Executive Summary
We are witnessing tremendous progress against the collection of diseases we call cancer. The rapid pace of these advances is attributable to research discoveries in basic, translational, clinical, and population science as well as technological innovations that are continually being translated to improvements in cancer prevention, detection, diagnosis, and treatment.
As the first and largest professional organization in the world whose mission is to prevent and cure all cancers, the American Association for Cancer Research (AACR) is dedicated to increasing public understanding of cancer and the importance of medical research for saving lives. It is also committed to advocating for increased federal funding to government entities that fuel progress against cancer, in particular, the National Institutes of Health (NIH), National Cancer Institute (NCI), United States (US) Food and Drug Administration (FDA), and Centers for Disease Control and Prevention (CDC).
The annual AACR Cancer Progress Report to Congress and the American public is a cornerstone of AACR’s educational and advocacy efforts. This fourteenth edition of the report highlights how medical research continues to extend and improve lives, like the lives of the courageous individuals featured in this report who have shared their experiences with cancer. It also underscores how federal funding for NIH, NCI, FDA, and CDC is vital if we are to maintain the momentum of progress against cancer for the benefit of all patients.
Cancer in 2024
The spectacular progress being made against cancer is resulting in a steady decline in cancer death rates, and a consistent rise in the number of people who live longer and fuller lives after a cancer diagnosis. In fact, the overall cancer death rate in the United States has fallen by 33 percent between 1991 and 2021, a reduction that translates into averting more than 4.1 million deaths from cancer. The drop in overall cancer mortality is attributable to reductions in smoking, as well as improvements in early detection and treatment of certain cancers.
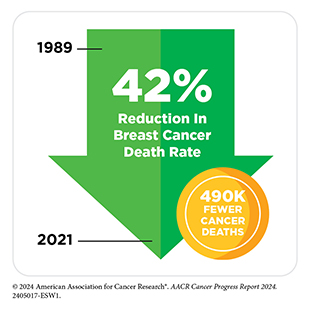
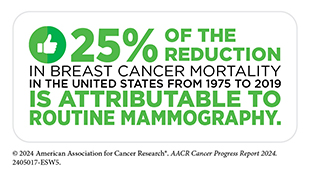
The steady decline in death rates for colorectal cancer and female breast cancer since the 1990s has helped drive down overall US cancer mortality. In addition, the decrease in US lung cancer death rate, the pace of which has accelerated in recent years, has contributed significantly to reducing the overall US cancer death rate in the past decade. Further contributing to the progress are the downward trends in death rates for leukemia, melanoma, and kidney cancer, attributable to breakthroughs in precision medicine.
Thanks to the research-driven advances, more than 18 million individuals with a history of cancer were alive in the United States as of January 1, 2022, and the number is projected to rise to 26 million by 2040.
Even though significant progress has been made, cancer continues to be an ongoing public health challenge in the United States and around the world. In the United States alone, it is estimated that more than two million new cancer cases will be diagnosed in 2024. Among the challenges we face is that the advances have not been uniform for all types and stages of cancer. As one example, while the overall cancer incidence in the United States has stabilized in recent years, cases of certain cancer types, such as pancreatic cancer, uterine cancer, and human papillomavirus (HPV)-associated oral cancers, are increasing. Furthermore, the age- and sex-specific incidence of certain cancers is on the rise. For instance, a growing concern among public health experts is the rising incidence of early-onset colorectal cancer—cancer in adults younger than 50 years.
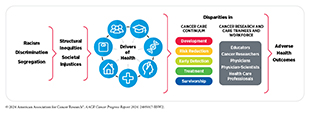
Another significant challenge we face is the disproportionately higher burden of cancer in US racial and ethnic minority groups and other medically underserved populations. Cancer disparities are driven largely by complex and interrelated structural and social factors. Increased collaboration among all stakeholders working toward the bold vision of health equity is vital if we are to ensure that research-driven advances against cancer benefit all patients, regardless of their race, ethnicity, age, sexual orientation, gender identity, socioeconomic status, or geographic location.
