- Cancer Development: Generating Knowledge
- Cancer Development: Interpreting Knowledge
- Changes That Contribute to Cancer Initiation
- Systems That Enable Cancer Progression
- Processes That Promote Cancer Growth and Metastasis
- Spotlight: Understanding the Biology of Childhood Cancers
- Chromosomal Rearrangements in Childhood Cancers
- The Promise of Precision Medicine for Childhood Cancers
- Cancer Development: Integrating Knowledge
Understanding the Path to Cancer Development
In this section, you will learn:
- Cancer is a group of diseases in which some of the body’s cells acquire changes that cause them to divide unchecked and spread to other parts of the body.
- Basic research plays a pivotal role in understanding how cancer develops and spreads.
- Changes inside the cell as well as in the tumor environment can influence cancer initiation and progression.
- Breakthroughs in technological advances have accelerated the identification of mechanisms that drive cancer.
- Integrating the knowledge of various aspects of cancer development has fueled the field of personalized medicine.
Precise molecular mechanisms control the growth and multiplication of normal cells. In cancer, these processes go awry, causing cells to divide uncontrollably and spread to other parts of the body. Cancer is a collection of related diseases that can affect almost any part of the body. During the course of cancer development, abnormal or damaged cells acquire characteristics that distinguish cancer cells from normal cells.
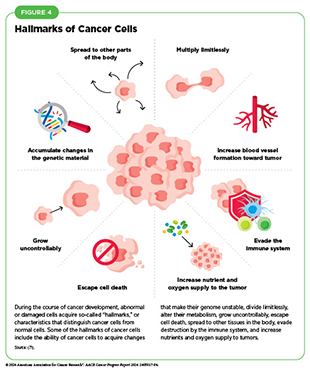
Hallmarks of cancer cells include their ability to multiply unchecked; acquire changes that make their genome unstable; ignore signals that stop normal cells from dividing or trigger death in old or damaged cells; utilize different metabolic strategies to sustain rapid growth; accumulate multiple genetic changes; leave the tissue of origin and spread to other sites; evade the immune system, which typically eliminates abnormal or damaged cells; and increase the supply of nutrients and oxygen to tumors (see Figure 4) (71)Hanahan D (2022) Cancer Discov, 12: 31..
Cancer Development: Generating Knowledge
Basic research is focused on understanding living systems and life processes. For example, basic research plays an essential role in characterizing normal cell behavior and in identifying the alterations that drive cancer initiation and progression. The knowledge gained from basic research has provided the foundation for advances against cancer. Recognizing the critical role of basic research in improving the overall health for all individuals, more than 50 percent of the National Institutes of Health (NIH) budget has been allocated to basic research every year since 2003 (72)National Institutes of Health. The Office of Budget. National Institutes of Health FY 2003 – FY 2023 Distribution of Budget Authority Percentages for Basic and Applied Research. Accessed: June 26, 2024..
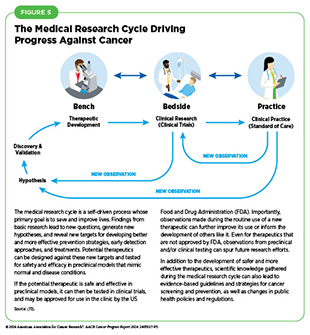
Basic research plays a central role in the medical research cycle (see Figure 5). Findings and hypotheses stemming from the medical research cycle are fundamental to understanding what triggers cancer development; how cancer evades the body’s defenses; and how cancer spreads within the body. This knowledge has led to improvements in the prevention of cancer, development of innovative imaging technologies, precise delivery of drugs to tumors, and selective and effective killing of cancer cells. Collectively, basic research-driven advances in cancer science and medicine are contributing to progress against cancer that is saving lives and improving health outcomes for countless patients.
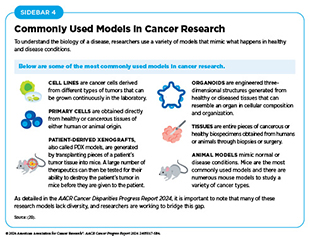
Researchers working across the fields of medicine study the significance of a new discovery in a wide range of models that mimic healthy and diseased conditions (see Sidebar 4). Findings from these studies can lead to the development of new anticancer drugs, innovative technologies, and strategies for cancer screening and prevention, each of which has the potential to improve public health.
Basic Research: Vital for Making Progress Against Cancer
Decades of basic research, and discoveries stemming from it, have provided the foundation for progress against cancer. A prime example is the finding in the 1950s of a biochemical process called phosphorylation (74)Fischer EH, et al. (1955) J Biol Chem, 216: 121.(75)Burnett G, et al. (1954) J Biol Chem, 211: 969.. The discovery of phosphorylation, the addition of phosphate groups to proteins and lipids, fundamentally changed the understanding of cellular regulation.
Phosphorylation is one of the most important modifications that modulate the activity of many proteins and lipids involved in cell division, growth, survival, and death. Research has shown that altered phosphorylation often leads to uncontrolled cell proliferation and survival, two major hallmarks of cancer (76)Singh V, et al. (2017) Protein J, 36: 1. DOI: 10.1007/s10930-017-9696-z..
Phosphorylation is a reversible process, which is mediated by specialized enzymes, called kinases and phosphatases. For example, in response to signals from outside the cell, protein kinases add phosphate groups to proteins and protein phosphatases remove them (77)Ubersax JA, et al. (2007) Nat Rev Mol Cell Biol, 8: 530. DOI: 10.1038/nrm2203.. Research has shown that alterations in kinases and phosphatases can lead to cancer development (78)Turdo A, et al. (2021) Front Cell Dev Biol, 9: 690306. DOI: 10.3389/fcell.2021.690306..
There are 518 protein kinases and 20 lipid kinases in human cells (79)Manning G, et al. (2002) Science, 298: 1912. DOI: 10.1126/science.1075762.. Thanks to decades of research, mechanisms by which kinases add phosphate groups to proteins and lipids are well understood (77)Ubersax JA, et al. (2007) Nat Rev Mol Cell Biol, 8: 530. DOI: 10.1038/nrm2203.. Furthermore, kinases (as well as phosphatases) are frequently mutated in cancer, and many of these mutations contribute to the onset and progression of cancer (78)Turdo A, et al. (2021) Front Cell Dev Biol, 9: 690306. DOI: 10.3389/fcell.2021.690306.. Knowledge gleaned from this research has established kinases as attractive drug targets for treatment of cancer (80)Bhullar KS, et al. (2018) Mol Cancer, 17: 48. DOI: 10.1186/s12943-018-0804-2.. For example, imatinib (Gleevec), which was approved by the US Food and Drug Administration (FDA) in 2001 to treat chronic myelogenous leukemia, is the first molecularly targeted anticancer drug against a protein kinase (81)Cohen P (2002) Nat Rev Drug Discov, 1: 309. DOI: 10.1038/nrd773.. As of June 2024, there are 70 FDA-approved anticancer drugs targeting various kinases for the treatment of different types of cancer (82)Roskoski R, Jr. (2024) Pharmacol Res, 200: 107059. DOI: 10.1016/j.phrs.2024.107059..
