthroughs led to the development of sotorasib (Lumakras), the first FDA-approved KRAS inhibitor for adults with KRAS G12C-mutated non–small cell lung cancer in 2021, nearly 40 years after the role of the KRAS gene in cancer was discovered. This milestone was quickly followed by the 2022 FDA approval of adagrasib (Krazati), a second KRAS inhibitor targeting the same mutation (see Sidebar 36).
As of June 30, 2025, sotorasib and adagrasib remain the only FDA-approved drugs targeting KRAS-mutated cancers, now approved for both lung and colorectal cancers, with ongoing research focused on enhancing their effectiveness and expanding their use across additional cancer types.
For example, a recent study of sotorasib-treated patients identified factors both within tumor cells and the surrounding environment that render KRAS-targeted treatments less effective, which may lead to new strategies for overcoming resistance to KRAS inhibitors (88)Skoulidis F, et al. (2025) Nat Med.. In another study, researchers uncovered a previously unknown mechanism by which RAS proteins promote cancer progression, opening new opportunities to target previously unrecognized pathways (89)Tripathi BK, et al. (2024) Nat Cancer, 5: 1902..
All the while, basic and translational research continue to drive the development of next-generation KRAS-targeting drugs, including small molecule inhibitors that target mutations other than G12C or work more broadly against multiple forms of KRAS—known as pan-KRAS inhibitors—which could help treat cancers beyond colorectal and lung. In addition to inhibitors, researchers are also developing protein degraders, a class of therapeutics that can eliminate KRAS proteins from cancer cells and immunotherapies designed to recognize and attack KRAS-mutated cells (see Sidebar 56).
Driven by the success of KRAS, researchers are now pursuing other challenging molecular targets, such as MYC and TP53, which are also commonly altered in many cancers. Decades of effort to inhibit MYC and TP53 are beginning to translate into tangible progress, with several compounds entering clinical trials and showing early signs of safety and efficacy (90)Whitfield JR, et al. (2025) Nat Rev Drug Discov, 24: 445.. Moreover, researchers have shown that many drugs targeting specific genetic alterations—such as those in the ALK, BRAF, NTRK, and RET genes—are effective across multiple tumor types, including lung, thyroid, melanoma, and in certain pediatric and rare cancers. In some cases, these therapies have enabled clinical trials and regulatory approvals for both adults and children simultaneously. As advances continue, the tools and insights gained from basic research are paving the way for innovative therapies aimed at tackling other difficult-to-treat cancers that have long resisted traditional approaches.
Cancer Development: Translating Knowledge to Understand the Biology of Cancer
Cancer is a collection of diseases characterized by the uncontrolled growth of cells, driven by disruptions to the molecular and cellular functions that regulate how cells grow, divide, and survive. Many of these changes are often just the first step in a complex and multistep process that is influenced by changes both inside and outside the cell.
As cancer progresses, cells accumulate additional changes that allow them to grow more aggressively, evade the immune system, and adapt to their environment. This continuous evolution leads to significant molecular and cellular differences among cells within a single tumor, a phenomenon known as tumor heterogeneity. Tumor heterogeneity also describes the differences that can exist between tumors arising in the same tissue type across different individuals, as well as among multiple tumors within an individual patient when the cancer has spread. These differences can affect how tumors grow and respond to treatment. Understanding the cellular and molecular factors inside and outside the cell that influence cancer initiation and progression is key to developing more effective strategies for treatment.
Influences Inside the Cell
Cells store their genetic instructions in a molecule called deoxyribonucleic acid (DNA). These instructions, encoded in DNA, guide the cell’s growth, function, and multiplication. DNA consists of two strands made up of repeating units called nucleotides, comprising one of four chemical bases—adenine (A), thymine (T), cytosine (C), or guanine (G). The two DNA strands pair together to form a double helical structure. The entirety of a person’s DNA is called the genome. In human cells, DNA resides within the cell’s nucleus, where it is tightly wrapped around proteins called histones. This DNA–protein complex, known as chromatin, is further compacted into larger structures called chromosomes. Most human cells contain 46 chromosomes.
Each chromosome contains hundreds to thousands of genes, which are segments of DNA that contain specific instructions. The cell uses a complex process, called transcription, to copy the instructions from genes into molecules known as messenger ribonucleic acid (mRNA). These mRNA molecules are then used in a separate process called translation to make proteins that perform a wide range of essential functions in the body. The following sections explore the types of changes within the cell that can lead to cancer development.
Genetic Alterations
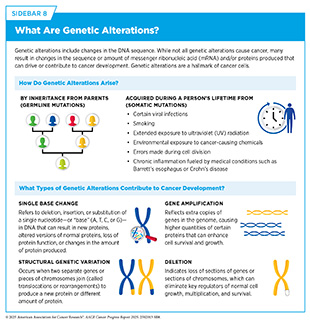
One of the hallmarks of cancer cells is alterations in the DNA sequence. Also called mutations, genetic alterations can change the sequence or amount of the resulting mRNA and protein, thus disrupting or modifying normal protein function and contributing to cancer development. Genetic alterations can be inherited from parents (germline mutations) or acquired throughout a person’s lifetime (somatic mutations) (see Sidebar 8). Whether inherited or acquired, genetic mutations tend to disrupt normal cellular functions. For example, mutations can activate oncogenes (genes that promote cell growth) or inactivate tumor suppressor genes (genes that restrict cell growth), both of which can lead to uncontrolled cell proliferation.
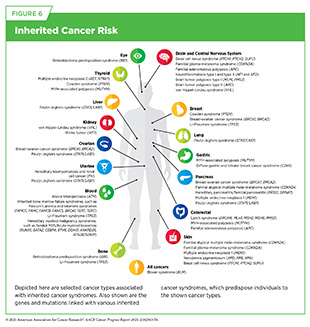
Germline mutations play a critical role in determining the inherited risk of developing cancer. About 10 percent of cancer cases are caused by germline mutations. These mutations occur in the body’s reproductive cells (egg or sperm) that are passed on from parents to children. Although not all germline mutations carried by a parent are passed down, those that are become incorporated into the DNA of every cell in the body of the offspring. These types of mutations can increase their risk of developing cancer; however, not all germline mutations contribute to cancer development. Genetic alterations not associated with an increased risk of cancer are referred to as benign variants, whereas those that contribute to cancer development are classified as pathogenic variants. However, even among pathogenic variants, some genetic alterations confer a higher risk of developing cancer than others (see Figure 6).