The burden of cancer and its economic toll, both on individuals and on the US health care system, are expected to rise in the coming decades, underscoring the urgent need for more research in medicine and public health to accelerate the pace of progress against cancer. The progress highlighted in this report was made as a direct result of the cumulative efforts of individuals working across the spectrum of medical research and the support from the federal government. Importantly, public sector funding from NIH and NCI directly benefits patients through the development of lifesaving anticancer therapeutics and preventive interventions. Continued federal investments in NIH, NCI, FDA, and CDC will help the medical research community maintain the momentum of scientific and technological innovation and ensure that we meet the President’s goal of reducing US cancer death rates by half by 2047.

The Landscape of Childhood, Adolescent and Young Adult (AYA) Cancers
This edition of the AACR Cancer Progress Report highlights the state of cancer in children and AYAs across the cancer continuum. A dedicated spotlight is included in all the relevant sections throughout the report.
Cancers are rare in children (14 years and younger), adolescents (15 to 19 years), and young adults (20 to 39 years). In the United States in 2024, approximately 9,620 children and 5,290 adolescents will be diagnosed with cancer. Leukemia and cancers of the nervous system, including brain tumors, are the most common cancers in children. Among adolescents, brain and nervous system tumors are the leading causes of new cancer cases, followed by lymphoma and leukemia. In contrast, young adults are most commonly diagnosed with solid tumors, including thyroid cancer, melanoma, and breast cancer.
Research over the past two decades has uncovered key biological differences between childhood and adult cancers. For example, emerging evidence indicates that genetic mutations inherited from parents play an important role in the development of childhood cancers. Another difference is the greater prevalence of structural DNA alterations, such as chromosomal rearrangements, in childhood cancers. Recent findings have identified nearly 300 fusion genes arising from chromosomal rearrangements that are associated with childhood cancers.
The knowledge that inherited genetic mutations play an important role in the development of childhood cancers is also helping researchers to develop surveillance strategies for monitoring and managing the risk of childhood cancers. For example, researchers have developed specific genetic tests, as well as surveillance recommendations, to monitor children who have Li–Fraumeni syndrome, a collection of diseases that is caused by inherited mutations in the TP53 gene and predisposes children to a wide range of early-onset cancers, including soft tissue sarcomas, osteosarcomas, breast cancer, brain tumors, and leukemia.
Modifiable risk factors play a far less critical role in the development of childhood cancers compared to cancers in adults. Regardless, there is some evidence that exposure to certain modifiable factors can increase the risk of cancer among children. AYA individuals diagnosed with cancer can be exposed to similar modifiable cancer risk factors as adults, and for a greater length of time compared to children. When combined with genetic predispositions, e.g., Lynch syndrome, such exposures can further increase the risk of cancer development.
According to NCI, an estimated 84,100 AYA individuals will be diagnosed with cancer in the United States in 2024, which is 4.2 percent of all cancer diagnoses. Although this is a small percentage compared to adults who receive a diagnosis of cancer, there has been a rise in certain types of early-onset cancers that are caused by a combination of factors including genetic predisposition, diet, microbiome, excess body weight, and environmental exposures. Infection with certain pathogens can also increase the risk of cancer in this group.