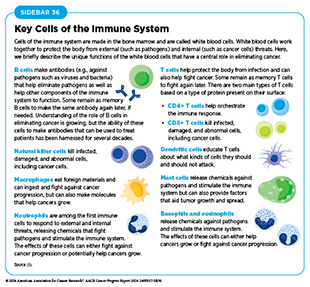
Another prominent example of the contributions of basic research to progress against cancer is the advent of modern immunotherapy, one of the most exciting new areas of cancer treatment. Groundbreaking basic research in the 1980s and 1990s uncovered the ways in which T cells function. T cells are immune cells that protect the body from infections and can also help fight cancer (see Sidebar 36). Intriguingly, some tumor cells have increased levels of certain proteins on their surface that attach to and activate “brakes” on T cells, thus stopping them from attacking cancer cells. These brakes are proteins on the surface of T cells and are called immune checkpoint proteins. Immune checkpoint inhibitors (ICIs) are a class of transformative new therapeutics that can release the brakes on T cells and trigger T cells to destroy cancer cells (83)Marin-Acevedo JA, et al. (2021) J Hematol Oncol, 14: 45. DOI: 10.1186/s13045-021-01056-8.. The first ICI, ipilimumab, was approved by FDA in 2011 to treat advanced-stage melanoma. Since then, FDA has approved 12 additional ICIs, and there is at least one ICI to treat more than 20 different types of cancer (see Releasing the Brakes on the Immune System).
Cancer Development: Interpreting Knowledge
Cancer is a collection of diseases with the common feature of uncontrolled growth of cells. Often, there are genetic alterations to the instructions encoded in a cell’s genetic material that disrupt tightly controlled functions, such as cell growth and division. Many of these changes are only the first step in a complex and multistep process that is influenced by changes both inside and outside the cell and that ultimately leads to the development of cancer.
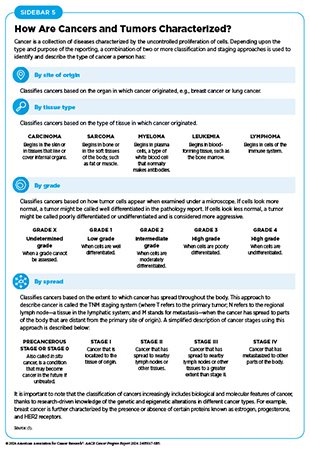
Researchers use several ways to characterize cancers, depending on the type and purpose of the research and/or reporting (see Sidebar 5). In most cases, several methods are simultaneously used to classify the type of cancer that a person has.
Changes That Contribute to Cancer Initiation
Deoxyribonucleic acid (DNA) constitutes the genetic material of cells and carries instructions for important cellular functions. DNA is a complex molecule that is made up of two strands, each of which is a string of four unique molecules called bases, designated A, T, C, and G. The two strands are paired together to form a double helix. The entirety of a person’s DNA is called the genome. In humans, DNA resides inside the nucleus and is wrapped around proteins called histones. The packaged DNA is called chromatin and is further compacted into structures called chromosomes. Nearly all human cells have 46 chromosomes.
Each chromosome contains hundreds to thousands of genes. The cell uses a complex process, called transcription, to copy the instructions or messages that are embedded in genes to make messenger ribonucleic acid (mRNA) molecules. Another complex process, called translation, copies the information in mRNAs to make proteins, which are functional units of the cell. The amount of mRNA or protein produced is influenced by cellular needs. The following sections describe the types of changes within a cell that may lead to cancer development.
Genetic Alterations
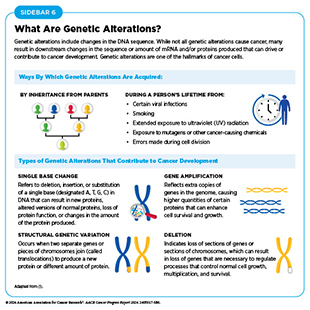
One of the hallmarks of cancer cells is alterations in the DNA sequence. Also called mutations, genetic alterations can change the sequence or the amount of mRNA and the resulting protein, thus disrupting or modifying normal protein function and contributing to cancer development. Genetic alterations can be inherited (also called germline mutations) or acquired during a person’s lifetime (also called somatic mutations) (see Sidebar 6). Not all genetic alterations lead to cancer.
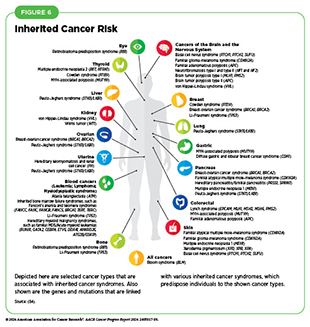
About 10 percent of cancer cases are caused by germline mutations. Germline mutations occur in a body’s reproductive cells (egg or sperm) that are passed on from parents to children and become incorporated into the DNA of every cell in the body of the offspring. These types of mutations can increase their risk of developing cancer, although not all germline mutations contribute to cancer development. Inherited genetic alterations that play a role in cancer development are among the pathogenic germline mutations (see Figure 6). However, even among pathogenic mutations, certain genetic alterations are more penetrant—meaning most people who carry the alteration will develop cancer—than others.
Technological advances in DNA sequencing have enabled a better understanding of germline pathogenic mutations and their association with a person’s risk of developing cancer. A recent study evaluating genome sequences of nearly 7,788 patients with lung cancer revealed that about 15 percent of the patients with lung cancer had well-described germline pathogenic mutations (85)Sorscher S, et al. (2023) JCO Precis Oncol, 7: e2300190. DOI: 10.1200/PO.23.00190.. Most of the mutations were found in genes needed for repairing damaged DNA, indicating these mutations may contribute to a person’s predisposition to lung cancer (85)Sorscher S, et al. (2023) JCO Precis Oncol, 7: e2300190. DOI: 10.1200/PO.23.00190..