Technological advances in DNA sequencing have enabled a better understanding of germline pathogenic mutations and their association with a person’s risk of developing cancer. A recent study analyzing whole-genome sequences from nearly 865,000 individuals identified germline mutations in six genes not previously associated with inherited cancer risk. These mutations were linked to either increased or decreased risk of developing specific cancers, including prostate, colorectal, thyroid, lung, melanoma, and breast cancers (91)Ivarsdottir EV, et al. (2024) Nat Genet, 56: 2422.. These findings provide new insights into how inherited mutations contribute to cancer susceptibility and may offer new opportunities to inform early detection strategies and therapeutic interventions.
In addition to large-scale sequencing efforts that identify potential risk-associated inherited genetic variants, emerging genome editing technologies are now making it possible to directly test the biological impact of these mutations. CRISPR-based systems are powerful and versatile gene editing tools that allows researchers to precisely modify DNA (see CRISPR in Cancer Research and Drug Development). In two recent studies, researchers used large-scale CRISPR-based screening to test nearly all possible single base changes in the BRCA2 gene, which is often linked to inherited risk in breast and ovarian cancers. This approach allowed them to assess the functional impact of thousands of previously unclear genetic changes, known as variants of unknown significance, and determine whether they were benign or pathogenic (92)Huang H, et al. (2025) Nature, 638: 528.(93)Sahu S, et al. (2025) Nature, 638: 538..
Findings from these studies provide a powerful resource for better understanding BRCA2 inherited mutations and enhancing clinical management for individuals who carry them.
Somatic, or acquired, mutations arise during an individual’s lifetime, often because of errors during normal cell division or in response to environmental exposures, lifestyle factors, or chronic health conditions. These mutations contribute to the unique genetic landscape of individual tumors, with distinct mutation patterns reflecting their potential causes, tissue of origin, and evolution over time.
Recent research has emphasized the importance of understanding how somatic mutation patterns can vary by age, as these differences may influence cancer development, progression, and response to treatment. A study examining early-onset colorectal cancer revealed that tumors diagnosed in younger adults often carry a different set of genetic alterations than those found in older patients (see The Growing Population Burden of Cancer and Sidebar 4). Some of these mutations may affect how tumors grow, while others are linked to how well patients respond to certain therapies (94)Monge C, et al. (2025) Cancers (Basel), 17.. The study also found that these molecular features can differ by ancestry, highlighting the need for inclusive research to better understand cancer in all populations. Similarly, a recent study of breast cancer found that tumors in older adults often carry different genetic changes than those in younger patients.
These age-related differences may affect how tumors behave and respond to treatment, especially in metastatic disease (95)Selenica P, et al. (2025) NPJ Breast Cancer, 11: 70.. Together, these studies suggest that age plays a critical role in shaping cancer biology, emphasizing the need for age-tailored approaches to diagnosis and treatment across cancer types.
In addition to advances in understanding how genetic mutations drive cancer development, researchers are increasingly exploring nontraditional DNA structures that have historically been understudied. In recent years, extrachromosomal DNA (ecDNA)—circular DNA fragments that exist outside chromosomes—has emerged as a key contributor to tumor growth, treatment resistance (see Sidebar 37), and poor clinical outcomes across cancer types (see Sidebar 9). By carrying genes that regulate cell growth and survival, ecDNA acts as a potent driver of cancer progression. Unlike chromosomal DNA, ecDNA does not follow the normal rules of inheritance during cell division, enabling rapid amplification of cancer-promoting genes and allowing cancer cells to adapt more easily to treatments and other stresses in the tumor microenvironment. Cutting-edge technologies and computational analysis tools have revealed that ecDNAs are present in approximately 17 percent of human cancers and are often associated with the overproduction of oncogenes, greater genetic heterogeneity within tumors, the ability of cancer cells to evade the immune system, and resistance to therapy (96)Hung KL, et al. (2024) Nature, 635: 201.(97)Bailey C, et al. (2024) Nature, 635: 193.(98)Weiser NE, et al. (2025) Cancer Discov, 15: 1105..
Reflecting the importance of ecDNA, the Cancer Grand Challenges, a global multidisciplinary research initiative, is funding research into how ecDNA forms, functions, and can be targeted in cancer (see Technological Innovations Fueling Precision Medicine). Just in the past year, notable progress has been made in ecDNA detection and our understanding of how it drives cancer (99)Giurgiu M, et al. (2024) Genome Res, 34: 1355.(100)Zhu K, et al. (2024) Genome Res, 34: 1344.. Researchers have recently developed an innovative animal model that allows ecDNA-driven cancer to be studied in real time, providing a powerful tool to uncover how these unique DNA elements contribute to tumor progression (101). New research has shown that ecDNA can lead to conflicts between the cell’s DNA copying and RNA producing machineries. This disruption creates unique vulnerabilities in a cancer cell that may be targeted with therapies, such as CHK1 inhibitors, which block a protein that helps cells repair DNA damage. A first-in-human clinical trial is now underway to evaluate CHK1 inhibitors in cancers driven by ecDNA (102)Tang J, et al. (2024) Nature, 635: 210.. Together, these discoveries establish ecDNA as a critical driver of cancer and an exciting new area for therapeutic development.
Researchers are building on the understanding of genetic alterations that drive cancer development to inform new strategies for cancer prevention, diagnosis, and treatment. Over the past two decades, FDA has approved numerous targeted therapies designed to inhibit the effects of specific genetic mutations found in cancer cells (103)National Cancer Institute. Targeted Therapy Drug List by Cancer Type. Accessed: June 5, 2025.. At the same time, genetic tests that detect germline mutations are helping individuals understand their inherited cancer risk, guiding clinical decisions, and supporting proactive health management for both patients and their families (see Genetic Insights Advancing Cancer Risk Reduction and Interception).