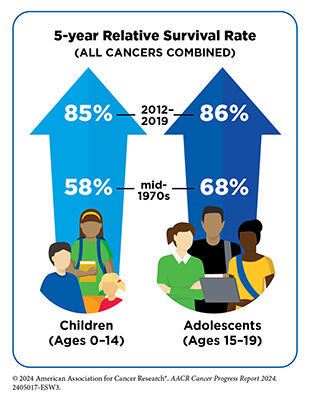
The unprecedented progress in the treatment of childhood and AYA cancers is reflected in the greater than 85 percent 5-year relative survival rates for all cancers combined among childhood and AYA patients. Many of the initial advances in the treatment of childhood cancers were made through intensification of cytotoxic chemotherapeutics, which while effective were associated with significant toxicities, including late and long-term adverse effects. With greater understanding of the biology of childhood and AYA cancers and innovations in technology, there is an increasing focus on utilizing personalized approaches to target cancers more precisely as well as on reducing treatment intensities among patients who have a favorable prognosis, to improve their quality of life. The National Cancer Institute is leading the efforts to harness the knowledge gleaned from genomic analyses of childhood cancers to develop strategies for precision or personalized medicine. In recent years, FDA has approved a broad array of precision therapeutics to treat a variety of childhood cancers.
Just in the 12 months covered by this report (July 1, 2023–June 30, 2024), FDA approved a new molecularly targeted therapy, tovorafenib (Ojemda), for the treatment of children with certain brain tumor types. During the same period, FDA also expanded the use of a molecularly targeted therapeutic, eflornithine (Iwilfin), previously approved for certain infectious and hormonal diseases, as the first treatment to reduce the risk of relapse in children with high-risk neuroblastoma. In addition, FDA expanded the use of a molecularly targeted therapeutic, repotrectinib
(Augtyro) previously approved for patients with certain type of lung cancer for the treatment of children with any solid tumor that has certain genetic alterations. Furthermore, several molecularly targeted therapeutics and immunotherapeutics that were already approved by FDA for use in adults with certain cancers received expanded approvals for the treatment of children with the same types of cancer. The promise of these therapeutics is illustrated through the personal experiences of Michael Methner and Parker Shaw.
Despite major progress, some cancers in children and AYA, such as sarcomas or certain brain tumors, have been difficult to treat and continue to have poor survival. Childhood and AYA cancer survivors such as Lourdes Monje also face unique challenges compared to their peers who have never been diagnosed with cancer. These challenges include long and late-term side effects from cancer and its treatments, financial toxicity, difficulty finding work, lower levels of educational attainment, psychosocial issues, and others. Because of the unique challenges faced by this population, researchers and care providers must ensure that the needs of this population are adequately addressed across the spectrum of cancer care.
Understanding the Path to Cancer Development
Seminal contributions from decades of research in basic, clinical, translational, and population science have established cancer as a collection of diseases that are characterized by uncontrolled cell division. A key insight from this knowledge is that different cancer types share many characteristics or hallmarks, including the ability of cancer cells to acquire changes that make their genome unstable, divide limitlessly, grow uncontrollably, escape cell death, spread to other tissues in the body, evade destruction by the immune system, and increase nutrients and oxygen supply to tumors.
Research has shown that hallmarks of cancer are primarily acquired through mutations in the genetic material of normal cells. There are two types of genetic mutations associated with cancer: inherited and somatic. Inherited mutations are passed on from parents to children and contribute to about 10 percent of all cancer cases. The remaining 90 percent of all cancer cases stem from somatic mutations, which are acquired throughout a person’s lifetime and can arise in multiple ways, such as from errors made during cell division, or due to exposure to modifiable risk factors including smoking, certain viral infections, and UV radiation and/or cancer-causing chemicals.
Cancer initiation, development, and progression are all multistep processes that are further influenced by changes inside and outside the cell. As the disease progresses, cancer cells acquire additional characteristics that enable them to establish mutually beneficial interactions with their surroundings, known as the tumor microenvironment. Research has shown that the tumor microenvironment affects the growth of cancer cells and cancer cells influence the tumor microenvironment to promote their survival.
Technological advances in understanding cancer at the levels of single cells and molecules have demonstrated that each patient’s cancer is unique. This important insight is the basis for precision medicine, or personalized medicine, which is broadly defined as treating patients based on molecular characteristics that distinguish them from other individuals with the same disease. Rapid developments in precision medicine are yielding new and effective anticancer therapeutics to treat cancer types for which there were no effective treatment options just two decades ago.