Somatic or acquired mutations occur over an individual’s lifetime due to errors arising during normal cell divisions or because of environmental exposures, lifestyle factors, and/ or chronic health conditions. Research has revealed that different tumors can also contain different somatic mutations, depending on their site of origin.
Researchers are leveraging the understanding of genetic mutations present in cancer cells to treat cancer. In the past two decades, FDA has approved a number of targeted therapeutics based on genetic mutation(s) present in the cancer (87)National Cancer Institute. Targeted Therapy Drug List by Cancer Type. Accessed: July 5, 2023.. Furthermore, genetic tests that identify germline mutations are helping to predict a person’s risk of developing cancer, evaluate the risk of cancer in family members, and make informed and active decisions about their health.
RNA Variations
Most human genes contain interspersed sequences called exons and introns. Exons contain the instructions for making proteins, while introns do not contain the information necessary to make a functional protein. The mRNA molecule that is initially transcribed from a gene contains both exons and introns. A “cut and paste” process, called splicing, removes introns and then joins exons together to produce an mRNA molecule that is subsequently translated into a functional protein. RNA splicing is mediated by specialized proteins and is critical for normal cellular functions.
Changes in proteins that mediate splicing can produce aberrant mRNA molecules, which subsequently make abnormal proteins that can fuel cancer development, lead to treatment resistance, and alter immune cell function (88)Bradley RK, et al. (2023) Nat Rev Cancer, 23: 135. DOI: 10.1038/s41568-022-00541-7.. Ongoing research is focused on understanding how cancer-related changes in RNA splicing can be leveraged for therapeutic purposes (89)Stanley RF, et al. (2022) Nature Cancer, 3: 536. DOI: 10.1038/s43018-022-00384-z..
In addition to mRNA, cells also produce RNA molecules that are not translated into proteins. These RNA molecules are called noncoding RNAs (ncRNAs), and they play important roles in normal cell functions as well as in cancer cells (90)Yan H, et al. (2021) Essays Biochem, 65: 625. DOI: 10.1042/EBC20200032.. Two major types of ncRNAs produced in cells are microRNAs (miRs or miRNAs) and long noncoding RNAs (lncRNAs). miRs are about 17 to 25 bases long and largely function by binding to mRNAs and blocking their translation into proteins (91)Ambros V (2004) Nature, 431: 350. DOI: 10.1038/nature02871.. lncRNAs are longer than 200 bases and regulate cellular functions in several ways, including functioning as signals for when a gene should be transcribed into mRNA, and guiding proteins to places within cells where they are needed (92)Statello L, et al. (2021) Nat Rev Mol Cell Biol, 22: 96. DOI: 10.1038/s41580-020-00315-9..
ncRNAs can either function to promote cancer development or prevent normal cells from becoming cancerous (93)Hayes J, et al. (2014) Trends Mol Med, 20: 460. DOI: 10.1016/j.molmed.2014.06.005.(94)Huarte M (2015) Nat Med, 21: 1253. DOI: 10.1038/nm.3981.. For example, miR-15 and miR-16, two well-studied miRs, inhibit cancer development by blocking the generation of proteins that help cancer cells grow as well as those that protect cancer cells from death (95)Liu T, et al. (2019) J Cell Physiol, 234: 5496. DOI: 10.1002/jcp.27342.. Loss of miR-15 and miR-16 promotes cancer cell growth and survival in several cancer types, including chronic lymphocytic leukemia, prostate cancer, and multiple myeloma (95)Liu T, et al. (2019) J Cell Physiol, 234: 5496. DOI: 10.1002/jcp.27342.. Similarly, HOTAIR, a well-studied lncRNA, is linked with multiple cancer types, including breast cancer, colorectal cancer, and glioma (96)Hakami MA, et al. (2024) Pathol Res Pract, 253: 154957. DOI: 10.1016/j.prp.2023.154957.. In colorectal cancer, the more HOTAIR cancer cells have, the more they become resistant to treatment, leading to poor outcomes (97)Cantile M, et al. (2024) Front Mol Biosci, 11: 1414651. DOI: 10.3389/fmolb.2024.1414651..
Research has shown that transcriptomics—the study of all RNA molecules in a cell—can help differentiate the types and levels of RNA that are present in healthy versus tumor tissues. Such information can reveal how different types of RNA contribute to cancer development and may identify RNA molecules that can be used to predict progression of cancer and response to treatment. Thanks to technological advances in RNA sequencing, researchers can now determine transcriptomes of single cells within a tumor. The in-depth knowledge gained from such studies is uncovering new mechanisms by which cancer develops, progresses, spreads to distant sites, and/or becomes resistant to treatment (98)Martinez-Ruiz C, et al. (2023) Nature, 616: 543. DOI: 10.1038/s41586-023-05706-4.(99)Wild SA, et al. (2022) Elife, 11: e80981. DOI: 10.7554/eLife.80981..
Protein Modifications
The complete set of proteins made by human cells is called the proteome and contains about 20,000 unique proteins. The proteome of cancer cells has revealed important information about cancer that may not have been apparent from genomic or transcriptomic analyses. As one example, bladder cancer is highly heterogeneous, and it is not easy to predict treatment response based on genomic and transcriptomic analyses alone. In a recent study, researchers evaluated the proteome from 242 tumors isolated from patients with bladder cancer and identified protein modifications that predict response to the treatment with higher accuracy (100)Dressler FF, et al. (2024) Nat Commun, 15: 4513. DOI: 10.1038/s41467-024-48096-5.. Further analysis suggested that some of the investigational anticancer drugs not currently in clinical use may be more effective in treating patients with bladder cancer (100)Dressler FF, et al. (2024) Nat Commun, 15: 4513. DOI: 10.1038/s41467-024-48096-5., indicating that studying the proteome can inform new treatment options.