RNA Variations
Gene expression is the process by which a cell reads the instructions in a gene to produce mRNA. Cells can regulate this process to increase or decrease mRNA production, depending on the cell’s needs. This regulation allows cells to fine-tune protein production as needed to respond to internal and external signals. In cancer, these regulatory processes often go awry. Cancer cells can increase the mRNA production for genes that promote survival and metastasis, while decreasing those that suppress growth.
Most human genes are made up of alternating segments known as exons and introns. Exons contain the instructions needed to make proteins, whereas introns do not. When a gene is transcribed into mRNA, the resulting transcript includes both exons and introns. Through a “cut and paste” process called RNA splicing, the introns are removed and the exons are joined together to produce an mRNA molecule that can then be translated into a functional protein. Cells also use a mechanism called alternative splicing to produce mRNAs with different combinations of exons from a single gene, generating multiple protein variants with distinct cellular functions.
In addition to mRNA, cells also produce non-coding RNAs (ncRNAs)—RNA molecules that do not encode proteins but play critical roles in regulating gene expression. These include microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs), each of which can drive normal cellular processes but also influence how tumors grow, interact with their environment, and resist treatment.
If errors occur during the RNA splicing process, faulty mRNAs can produce dysfunctional proteins. In a recent study, aberrant splicing was found to play a critical role in driving tumor heterogeneity, proliferation, invasion, and therapeutic resistance in gliomas (104)Ben Mrid R, et al. (2025) Cell Death Discov, 11: 50.. Researchers are exploring the potential of targeting splicing mechanisms as innovative therapeutic strategies to restore normal splicing patterns or selectively eliminate aberrant splice variants that are associated with cancer (105)Anczukow O, et al. (2024) Nat Rev Cancer, 24: 887..
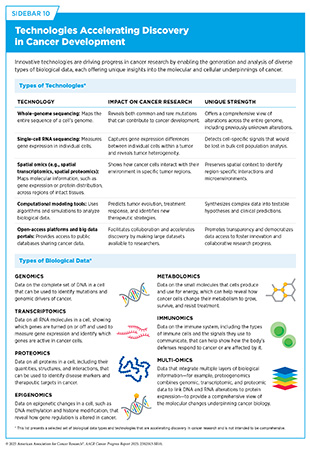
Understanding how tumors change at the RNA level provides vital insights into how cancer develops, evolves, and spreads. Technologies such as transcriptomics and single-cell RNA sequencing are more often being used to identify and understand RNA-level changes by mapping how genes are expressed within individual tumor cells and across different regions of a tumor (see Sidebar 10).
In a recent study, researchers conducted a large-scale transcriptomic analysis across multiple cancer types to understand how tumors adapt as they metastasize to new tissues. They found that while primary tumors largely resemble their tissue of origin, metastatic tumors exhibit gene expression profiles that resemble the tissues they spread to, particularly in metabolic and immune pathways (106)Sanghvi N, et al. (2024) Sci Adv, 10: eadn0220.. In addition, the study suggests that certain pathways in primary tumors already show gene expression patterns more similar to the metastatic target tissues than to the tissue of origin, highlighting transcriptomic changes that may drive or support metastasis.
In glioblastoma multiforme (GBM), a highly aggressive brain cancer, researchers used single-cell RNA sequencing to track tumor development from its earliest stages and uncovered a unique population of tumor cells with a neural crest–like identity, normally seen during early development or injury repair (107)Hamed AA, et al. (2025) Nature, 638: 499.. These findings suggest that GBM may co-opt the brain’s natural healing response to fuel tumor initiation.
Protein Modifications
The full set of proteins expressed by the cell, known as the proteome, contains approximately 20,000 unique proteins. Proteomics is the large-scale study of proteins, and research into the proteome of cancer cells has revealed important information about cancer that may not have been apparent from genomic or transcriptomic analyses alone. For example, using large-scale proteomic profiling, researchers recently identified three clinically distinct molecular subtypes of prostate cancer and developed a 16-protein panel that predicts recurrence more accurately than existing clinical methods. The study also found that two proteins from the panel play functional roles in promoting tumor aggressiveness, providing new potential molecular targets for prostate cancer therapy (108)Sun R, et al. (2024) Cell Rep Med, 5: 101679..
Proteogenomic research, which integrates proteomics, genomics, and transcriptomics, provides a comprehensive view of protein-level changes driving cancer that may be missed by DNA or RNA analysis alone (see Sidebar 10). By analyzing proteogenomic data from over 1,000 patients across 10 cancer types, researchers identified proteins that cancer cells rely on to survive and grow. While some of these proteins are already targetable by available cancer drugs, others represent new opportunities for drug development or repurposing (109)Savage SR, et al. (2024) Cell, 187: 4389.. These findings underscore the promise of proteogenomics in advancing precision medicine and guiding the development of more effective, targeted therapies.
The functions of many proteins are regulated by posttranslational modifications (PTMs), which are reversible chemical changes that modulate protein function, localization, and stability. In a recent study of more than 1,000 cancer patients across multiple tumor types, researchers introduced a precision proteogenomic approach to show that inherited genetic variants can change PTMs and protein stability, helping to explain differences between tumors that are not captured by gene expression alone (110)Martins Rodrigues F, et al. (2025) Cell, 188: 2312..
Proteins carry out nearly all the cell’s functions and must be located in the right place, in the correct 3D structure, and precisely controlled to function correctly. When proteins are no longer needed or become damaged, cells rely on carefully controlled degradation systems to remove them. Impaired protein degradation can contribute to cancer progression. A recent study showed that chaperone-mediated autophagy Group 3216, Grouped object(CMA), a process that breaks down specific cellular proteins, helps suppress tumor spread by degrading proteins that promote metastasis. Researchers found that loss of CMA promotes epithelial-to-mesenchymal transition, a process by which cancer cells become more flexible and able to move, which in turn drives more aggressive tumor growth (111)Zhou X, et al. (2025) EMBO Mol Med, 17: 747.. These findings demonstrate a mechanistic link between impaired protein degradation and metastatic progression, revealing the therapeutic potential of processes that cause controlled protein degradation in cancer.