The functions of many proteins are controlled by posttranslational modifications (PTMs), which are characterized by reversible addition and removal of molecules, such as phosphate (see Basic Research: Vital for Making Progress Against Cancer). Proteins undergo PTMs depending upon cellular needs and they are necessary for normal cellular functions, such as responding to signals from outside the cell (101)Uversky VN. Posttranslational Modification. Academic Press: Elsvier; 2013.. Researchers estimate that there are more than 400 different types of PTMs that modulate various aspects of protein functions (102)Wang H, et al. (2023) Cancer Gene Ther, 30: 529. DOI: 10.1038/s41417-022-00464-3.. Changes in normal PTMs of proteins can contribute to cancer (102)Wang H, et al. (2023) Cancer Gene Ther, 30: 529. DOI: 10.1038/s41417-022-00464-3.. In a recent study, researchers analyzed PTM profiles from 1,110 patients across 11 cancer types (103)Geffen Y, et al. (2023) Cell, 186: 3945. DOI: 10.1016/j.cell.2023.07.013.. Findings of the study revealed that different types of PTMs are associated with different hallmarks of cancer. For example, the researchers found that altered phosphorylation was associated with tumors in which machinery to repair DNA was defective, while altered acetylation, another type of PTM, was more prevalent in tumors with altered metabolism (103)Geffen Y, et al. (2023) Cell, 186: 3945. DOI: 10.1016/j.cell.2023.07.013..
Epigenetic Changes
Epigenetic modifications change the structure of DNA without altering the DNA sequence. These changes involve the addition or removal of chemical marks on DNA or the PTMs of histones, which are the proteins that package DNA into chromosomes. Specialized proteins facilitate the addition or removal of these unique modifications on DNA and histones (104)Lu Y, et al. (2020) Mol Cancer, 19: 79. DOI: 10.1186/s12943-020-01197-3.. Epigenetic alterations are influenced by aging, environmental exposures (e.g., air pollution), behavioral risk factors (e.g., smoking), and chronic stress (e.g., systemic racism). Furthermore, these modifications are heritable and can contribute to a person’s risk of developing cancer.
Epigenetic modifications determine when and how genes are activated or silenced. For example, depending on cellular needs and in response to signals from outside the cell, epigenetic changes can make genes accessible to the machinery that makes mRNA. Unlike genetic mutations, epigenetic changes are typically reversible.
The entirety of epigenetic changes within a cell is called the epigenome. Research has significantly advanced our understanding of how the epigenome is modified in cancer and how these changes contribute to cancer (105)Yu X, et al. (2024) Cell Death Discov, 10: 28. DOI: 10.1038/s41420-024-01803-z.. The reversible nature of epigenetic modifications has made them compelling targets for drug development. Consequently, several anticancer drugs that modify the cancer epigenome have been developed and approved by FDA (105)Yu X, et al. (2024) Cell Death Discov, 10: 28. DOI: 10.1038/s41420-024-01803-z..
Understanding of the epigenome and the proteins that regulate it is being used to further comprehend cancer development at a molecular level (106)Cheng MW, et al. (2023) Commun Biol, 6: 1138. DOI: 10.1038/s42003-023-05459-w.. For instance, in a recent study, researchers evaluated the role of the epigenome at various steps during cancer development across 11 cancer types (107)Terekhanova NV, et al. (2023) Nature, 623: 432. DOI: 10.1038/s41586-023-06682-5.. Findings of the study showed that unique sets of proteins that regulate the epigenome are associated with the onset of cancer, how cancer progresses, and how it metastasizes (107)Terekhanova NV, et al. (2023) Nature, 623: 432. DOI: 10.1038/s41586-023-06682-5.. These discoveries are paving the way for identifying new therapeutic targets and accelerating the development of new drugs to treat cancer.
Systems That Enable Cancer Progression
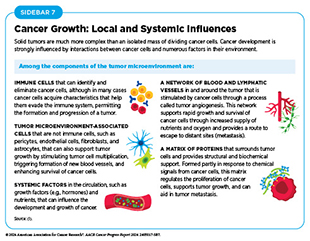
A hallmark of cancer is the ability of tumor cells to break away from the primary tissue and travel to other parts of the body. Systems that enable cancer to spread from the primary tissue site to other organs of the body include the blood system, the lymphatic system, and the immune system (see Sidebar 7).
The Blood System
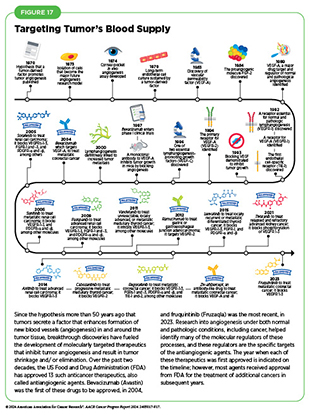
Angiogenesis is the formation of new blood vessels, which occurs throughout life. Multiple chemical signals in the body control this essential process. Cancer cells acquire the ability to promote angiogenesis toward and within a tumor to meet the high demand of oxygen and nutrients needed to fuel rapid tumor growth.
Decades of research have revealed multiple proteins and chemicals that regulate normal blood vessel formation, and whose functions are hijacked by cancer cells to increase tumor angiogenesis (108)Liu ZL, et al. (2023) Signal Transduct Target Ther, 8: 198. DOI: 10.1038/s41392-023-01460-1.. For example, vascular epidermal growth factor (VEGF) and its cell surface binding partner, called the VEGF receptor, are necessary for angiogenesis and play a crucial role in the growth of cells that line the inside of blood vessels. Cancer cells can produce and release high levels of VEGF, thus directing the formation of new blood vessels in and around tumors (109)Apte RS, et al. (2019) Cell, 176: 1248. DOI: 10.1016/j.cell.2019.01.021..
Over the past two decades, several drugs that block angiogenesis have been approved to treat cancer. In 2004, FDA approved the first anti-angiogenic drug bevacizumab (Avastin), which blocks VEGF. Since then, FDA has approved 12 different anticancer therapeutics targeting proteins that promote angiogenesis, to treat 13 different cancer types (see Figure 17) (73)American Association for Cancer Research. AACR Cancer Progress Report 2021. Accessed: June 30, 2023..
The Lymphatic System
The lymphatic system consists of an extensive network of vessels, called lymph vessels or lymphatic vessels; small bean-shaped structures, called lymph nodes; and other organs such as the spleen, thymus, tonsils, and adenoids. The lymphatic system maintains total body fluid levels, removes cellular waste from tissues, detects pathogens, absorbs fats, and produces immune cells and antibodies in the lymph nodes.
One of the ways cancer cells travel to other parts of the body is through the lymphatic system. When spreading to other body parts through the lymphatic system, cancer cells can accumulate in one or more of the nearest lymph nodes. The presence of cancer cells in lymph nodes is one of the ways to determine the stage and/or the extent of cancer (see Sidebar 5).