Epigenetic Changes
Epigenetic modifications are chemical changes that regulate gene activity without altering the underlying DNA sequence. The entirety of epigenetic changes within a cell is called the epigenome. These changes involve the addition or removal of chemical marks on DNA and modifications to histones, the proteins that package DNA into chromosomes. Epigenetic alterations can be shaped by aging, environmental exposures, lifestyle factors, and chronic stress. In healthy cells, these mechanisms tightly regulate gene expression, but in cancer, this regulation is disrupted.
Epigenetic changes are now being linked with different aspects of cancer development. For example, researchers have identified that brain metastases rely on epigenetic alterations, which lead to changes in gene activity by turning genes on or off, enabling cancer cells to survive and thrive in the brain’s unique microenvironment (112)Powell AM, et al. (2025) Oncogene..
One common type of epigenetic alteration is DNA methylation, in which methyl groups are added to specific regions of the genome to regulate whether nearby genes are active or silenced. In a recent study, researchers developed a DNA methylation–based model using tumor and plasma samples from patients with lung cancer to accurately predict the risk of brain metastases (113)Zuccato JA, et al. (2025) Nat Med, 31: 116.. This study highlights the clinical potential of DNA methylation profiles as noninvasive biomarkers—measurable molecular markers that can indicate disease presence, risk, or treatment response—for identifying patients at risk for brain metastases, supporting earlier intervention and more personalized care.
Epigenetics also plays a central role in how tumors evolve and resist treatment. In a recent study, researchers used Hi-C, a high-throughput genomic and epigenomic technology that captures the spatial organization of chromatin in a cell, to examine tumor and nearby normal brain tissue from nine patients with GBM. By mapping the 3D organization of DNA, they found that changes in the genome’s structure can disrupt normal gene regulation by altering which DNA regions interact. These changes can switch genes on or off inappropriately, potentially driving tumor growth (114)Wang Q, et al. (2025) Sci Adv, 11: eadn2830.. These findings reveal that the epigenetic landscape of GBM is shaped by the tumor genome’s 3D structure and point to new therapeutic vulnerabilities that could be targeted to disrupt these altered regulatory patterns.
Epigenetic regulation is also influenced by aging and the immune system. Researchers often refer to epigenetic clocks as an estimate of a person’s biological age based on their DNA methylation patterns. When an individual’s biological age outpaces their actual chronological age—known as epigenetic age acceleration—it may signal increased cancer risk. A recent study found that aging of the immune system leads to epigenetic changes that weaken the body’s ability to fight cancer, allowing immunosuppressive cells to build up and support tumor growth (115)Park MD, et al. (2024) Science, 386: eadn0327.. In contrast, another study showed that aging may help protect lungs from developing cancer by weakening the ability of certain lung cells to become cancerous. This protective effect was linked to aging-associated epigenetic changes that lead to the loss of cancer-promoting features of lung cells (116)Zhuang X, et al. (2025) Nature, 637: 184.. Together, these findings highlight the complex role of epigenetic regulation in cancer, showing that aging-related changes can either suppress tumor initiation or promote tumor growth, depending on the cellular context. This may help explain why, although cancer risk generally increases with age, incidence declines slightly among the oldest individuals (117)Ledford H (2024) Nature, 631: 261..
Research has significantly advanced our understanding of how the epigenome is modified in cancer and how these changes contribute to cancer development. A unique feature of epigenetic changes is that they are dynamic and reversible, making them attractive targets for cancer treatment. Consequently, several anticancer drugs that modify the cancer epigenome have been developed and approved by FDA.
Influences Outside the Cell
In addition to alterations inside the cell, influences outside the cell contribute to cancer development, progression, and response to therapy (see Sidebar 11). These external factors include systemic influences, such as hormones and nutrients in the circulation, as well as local tissue environments that interact continuously with tumor cells. A hallmark of cancer is the ability of tumor cells to break away from the primary tissue and travel to other parts of the body (84)Hanahan D (2022) Cancer Discov, 12: 31.. This process—known as metastasis—is enabled by circulatory systems, such as the blood and lymphatics, which can provide physical routes through which cancer can spread, and the immune system, which can either suppress or support tumor progression, depending on how it is activated or suppressed.
Emerging evidence also shows the nervous system plays an active role in the ability of tumor cells to invade surrounding tissues and communicate with distant organs, influencing tumor growth, invasion, and metastasis. The microbiome—comprising a diverse community of microorganisms residing on and in the body—can influence cancer development by modulating inflammation, immune responses, and systemic metabolism. The tumor microenvironment encompasses the cellular and structural context in which a tumor exists and shapes how cancer evolves and responds to treatment. The following sections describe the influences outside the cell that can contribute to cancer development.
Circulatory and Immune Systems
The circulatory and immune systems—including the blood, lymphatic, and immune networks—play essential and interconnected roles in cancer initiation, progression, and response to treatment. These systems influence how tumors grow, spread, and interact with their surrounding environment, shaping both disease course and therapeutic outcomes.
The blood system, composed of blood vessels and the cells and signals that circulate within them, is essential for delivering oxygen and nutrients throughout the body. It also supports immune surveillance, the process by which the immune system constantly monitors the body for the presence of abnormal or damaged cells, including cancer cells. Angiogenesis is the formation of new blood vessels, a process that occurs naturally throughout a person’s life. Multiple chemical signals in the body control this essential process. Cancer cells acquire the ability to promote angiogenesis toward and within a tumor to meet the high demand of oxygen and nutrients needed to fuel rapid tumor growth.
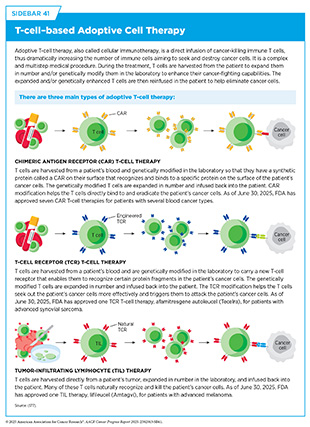
Decades of research have revealed multiple proteins and chemicals that regulate normal blood vessel formation, many of which are hijacked by cancer cells to promote tumor angiogenesis. For example, the protein vascular endothelial growth factor (VEGF) and its cell surface binding partner, called the VEGF receptor, are necessary for angiogenesis and play a crucial role in the growth of cells that line the inside of blood vessels. Cancer cells can produce and release high levels of VEGF, thus directing the formation of new blood vessels in and around tumors. VEGF can also limit the function of certain cancer immunotherapies known as chimeric antigen receptor (CAR) T cells (see Sidebar 41). In a recent study, researchers engineered CAR T cells to secrete a VEGF-blocking molecule directly within tumors, enhancing both their antitumor activity and their ability to infiltrate solid tumors (119)Supper VM, et al. (2025) Cancer Immunol Res..
Over the past two decades, several drugs that block angiogenesis have been approved to treat cancer. More recently, advances in our understanding of the cellular and molecular mechanisms driving angiogenesis are informing the development of novel therapeutic strategies. In one recent study, researchers showed that low doses of certain chemotherapeutic agents can inadvertently enhance tumor growth and metastasis in mouse models (see Sidebar 6). This effect was driven by increased angiogenesis and immune suppression through increased circulation of proangiogenic and immunosuppressive bone marrow-derived cells, underscoring the importance of optimal dosing strategies to avoid unintended pro-tumorigenic effects (120)You H, et al. (2024) Front Pharmacol, 15: 1414832.. Researchers have also shown that combining antiangiogenic agents with cancer immunotherapies known as immune checkpoint inhibitors (ICIs) could further improve immunotherapy outcomes, offering therapeutic opportunities for more precisely disrupting tumor blood supply and enhancing treatment efficacy (121)Yang S, et al. (2025) Clin Transl Med, 15: e70313..
The lymphatic system is an extensive network of vessels—known as lymphatic or lymph vessels—along with lymph nodes and several organs including the spleen, thymus, tonsils, and adenoids. It plays an important role in maintaining total fluid balance, removing cellular waste from tissues, detecting pathogens, and producing immune cells and antibodies within lymph nodes.
One way that cancer cells travel to other parts of the body is through the lymphatic system. To initiate this process, tumor cells release signaling molecules that help them move toward nearby lymphatic vessels. As they enter the lymphatic network, cancer cells can accumulate in one or more regional lymph nodes. The detection of cancer cells in lymph nodes is a key factor in determining the stage and extent of disease. From the lymph nodes, cancer cells can enter the bloodstream or continue through the lymphatic system, enabling their spread to distant organs and tissues.
Tumors can also stimulate the formation of new lymphatic vessels—a process known as lymphangiogenesis—by releasing proteins, such as a form of the VEGF protein called VEGF-C. These proteins promote lymphatic vessel remodeling and increase opportunities for cancer cells to access and spread through the lymphatic system, particularly to nearby lymph nodes.
Researchers have found that while tumor-associated lymphangiogenesis is often linked to cancer progression, it may also help the immune system fight cancer. In melanoma and colorectal cancer, more lymphatic vessels in primary tumors were found to correlate with more inflammation and increased immune cell activity in the tumor. Furthermore, VEGF-C expression in metastatic melanoma was associated with better outcomes following treatments with immunotherapeutics (122)Sun M, et al. (2025) J Exp Med, 222.. These findings suggest that changes to the structure and function of lymphatic vessels in tumors may promote immune activation and improve response to immunotherapy.
The immune system plays a critical role in fighting infections and other diseases, including cancer. Multiple cell types, tissues, and organs make up the immune system, and they work together to detect and remove pathogens, as well as abnormal or damaged cells from the body. The immune system has two main components. The innate immune system is the body’s first line of defense that provides a general, nonspecific response to pathogens. It includes physical barriers, such as the skin and mucous membranes, as well as certain immune cells and molecules that quickly respond to and kill a broad range of pathogens. In contrast, the adaptive immune system provides a specific response against pathogens and remembers them for a faster response in case of a future encounter. Some key immune cells include dendritic cells that detect threats and alert the immune system to respond, T cells that can kill infected or abnormal cells and help coordinate the immune response, and B cells that produce antibodies to neutralize pathogens (see Sidebar 39).
As cancer progresses, some cancer cells acquire properties that allow them to evade immune detection and destruction. Researchers have identified several mechanisms by which these cells evade the immune system, including activating brakes on the system to suppress T-cell responses and recruiting immunosuppressive cells. Another way cancer cells escape immune detection involves the release of proteins that impair immune cell function. In a recent study, researchers showed that melanoma cells can secrete a protein that disrupts dendritic cell function and impairs T-cell activation, thus promoting immune resistance and disease progression (123)Catena X, et al. (2025) Nat Cancer, 6: 682..
In other cases, cancer cells can disrupt cellular machinery used by immune cells to recognize damaged or abnormal cells. For example, a recent study revealed that breast cancer cells can reprogram surrounding immune cells through metabolic cross-talk, manipulating the nutrients available in their environment to transform immune cells that would normally fight cancer into ones that help it grow. This reprogramming suppresses T cell response and reshapes the tumor microenvironment (124)Zhu Y, et al. (2025) Cancer Cell, 43: 1045.. Identifying immune evasion strategies can reveal new targets for therapeutic development and improve the effectiveness of immune-based cancer therapies.
A recent study demonstrated that the dynamic relationship between cancer and the immune system can be shaped by a person’s inherited mutations, which influence how well the immune system recognizes proteins derived from certain cancer-driving genes. For example, researchers found that individuals whose immune systems could more effectively recognize HER2 protein were less likely to develop HER2-positive breast cancer. These findings suggest that inherited genetic factors can shape how the immune system detects and responds to cancer, thereby influencing both cancer risk and tumor evolution (125)Houlahan KE, et al. (2024) Science, 384: eadh8697..
Researchers continue to explore therapeutics that target immune escape mechanisms. ICIs, for example, block proteins that tumors use to suppress T cell activity, unleashing the immune response (see Sidebar 40). In a recent study, researchers discovered that a protein called ID3 helps maintain a pool of specialized T cells that can sustain immune responses against cancer over time. Boosting ID3 activity improved tumor control in preclinical models, highlighting its promise as a way to strengthen response to immunotherapies (126)Gago da Graca C, et al. (2025) Sci Immunol, 10: eadn1945.. As our understanding of the complex interactions between cancer and the immune system continues to grow, so does the potential to develop more effective and lasting therapies.