Cancer cells shed by the tumor release certain molecules that help them move toward the lymphatic system. In addition, cancer cells adopt mechanical changes that facilitate their entry into the lymphatic system (110)Zhou H, et al. (2021) Cells, 10: 627. DOI: 10.3390/cells10030627.. Once inside the lymphatic system, cancer cells acquire additional properties that make them more aggressive and facilitate their spread to other parts of the body (111)Padera TP, et al. (2016) Annu Rev Biomed Eng, 18: 125. DOI: 10.1146/annurev-bioeng-112315-031200..
Researchers are working on ways to leverage the lymphatic system for targeted delivery of anticancer therapy to lymph nodes where cancer cells can accumulate. Furthermore, ongoing work is focused on developing drugs that can prevent cancer cells from reaching and entering the lymphatic system (110)Zhou H, et al. (2021) Cells, 10: 627. DOI: 10.3390/cells10030627..
The Immune System
The immune system helps the body fight infections and other diseases, including cancer. Multiple cell types, tissues, and organs make up the immune system and they work in concert to detect and remove pathogens as well as abnormal or damaged cells from the body. There are two main components of the immune system. The innate immune system is the body’s first line of defense that provides a general, nonspecific response to pathogens. It includes physical barriers like the skin and mucous membranes, as well as certain immune cells and molecules that quickly respond to and kill a broad range of pathogens. In contrast, the adaptive immune system provides a specific response against pathogens and remembers them for a faster response in the future. This includes B cells that produce antibodies to neutralize pathogens, as well as T cells that can kill infected cells and/or help coordinate other immune responses (see Sidebar 36).
The immune system constantly monitors the body for the presence of abnormal or damaged cells, including cancer cells, in a process called cancer immune surveillance (112)Hiam-Galvez KJ, et al. (2021) Nat Rev Cancer, 21: 345. DOI: 10.1038/s41568-021-00347-z.. However, as cancer progresses, some cancer cells obtain properties that help them evade the immune system. Research has revealed several ways cancer cells evade the immune system (113)Spranger S, et al. (2018) Annu Rev Canc Biol, 2: 213. DOI: 10.1146/annurev-cancerbio-030617-050606.. In some cases, cancer cells disrupt the cellular machinery that helps immune cells recognize damaged or abnormal cells. In others, cancer cells exhibit increased levels of proteins that function as brakes on the immune system. And in yet other cases, cancer cells release molecules that prevent the immune cells from becoming fully functional (114)Kim SK, et al. (2022) Front Pharmacol, 13: 868695. DOI: 10.3389/fphar.2022.868695.. Increasing evidence also suggests that certain immune cells present in the tumor microenvironment promote tumor growth (115)Binnewies M, et al. (2018) Nat Med, 24: 541. DOI: 10.1038/s41591-018-0014-x..
The Microbiome
All microorganisms (e.g., bacteria and fungi) and viruses that live in the gut, skin, and mouth, and other sites in the body, collectively make up the human microbiome. Research has shown that the microbiome plays a critical role in health outcomes (116)Ogunrinola GA, et al. (2020) Int J Microbiol, 2020: 8045646. DOI: 10.1155/2020/8045646.(117)Pflughoeft KJ, et al. (2012) Annu Rev Pathol, 7: 99. DOI: 10.1146/annurev-pathol-011811-132421.(118)de Vos WM, et al. (2022) Gut, 71: 1020. DOI: 10.1136/gutjnl-2021-326789..
Most of the microorganisms in the human microbiome are beneficial to health, but some are potentially harmful. Accumulating evidence suggests that the balance between helpful and potentially harmful microorganisms in the gut microbiome contributes to overall health, while an imbalance contributes to a number of diseases, including cancer (119)Kho ZY, et al. (2018) Front Microbiol, 9: 1835. DOI: 10.3389/fmicb.2018.01835.. In cancer, the microbiome can influence progression and spread of the disease through interactions among microorganisms, between the microbiome and the patient’s immune system, and through secretion of molecules (120)Schwabe RF, et al. (2013) Nat Rev Cancer, 13: 800. DOI: 10.1038/nrc3610.(121)Cullin N, et al. (2021) Cancer Cell, 39: 1317. DOI: 10.1016/j.ccell.2021.08.006.. Furthermore, microorganisms living in different parts of the body can be associated with specific cancer types. For example, research has shown that specific types of bacteria present in the mouth are prevalent in patients with oral cancer. Conversely, an abundance of another type of bacteria is correlated with better overall survival in patients with oral cancer (122)Baker JL, et al. (2024) Nat Rev Microbiol, 22: 89. DOI: 10.1038/s41579-023-00963-6.. As another example, the interplay between the vaginal microbiome and human papillomavirus (HPV) in cervical precancers and cancer development is an area of ongoing research (123)Kyrgiou M, et al. (2022) Semin Cancer Biol, 86: 189. DOI: 10.1016/j.semcancer.2022.03.005.(124)Sharifian K, et al. (2023) Virol J, 20: 73. DOI: 10.1186/s12985-023-02037-8..
Research has shown that the microbiome can inform response to treatments and predict health outcomes (125)Chen Y, et al. (2022) Front Immunol, 13: 935846. DOI: 10.3389/fimmu.2022.935846.. For example, a recent study analyzing microbiomes of more than 4,000 tumors showed that a specific type of bacteria was associated with resistance to immune checkpoint inhibitor treatment in patients with lung cancer (126)Battaglia TW, et al. (2024) Cell, 187: 2324. DOI: 10.1016/j.cell.2024.03.021.. These findings and those from similar studies suggest that modulating the microbiome can boost the effectiveness of certain anticancer treatments such as immunotherapies (127)Villemin C, et al. (2023) Trends Immunol, 44: 44. DOI: 10.1016/j.it.2022.11.002..
It is clear that targeting the microbiome may help improve health outcomes for patients with cancer. Researchers are actively working to address many outstanding questions, such as the interplay between the microbiome and the host before such interventions can become a part of routine clinical care (128)Byrd D, et al. (2024) Nat Rev Cancer, 24: 89. DOI: 10.1038/s41568-023-00638-7.(129)Ahmad S, et al. (2022) World J Gastroenterol, 28: 2782. DOI: 10.3748/wjg.v28.i25.2782..
Processes That Promote Cancer Growth and Metastasis
Cancer metastasis refers to the spread of cancer cells from the tissue where they first originated to another part of the body. During metastasis, cancer cells break away from the original (primary) tumor site, travel through the blood or lymphatic system, and form a new tumor in other organs or tissues of the body. Although the new, metastatic tumor acquires many additional alterations during the course of cancer development, it remains the same type of cancer as the primary tumor. For example, if breast cancer spreads to the bone, the cancer cells in the bone are breast cancer cells, not bone cancer cells.