The Nervous System
The nervous system is becoming more recognized as a key regulator of cancer development. Research has uncovered dynamic interactions between the nervous system and cancer as drivers of tumor growth, metastasis, immune evasion, and resistance to therapy, giving rise to the emerging interdisciplinary field of cancer neuroscience. In recognition of the complexity and clinical importance of this topic, the Cancer Grand Challenges initiative recently launched a new challenge to catalyze research efforts aimed at unraveling interactions between the nervous system and cancer (see Technological Innovations Fueling Precision Medicine).
The nervous system—composed of the central nervous system (CNS), which includes the brain and spinal cord, and the peripheral nervous system (PNS), a network of nerves that connects the CNS to the rest of the body—uses electrical signals and chemicals to regulate and coordinate essential functions. Interactions between the nervous system, the immune system, and cancer take place both within the local tumor microenvironment and systemically throughout the body.
Nerve cells, or neurons, interact with cancer cells by releasing signaling molecules and transmitting electrical signals through direct contact, which influence nearby immune and stromal cells—the supportive cells that make up certain types of connective tissue—within the tumor microenvironment. Using an innovative single-cell transcriptomic technology (see Sidebar 10), researchers recently identified distinct subtypes of peripheral neurons that infiltrate pancreatic tumors, and investigated how these neurons interact with other cells in the tumor microenvironment. They found that pancreatic cancer cells reprogram these neurons, creating a network of interactions between nerve cells, cancer cells, and the surrounding microenvironment that influences tumor growth, immune infiltration, and therapy response. Blocking nerve signals using therapeutics created a more immune-friendly environment and enhanced the effectiveness of ICIs, opening a new avenue for cancer treatment (127)Thiel V, et al. (2025) Nature, 640: 1042..
In another study, researchers discovered a direct connection between nerve cells and gastric cancer cells that was mediated by CGRP—a chemical released by nerve cells that promotes tumor growth, metastasis, and immunosuppression. Inhibiting CGRP signaling with a migraine drug, rimegepant (Nurtec ODT), significantly reduced tumor burden and prolonged survival in preclinical models (128)Zhi X, et al. (2025) Nature, 640: 802.. These findings highlight cancer–nerve interactions as promising targets for therapeutic intervention.
Other cancer cells appear to acquire neuron-like features themselves. Recent research revealed that neuroendocrine cells—cells that release hormones into the blood in response to stimulation by the nervous system—in small cell lung cancer can produce electrical signals that directly promote tumor progression and aggressiveness. Even cells that do not produce electrical signals support this activity by supplying nutrients to cancer cells, which help fuel the high energy demands of electrical signaling (130)Peinado P, et al. (2025) Nature, 639: 765.. These findings highlight new insights into tumor growth driven by electrical activity.
Scientists are uncovering new ways in which the nervous system influences cancer progression. Factors such as nerve activity, brain chemical signaling, and disruption to the body’s internal clock are being explored for their roles in promoting cancer cell proliferation, survival, and immune evasion.
Microbiome
The human microbiome is made up of all the microbes—bacteria, fungi, viruses, and other microorganisms—that live on and in the skin, mouth, gut, and other sites in the body. While many early studies focused on the gut microbiome, scientists are now recognizing the importance of microbiomes in other tissues and organs, each with its own distinct microbial communities. Studies have shown that microbiomes throughout the body play essential roles in digestion, metabolism, immune system regulation, and protection against pathogens. A growing body of research suggests that these microbial communities may also influence cancer development and treatment response by shaping immune activity, inflammation, and the tumor microenvironment (84)Hanahan D (2022) Cancer Discov, 12: 31..
A recent study highlighted how changes in the composition of the gut microbiome contribute to the initiation and progression of colorectal, gastric, liver, and pancreatic cancers. Beyond its role in cancer development, the microbiome can also shape responses to treatment by enhancing drug metabolism, reducing toxicity and side effects, and promoting antitumor immunity (131)Nobels A, et al. (2025) Nat Metab, 7: 895.. For example, a recent study found that using low doses of radiation to the gut improved the effectiveness of PD-L1 immunotherapy in patients with metastatic cancer, but this response was strongly influenced by the composition of each patient’s gut microbiome (132)Chen J, et al. (2025) Cancer Cell, 43: 361..
An analysis of 175 patients with melanoma revealed that low gut microbial diversity was associated with poor response to immunotherapy (133)Gilbert JA, et al. (2025) Nat Med, 31: 1099.. Higher abundance of specific gut-associated bacteria was linked to better outcomes, including survival without signs of cancer progression for at least 12 months and a more active immune response against cancer. In contrast, the use of broad-spectrum antibiotics, which can reduce microbial diversity and eliminate beneficial bacteria, was associated with poorer outcomes from immune checkpoint blockade. These findings suggest that the gut microbiome may influence how well cancer therapies work, and larger studies are needed to further establish the role of modulating the microbiome through lifestyle and therapeutic interventions, such as dietary changes or probiotic use, to improve cancer treatment outcomes.
Efforts to understand the impact of the gut microbiome on cancer have led to large-scale international collaborations, such as the ONCOBIOME network, to investigate the gut microbiome’s role in cancer development and treatment response, with the goal of using the microbiome to develop effective ways of diagnosing and treating cancer. Analyzing over 12,000 fecal samples from over 9,800 patients with cancer and healthy donors, researchers characterized how microbial communities influence the immune system and therapeutic outcomes. The ONCOBIOME initiative also developed diagnostic tools that helped identify microbes with the ability to activate or suppress immune response, laying the groundwork for personalized microbiome-based therapeutic interventions (135)Zitvogel L, et al. (2025) Nat Med, 31: 1085..
Beyond the gut, researchers recently showed that breast tissue has a distinct modifiable microbiome population. They found that tamoxifen, a common hormone therapy for breast cancer, alters the breast tissue microbiome, particularly by increasing beneficial bacteria, such as Lactobacillus spp. This increase in beneficial microbes can suppress tumor growth and reduce proliferation in estrogen receptor–positive breast cancer models (136)Arnone AA, et al. (2025) Cell Rep Med, 6: 101880.. These findings suggest that the breast microbiome actively influences tumor metabolism and progression, and that targeting it may enhance breast cancer prevention and treatment strategies.