According to the most recent estimates available, there were 623,405 people living with metastatic breast, prostate, lung, colorectal, or bladder cancer or metastatic melanoma in the United States in 2018, and that number is expected to increase to 693,452 by 2025 (10)Gallicchio L, et al. (2022) J Natl Cancer Inst, 114: 1476. DOI: 10.1093/jnci/djac158.. The 5-year survival rates are significantly lower in patients with metastatic cancer compared to those with localized cancer (2)American Cancer Society. Cancer Facts and Figures 2024. Accessed: July 10, 2024.. Although the causes of death in patients with cancer are complex and multifaceted, patients with metastatic disease are significantly more likely to die compared to those whose cancer has not metastasized (130)Boire A, et al. (2024) Nat Rev Cancer, 00: s41568. DOI: 10.1038/s41568-024-00708-4.. Furthermore, chances of a cure are limited in patients with metastatic cancer. Complex processes facilitate cancer metastasis and finding additional ways to effectively treat patients with advanced stage disease are active areas of research.
Tumor Evolution and Heterogeneity
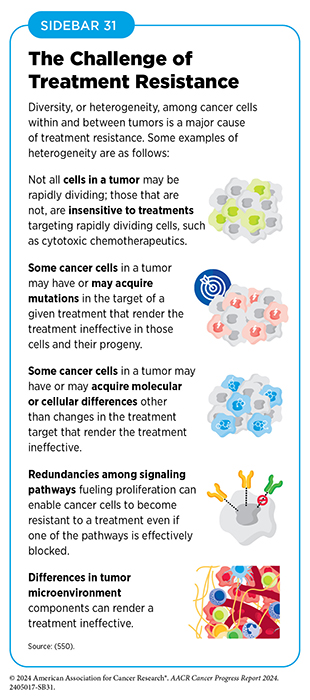
Tumors are highly heterogeneous where (i) the cancer cells within them have distinct alterations and features within a tumor; (ii) there can be significant differences between tumors of the same type in different patients; and (iii) there are major differences between a primary (original) tumor and the metastatic tumor. Tumor heterogeneity arises from acquisition of new alterations in the genomes, epigenomes, transcriptomes, and proteomes of cancer cells as they divide. Over time, these changes accumulate and contribute to the genetic diversity of cancer cells within the tumor. Furthermore, the tumor microenvironment can influence cancer cell behavior, leading to distinct subpopulations of cancer cells within the same tumor. Collectively the process by which tumors acquire heterogeneity is called tumor evolution (131)Zahir N, et al. (2020) Nat Genet, 52: 759. DOI: 10.1038/s41588-020-0668-4.(132)de Visser KE, et al. (2023) Cancer Cell, 41: 374. DOI: 10.1016/j.ccell.2023.02.016.(133)Bailey C, et al. (2021) Cancer Discov, 11: 916.. The heterogeneity of cancer cells in the tumor enables some cancer cells to acquire properties that facilitate their spread to other parts of the body. Tumor heterogeneity also poses significant challenges for cancer treatment. The presence of diverse subpopulations of cancer cells within a tumor can lead to differential responses to therapy, with some subpopulations being more resistant to treatment than others. This can result in treatment failure and disease recurrence (134)Dagogo-Jack I, et al. (2018) Nat Rev Clin Oncol, 15: 81. DOI: 10.1038/nrclinonc.2017.166. (see Sidebar 31)
Two recent studies using advanced techniques have developed a comprehensive three-dimensional map of glioma, which revealed interesting details of tumor heterogeneity in this deadly type of brain cancer that has no effective treatment available (135)Mathur R, et al. (2024) Cell, 187: 446. DOI: 10.1016/j.cell.2023.12.013.(136)Greenwald AC, et al. (2024) Cell, 187: 2485. DOI: 10.1016/j.cell.2024.03.029.. In one study, researchers combined a precision surgical procedure with single cell genomic, transcriptomic, and epigenomic analyses to identify molecular pathways that contribute to the heterogeneity of glioblastoma multiforme, a type of glioma (135)Mathur R, et al. (2024) Cell, 187: 446. DOI: 10.1016/j.cell.2023.12.013.. The second study found that gliomas are composed of subpopulations of cells that share similar molecular traits and that certain subpopulations always exist in proximity to each other. Researchers found that low oxygen conditions, a hallmark of cancer, play a significant role in this complex organization (136)Greenwald AC, et al. (2024) Cell, 187: 2485. DOI: 10.1016/j.cell.2024.03.029.. Similar analyses are being performed for many cancers, including other aggressive and difficult-to-treat diseases, such as certain types of skin cancer (137)Taylor MA, et al. (2024) Science, 384: eadi7453. DOI: 10.1126/science.adi7453., pancreatic cancer (138)Braxton AM, et al. (2024) Nature, 629: 679. DOI: 10.1038/s41586-024-07359-3., small cell lung cancer (139)George J, et al. (2024) Nature, 627: 880. DOI: 10.1038/s41586-024-07177-7., and certain types of breast cancer (140)Nishimura T, et al. (2023) Nature, 620: 607. DOI: 10.1038/s41586-023-06333-9..
In-depth understanding of tumor heterogeneity helps develop treatment strategies that are more effective. For example, using combinations of drugs that target different characteristics of a tumor can help overcome treatment resistance. Furthermore, the cellular and molecular profile of a patient’s tumor can help develop personalized treatment plans that are more likely to be effective.
Epithelial-to-mesenchymal Transition
Epithelial-to-mesenchymal cell transition, or EMT, is an essential developmental process (141)Castaneda M, et al. (2022) Semin Cancer Biol, 87: 17. DOI: 10.1016/j.semcancer.2022.10.006.. Epithelial cells are tightly connected with each other and form the covering of all body surfaces, line body cavities and hollow organs, and are the major tissue in glands. In EMT, epithelial cells acquire the properties of another type of cell called mesenchymal cells, which form the connective tissue, blood vessels, and lymphatic tissue and have the ability to migrate within the body. This transition allows epithelial cells to move within the embryo during the formation of organs (141)Castaneda M, et al. (2022) Semin Cancer Biol, 87: 17. DOI: 10.1016/j.semcancer.2022.10.006.. EMT is also essential for wound healing and tissue regeneration throughout life (142)Marconi GD, et al. (2021) Cells, 10: 1587. DOI: 10.3390/cells10071587..
Roughly 90 percent of cancers develop in epithelial cells (143)Holly JM, et al. (2013) Cancer Metastasis Rev, 32: 673. DOI: 10.1007/s10555-013-9445-5.. Cancers that develop in epithelial cells hijack pathways fundamental for EMT. This hijacking of EMT pathways by cancer cells is one of the hallmarks of cancer and plays a central role in metastasis (144)Pastushenko I, et al. (2019) Trends Cell Biol, 29: 212. DOI: 10.1016/j.tcb.2018.12.001.. Research has established that EMT is regulated by several proteins that promote cancer cell division, survival, and mobility, and enable metastasis (145)Brabletz T, et al. (2018) Nat Rev Cancer, 18: 128. DOI: 10.1038/nrc.2017.118.(146)Fischer KR, et al. (2015) Nature, 527: 472. DOI: 10.1038/nature15748.. Recent studies have found that EMT also plays a critical role in the ability of cancer cells to evade the immune system (147)Wang G, et al. (2021) NPJ Precis Oncol, 5: 56. DOI: 10.1038/s41698-021-00200-4..
Ongoing research is exploring whether therapeutically targeting proteins involved in EMT could improve clinical outcomes. For example, findings from two recent studies show that the levels of netrin-1, a protein that is normally present during embryonic development and is involved in blood vessel formation, cell survival, and brain development, are increased in cancer cells undergoing EMT (148)Lengrand J, et al. (2023) Nature, 620: 402. DOI: 10.1038/s41586-023-06372-2.(149)Cassier PA, et al. (2023) Nature, 620: 409. DOI: 10.1038/s41586-023-06367-z.. Researchers found that blocking the activity of netrin-1 not only blocked EMT but also inhibited
tumor growth in endometrial cancer (149)Cassier PA, et al. (2023) Nature, 620: 409. DOI: 10.1038/s41586-023-06367-z. and skin cancer (148)Lengrand J, et al. (2023) Nature, 620: 402. DOI: 10.1038/s41586-023-06372-2..
Tumor Microenvironment
Cancer cells interact with and alter their surrounding cells and tissues to maintain their uncontrolled growth, accumulate within their primary site, and spread to other organs. The combination of cells, molecules, and blood vessels that support and sustain cancer cells is known as the tumor microenvironment. This environment plays a crucial role in influencing tumor growth and metastasis, while cancer cells, in turn, can modify the tumor microenvironment to enhance their survival and proliferation.
Cancer cells secrete molecules that modify their surroundings to ensure an adequate supply of nutrients and oxygen and to provide structural support (see Sidebar 7). Furthermore, the tumor microenvironment can adapt in ways that impede the effectiveness of immune cells or anticancer drugs, making it more difficult to target and destroy tumor cells (150)Jin MZ, et al. (2020) Signal Transduct Target Ther, 5: 166. DOI: 10.1038/s41392-020-00280-x.(151)Anderson NM, et al. (2020) Curr Biol, 30: R921. DOI: 10.1016/j.cub.2020.06.081..
The importance of the tumor microenvironment in cancer initiation, progression, metastasis, and as a barrier to treatment, has made it a significant focus for therapeutic development. Researchers are exploring ways to modify immune cells to enable them to penetrate the tumor microenvironment and effectively kill cancer cells (152)Bejarano L, et al. (2021) Cancer Discov, 11: 933.. One such approach involves isolating T cells from within a patient’s tumors, expanding them in the laboratory, and injecting them back into the patient. Because these T cells have already acquired the properties that enable them to infiltrate the tumor, they can overcome barriers posed by the tumor microenvironment effectively. The potential of this approach is underscored by FDA approval in February 2024 of the first such therapeutic (see Boosting the Cancer-killing Power of Immune Cells) (153)No Authors (2024) Nat Biotechnol, 42: 349. DOI: 10.1038/s41587-024-02195-2.. Additionally, therapies aimed at inhibiting tumor angiogenesis—thereby cutting off the tumor’s supply of oxygen and nutrients—have shown considerable promise in targeting the tumor microenvironment and impeding tumor growth (152)Bejarano L, et al. (2021) Cancer Discov, 11: 933..

Understanding the Biology of Childhood Cancers
Research over the past two decades has uncovered the molecular underpinnings of childhood cancer and has revealed features that distinguish childhood cancers from adult cancers (see Sidebar 8). At the molecular level, there is an increasing recognition that germline mutations play a pivotal role in childhood cancer, with studies indicating that at least 10 percent to 15 percent of childhood cancers are driven by germline mutations (154)Zhang J, et al. (2015) N Engl J Med, 373: 2336. DOI: 10.1056/NEJMoa1508054.(155)Parsons DW, et al. (2016) JAMA Oncol, 2: 616. DOI: 10.1001/jamaoncol.2015.5699.(156)Fiala EM, et al. (2021) Nat Cancer, 2: 357. DOI: 10.1038/s43018-021-00172-1.. Furthermore, structural alterations in DNA, such as chromosomal rearrangements and chromosomal translocations, play a central role in the initiation and progression of childhood cancers.
Chromosomal Rearrangements in Childhood Cancers
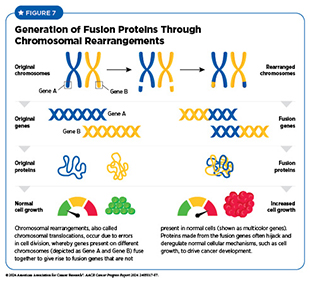
Chromosomal rearrangements are structural variations in which two genes, present on two different chromosomes (more common) or on the same chromosome (less common), fuse together to make a fusion gene that is not present in normal cells. This rearrangement not only disrupts functions of the proteins encoded by genes involved in chromosomal translocations, but also produces an entirely new protein from the fusion gene that can drive cancer initiation and progression (see Figure 7).
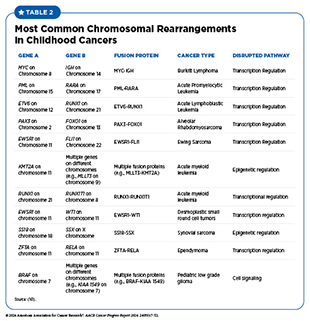
Chromosomal rearrangements occur due to errors during cell division. For example, breaks in the DNA strands during cell division can lead to incorrect repair and fusion of pieces of chromosomes. Chromosome breaks leading to fusion proteins are very common in childhood cancers and have been identified in many leukemias, solid tumors, and brain cancers in children (see Table 2). Findings from a large study evaluating transcriptomic sequencing data from 5,190 children with cancer identified 272 fusion genes. Researchers also discovered multiple mechanisms by which these fusion genes can lead to cancer in children, including disrupted gene regulation and altered RNA splicing (see RNA Variations) (160)Liu Y, et al. (2023) Nat Commun, 14: 1739. DOI: 10.1038/s41467-023-37438-4..