Emerging research has also shown that the oral microbiome may play a direct role in the development of pancreatic cancer, one of the most lethal cancer types. Certain oral bacteria have been identified within pancreatic tumors and may promote cancer progression by inducing chronic inflammation and disrupting immune function (137)Guo X, et al. (2025) Mol Med, 31: 103.. However, more research is needed to further characterize the microbiome inside tumors and determine their influence on cancer development.
The fungal microbiome, or mycobiome, is emerging as another contributor to cancer initiation, progression, and treatment outcomes. Researchers are exploring how fungi influence the tumor microenvironment, and whether this knowledge can be used to develop effective treatments for cancer (138)Bilal H, et al. (2025) Cancer Res, 85: 413.. For example, a recent study identified different types of fungi, such as Candida and Malassezia, across various cancer types, where they promote inflammation and immune suppression (139)Ding T, et al. (2025) Mol Cancer, 24: 18.. As technologies for fungal detection and analysis improve, it will be important to evaluate whether the mycobiome can be leveraged to diagnose and treat cancer.
It is becoming increasingly clear that targeting the microbiome may help improve health outcomes for patients with cancer. Efforts are now underway to manipulate the microbiome through strategies such as specifically designed mixtures of beneficial bacteria (probiotics), dietary supplements that nourish helpful microbes (precision prebiotics), and engineered bacterial species that deliver therapies or modulate immune signaling. In parallel, microbiome profiling may also become an integral component of predicting how patients may respond to cancer treatment, particularly immunotherapy.
Tumor Microenvironment
The tumor microenvironment (TME)—composed of cancer and non-cancer cells, blood vessels, signaling molecules, and structural components—is a dynamic ecosystem that influences tumor growth, metastasis, and response to treatment. Cancer cells shape this environment by releasing molecules that secure nutrients, oxygen, and structural support essential for their growth (see Sidebar 11). The full collection of proteins and molecules released by cells into their surroundings is known as the secretome. Tumor cells often alter the composition of their secretome, affecting the behavior of nearby healthy cells and influencing distant tissues to support tumor progression.
Understanding the TME is essential for advancing cancer therapies, as it reveals how the surrounding cellular and structural environment supports tumor growth and may be targeted to improve therapeutic outcomes.
Recent advances in single-cell and spatial transcriptomic technologies (see Sidebar 10) are transforming our understanding of the TME by helping map the cellular and molecular interactions that occur within and around tumors. These technologies can determine how cells organize and communicate across three dimensions within the microenvironment, uncovering evolving processes that promote tumor growth, metastasis, and resistance to therapy. In a recent study, researchers used single-cell and spatial transcriptomics to uncover treatment-induced remodeling of the TME in pancreatic cancer and found that elevated signaling of IL-6, which helps cancer cells communicate with their surroundings, promotes resistance to cancer treatment through enhanced cell survival and epithelial-to-mesenchymal transition (141)Shiau C, et al. (2024) Nat Genet, 56: 2466..
Studying the TME in cancers that metastasize to the brain may offer valuable insights into why these tumors are often difficult to treat and resistant to standard therapies. To better understand this environment, researchers are using advanced single-cell and spatial tools that analyze individual cells and their activity within the tumor’s structure. In two recent studies, researchers built detailed maps of brain metastases across several cancer types, revealing features that help tumors survive in the brain microenvironment (142)Tagore S, et al. (2025) Nat Med, 31: 1351.(143)Xing X, et al. (2025) Cancer Cell.. These findings highlight how studying tumor cells and their surroundings can reveal molecular underpinnings of tumor growth and resistance to treatment, and may point to new ways to improve therapy.
Within the TME, the tumor immune microenvironment (TIME) specifically refers to the network of immune cells and immune-modulating factors that interact with the tumor. The TIME plays an important role in cancer progression, and a deeper understanding of the processes within this environment is essential for developing effective immunotherapies.
Emerging research suggests that age-related weakening of the immune system, known as immune aging, makes it harder for the body to detect and eliminate cancer cells and may alter the TIME in ways that support tumor growth and treatment resistance. These changes are driven by several interconnected biological processes, including cellular senescence—a state in which cells permanently stop dividing in response to stress, damage, or aging signals but remain metabolically active—and chronic inflammation. As senescent cells accumulate within aged tissues and secrete pro-inflammatory factors, they weaken immune system function, making it harder for the body to remove both senescent and malignant cells (144)Bracken OV, et al. (2025) Nat Rev Drug Discov, 24: 300.. Therapies that target senescent cells, reduce inflammation, or enhance immune activity may reshape the aging TIME and improve clinical outcomes.
Cancer Development: Integrating Knowledge to Advance Precision Medicine
Breakthrough discoveries and technological innovations have significantly advanced our understanding of cancer initiation and progression, enabling the development of a myriad of effective anticancer therapies in recent years (see Sidebar 12). A crucial insight stemming from this knowledge is the understanding that each patient’s cancer is unique.
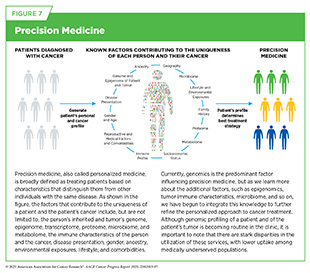
This understanding has paved the way for precision medicine, also called personalized medicine, which is broadly defined as treating patients based on characteristics that distinguish them from other individuals with the same disease (see Figure 7). For patients with cancer, precision medicine means using molecular characteristics of the tumor, such as the genome sequence of cancer cells or features of the tumor-associated immune cells, to make a diagnosis, plan treatment, and predict and monitor treatment response.
Technological Innovations Fueling Precision Medicine
By leveraging emerging technologies and multi-omic data (see Sidebar 10), researchers are generating a complete picture of the cellular and molecular characteristics that drive cancer. This comprehensive approach, coupled with powerful computational tools and shared research platforms, is enabling deeper integration of cancer biology insights and driving the development of more personalized therapies, refining tumor classification, revealing novel therapeutic targets, and addressing key challenges such as treatment resistance.