Understanding chromosomal rearrangements and their impact on cellular pathways is crucial for the diagnosis and treatment of childhood cancers. Targeted therapies that specifically address these molecular alterations have the potential to improve treatment outcomes and reduce adverse effects associated with chemotherapy or radiotherapy.
The Promise of Precision Medicine for Childhood Cancers
Precision medicine, or personalized medicine, means that patient treatment is based on characteristics that distinguish them from other individuals with the same disease (see Cancer Development: Integrating Knowledge). Precision medicine has shown great promise in treating childhood cancers, as underscored by NCI’s precision medicine initiatives focused on childhood cancer (see Sidebar 9).
One example of how precision medicine is accelerating the pace of progress against childhood cancers is the use of new strategies to assess the risk status in medulloblastoma, the most common childhood brain tumor (163)Rossi A, et al. (2008) Clin Cancer Res, 14: 971.. Historically, the risk stratification of medulloblastoma tumors was assessed by the morphology of the cancer cells and how much surgical removal of the tumor was performed. The integration of epigenomics, such as DNA methylation profiles, has led to the identification of distinct molecular subtypes of the disease, and has significantly improved the decision-making for treatment, such as using less intense treatments for children with cancer who have more favorable molecular characteristics. Because of its impact on improving health outcomes for patients, molecular profiles are now a part of the World Health Organization’s classification and risk assessment for the disease (164)Gajjar A, et al. (2021) J Clin Oncol, 39: 822. DOI: 10.1200/JCO.20.01372..
Another example of how precision medicine is improving outcomes for children with cancer is the NCI Therapeutically Applicable Research to Generate Effective Treatments (TARGET) program. Using gene expression analyses, researchers confirmed that some children with acute lymphoblastic leukemia (ALL) have the BCR-ABL fusion gene, also called the Philadelphia chromosome, which was originally identified by imaging and other approaches. Additional analyses revealed that children with this genetic alteration have poor outcomes, leading to clinical trials evaluating the efficacy of molecularly targeted therapeutics that block the activity of the BCR-ABL fusion protein (165)Roberts KG, et al. (2014) N Engl J Med, 371: 1005. DOI: 10.1056/NEJMoa1403088.. Other advances in the development of molecularly targeted therapy through genomics are highly effective therapeutics being used in the clinic to treat TRK fusion-positive cancers, ALK fusion-positive cancers, and ALK-driven neuroblastoma (158)Gore L, et al. (2024) Cell, 187: 1584. DOI: 10.1016/j.cell.2024.02.039..
Cancer Development: Integrating Knowledge
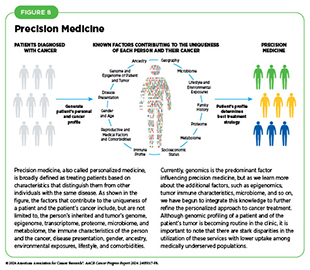
Breakthrough discoveries and technological innovations have significantly advanced the understanding of cancer initiation and progression, enabling the development of a myriad of effective anticancer therapies in recent years. A crucial insight stemming from this knowledge is the understanding that each patient’s cancer is unique at the molecular level. This understanding has paved the way for precision medicine, also called personalized medicine, which is broadly defined as treating patients based on characteristics that distinguish them from other individuals with the same disease (see Figure 8). For patients with cancer, precision medicine means using molecular characteristics of the tumor, such as the genome sequence of cancer cells, to make a diagnosis, plan treatment, evaluate whether treatment is working, and/or predict outcome.
In recent years, FDA has approved a number of anticancer therapeutics that are developed on the basis of genetic alterations that drive the characteristics of cancers, and many molecularly targeted drugs are being used to treat cancers that originate from different organs but share similar genetic characteristics (166)Malone ER, et al. (2020) Genome Med, 12: 8. DOI: 10.1186/s13073-019-0703-1.(167)Adashek JJ, et al. (2021) Trends Cancer, 7: 15. DOI: 10.1016/j.trecan.2020.08.009.. Furthermore, researchers are leveraging the ever-increasing information about a patient’s tumors to develop effective treatment plans for patients with cancer who develop resistance to available treatment options. For example, although many molecularly targeted therapies and immunotherapeutics are available for the treatment of non–small cell lung cancer (NSCLC), patients eventually develop resistance to these treatments and experience adverse health outcomes. One of the ways tumors become resistant to the treatment with precision therapeutics is by mutations in proteins that help cells repair damaged DNA. Based on this knowledge, researchers used a combination of an immunotherapeutic and a molecularly targeted therapeutic developed against the mutated form of protein involved in DNA repair. Results show that the treatment combination significantly improved the overall survival in patients harboring the mutated form of protein involved in DNA repair to 22.8 months from 8.4 months with standard of care treatment (168)Besse B, et al. (2024) Nat Med, 30: 716. DOI: 10.1038/s41591-024-02808-y..
The exciting new frontier of precision medicine is integrating all the information of a patient’s tumor gleaned from analyzing the genome, epigenome, proteome, transcriptome, microbiome, and immune system, among other aspects, to develop a personalized treatment plan. In fact, researchers are already integrating multiple aspects of a patient’s tumor to improve cancer diagnosis; identify precise drug targets; and predict treatment responses and outcomes more accurately (169)Mani DR, et al. (2022) Nat Rev Cancer, 22: 298. DOI: 10.1038/s41568-022-00446-5..
Precision medicine holds immense promise to deliver better outcomes with reduced toxicity for patients with cancer. However, many questions remain unanswered, such as the cost-effectiveness of multidimensional profiling that is a prerequisite for personalized treatments and the extent to which such profiling improves outcomes for individuals (170)Wahida A, et al. (2023) Nat Rev Cancer, 23: 43. DOI: 10.1038/s41568-022-00529-3.. It is vital that stakeholders across cancer science, medicine, and public health work together to ensure that all patients with cancer can equitably benefit from breakthroughs being made in cancer care by precision medicine approaches (171)Mateo J, et al. (2022) Nat Med, 28: 658. DOI: 10.1038/s41591-022-01717-2..
Next Section: Reducing the Risk of Cancer Development Previous Section: Cancer in 2024