Large-scale data-sharing initiatives, such as The Cancer Genome Atlas (TCGA) and the Cancer Dependency Map (DepMap), have laid the groundwork for a more comprehensive understanding of cancer biology. TCGA has enabled an extensive catalog of genetic, epigenetic, and molecular changes, or “maps,” across patient tumors, thus proving a reference point for distinguishing tumor subtypes and identifying common oncogenic pathways. DepMap has complemented this effort by providing a large-scale data repository for genetic and molecular vulnerabilities, or weak points that cancer cells rely on for survival, across a wide array of cancer models routinely used by researchers (see Sidebar 6) (146)Arafeh R, et al. (2025) Nat Rev Cancer, 25: 59.. Harnessing the strengths of both TCGA and DepMap, researchers have recently developed an artificial intelligence (AI) tool that identifies critical genes or pathways that tumors depend on to grow and survive (see New Advances in Artificial Intelligence and see Sidebar 54) (147)Shi X, et al. (2024) Nat Cancer, 5: 1176..
These initiatives have also transformed our understanding of cancer biology by promoting structured, large-scale collaboration across institutions. While the value of collaborative frameworks was established by efforts like the Human Genome Project and TCGA, researchers have recently proposed a framework that builds on this by defining specific “Hallmarks of Cancer Collaboration” to help modern cancer teams align incentives, share resources, and pursue common goals. This framework emphasizes the need for deliberate, coordinated efforts to translate big data into meaningful clinical advances (148)Boehm JS, et al. (2024) Cancer Discov, 14: 563..
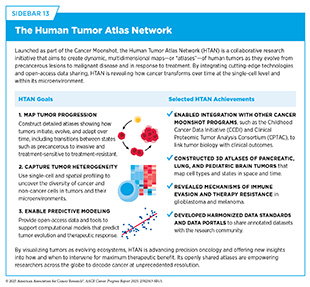
The Human Tumor Atlas Network (HTAN) is another example of a next-generation, collaborative, data-intensive cancer research initiative. HTAN projects apply advanced technologies that examine individual cells and their molecular features within the structure of tumors through single-cell and spatial multi-omic technologies—including transcriptomics, proteomics, epigenomics, and advanced imaging—to map the cellular and molecular architecture of tumors throughout the course of disease progression and treatment (149)Naddaf M (2024) Nature, 635: 14.(150)de Bruijn I, et al. (2025) Nat Methods, 22: 664.(151)Iglesia MD, et al. (2024) Nat Cancer, 5: 1713.. The comprehensive tumor maps, or atlases, are helping researchers understand how distinct cell types and cellular states arise during tumor evolution and the emergence and expansion of therapy-resistant cell populations (151)Iglesia MD, et al. (2024) Nat Cancer, 5: 1713.(152)Mo CK, et al. (2024) Nature, 634: 1178.(153)Sims Z, et al. (2024) Commun Biol, 7: 409..
HTAN studies also reveal how the TME adapts in response to therapy, including changes in immune cell infiltration, fibroblast activity, and extracellular matrix remodeling (154)Pang M, et al. (2025) Nat Methods, 22: 348.(155)Baker GJ, et al. (2024) Nat Methods, 21: 2248.. In parallel, HTAN studies are uncovering how the ability of cancer cells to change their identity—such as by mimicking developmental programs or reverting to a less specialized state—and switching between cell types contribute to therapy resistance and tumor recurrence (156)Ma C, et al. (2024) Nat Methods, 21: 2239.(157)Esplin ED, et al. (2024) Nat Cancer, 5: 1737.. By capturing the complexity of tumors over time and space, HTAN is informing efforts to design treatment strategies that can be modified based on tumor stage, molecular characteristics, and response to therapy (see Sidebar 13) (158)Kaur H, et al. (2024) Commun Biol, 7: 1295.(159)Zhu Y, et al. (2024) Nat Cancer, 5: 1697..
Precision oncology initiatives are increasingly integrating multidimensional profiling into patient care to support real-world clinical decision-making. Large-scale efforts such as NCI’s Childhood Cancer Data Initiative (CCDI), United Kingdom’s 100,000 Genomes Project, and Australia’s Zero Childhood Cancer Program demonstrate how whole-genome, exome, and transcriptome sequencing can inform treatment decisions (160)Kinnersley B, et al. (2024) Nat Genet, 56: 1868.(161)Lau LMS, et al. (2024) Nat Med, 30: 1913.. These studies reveal that many actionable biomarkers, including structural alterations, mutational signatures, and RNA expression changes, are only detectable through comprehensive multi-omic analyses. Together, these initiatives are transforming cancer care by enabling more precise, data-driven treatment strategies tailored to the unique molecular profile of each patient.
Collaborative research initiatives, such as the Cancer Grand Challenges, underscore the vital role of integrative research in addressing cancer’s most complex and unresolved questions. Launched as a global partnership between the National Cancer Institute (NCI) and Cancer Research UK, Cancer Grand Challenges brings together multidisciplinary teams from around the world to tackle scientific challenges in cancer research too vast for any single laboratory or institution to address (see Sidebar 14). These challenges focus on critical unmet needs that demand integrated research spanning cancer biology, environmental exposures, and social drivers of health (SDOH), such as determining the cause of early-onset cancers (see The Growing Population Burden of Cancer), understanding the biology of ecDNA, addressing cancer cachexia (see Addressing the Challenges Faced by Survivors), developing novel therapies for pediatric cancers, and uncovering the dynamic interactions between the nervous system and cancer. By supporting bold, collaborative science that spans basic, translational, clinical, and population-based research, this initiative highlights the importance of integrating knowledge across domains to advance cancer prevention, detection, and treatment for all patients.
As precision oncology advances, it is essential to ensure that its benefits are accessible to all patients. Recent studies emphasize the need to diversify genomic datasets and include ancestral and environmental variables in both research and clinical implementation. Integrating insights from evolutionary biology, more complete references of human genetic variation (pangenomes), and global biobanks are essential to addressing historical biases in cancer genomics and ensuring that biomarker discovery and risk prediction are applicable and effective across diverse populations (162)Palma-Martinez MJ, et al. (2025) Nat Med, 31: 751.. Moving forward, broadening representation in studies and making molecular testing and new treatments more accessible across patient populations will be essential to realizing the full potential of precision medicine for all patients (see Rapidly Delivering Safe and Effective Therapies to Patients).
Next Section: Reducing the Risk of Cancer Development Previous Section: Cancer in 2025