Reducing the Risk of Cancer Development
Research in basic, translational, and population sciences has broadened our understanding of the factors that increase an individual’s risk of developing cancer. It is estimated that 40 percent of all cancer cases in the United States are attributable to preventable causes. Many of these risk factors are modifiable, such as reducing tobacco use, avoiding an unhealthy diet, staying physically active, limiting exposure to UV radiation, reducing or eliminating alcohol consumption, and preventing and treating cancer-causing pathogenic infections.
Between 1991 and 2021 the overall cancer mortality in the United States declined by 33 percent, in part due to the implementation of public health campaigns and policy initiatives that helped reduce smoking and increase early detection of cancers. Although smoking rates have declined, the increasing prevalence of other risk factors, including obesity among US children and adults, are cause for public health concern. Additionally, there is a lack of widespread utilization in the United States of preventive interventions, such as vaccination against cancer-causing pathogens, including HPV, which is the primary cause of cervical cancer.
Environmental risk factors, such as air pollution, water contamination, and naturally occurring radon gas, also increase a person’s risk for certain types of cancers, such as lung cancer. There is also an increasing recognition that endocrine-disrupting chemicals, such as those found in hair straightening products, food packaging, and many other consumer products, can increase the risk of certain diseases, including cancers of the breast and thyroid.
Exposure to elevated levels of carcinogens in certain occupations, such as firefighting or welding, can increase the risk of certain types of cancer. Furthermore, occupations that involve night shift work, which can disrupt the body’s natural sleep patterns, as well as the lack of sleep due to overwork, can increase an individual’s risk of developing cancer. Finally, hormonal factors that result from normal physiology, such as pregnancy and breastfeeding can also increase or decrease the risk of developing certain types of cancer.
As we learn more about cancer risk factors and identify segments of the US population who are exposed to elevated levels of these factors, new and equitable policies must be developed and implemented to reduce cancer risk and improve the health of all populations, including those exposed to environmental and occupational cancer risk factors.
Screening for Early Detection
Cancer screening refers to checking for cancer, or for abnormal cells that may become cancerous, in people who do not have any signs or symptoms of the disease. Cancer screening can help detect cancer at the earliest possible stage when it can be treated successfully, with a higher likelihood of cure. Accruing evidence shows that cancer screening saves lives and reduces the burden of the disease at a population level.
In the United States, the US Preventive Services Task Force, a panel of experts in preventive medicine, periodically issues evidence-based screening recommendations for cancers of breast, cervix, colon and rectum, lung and bronchus, and prostate. Key considerations that determine who should receive screening and for which cancer include biological sex and age of the individual, as well as genetic, environmental, behavioral, and social influences.
Cancer screening is a multistep process that includes receiving the recommended test, as well as follow-up care if the initial test shows abnormal findings. Unfortunately, disadvantaged segments of the US population experience inequities in receiving the recommended cancer screening and follow-up care. There are several reasons for low rates of cancer screening, including social and structural barriers; bias and discrimination against marginalized populations in the health care system; mistrust of health care professionals among minoritized populations; lack of access to quality health insurance; low health literacy; and miscommunication between patients and providers.
Researchers have identified evidence-based interventions that are proving effective in increasing adherence to recommended screening guidelines and follow-up care. These interventions include using electronic health records to educate and inform patients and providers about routine cancer screening; reducing structural barriers so that it is easier for people to take the routine cancer screening test; and implementing culturally tailored strategies to build trust between patients and providers.
Researchers are also cautiously optimistic about the potential of recent technological advances, such as implementation of artificial intelligence (AI) and minimally invasive screening tests, in improving early detection of cancers. In recent years, FDA has approved several AI-assisted medical devices to aid clinicians in cancer diagnosis. During the 12 months covered by this report (July 1, 2023–June 30, 2024), FDA also approved two minimally invasive tests for inherited risk prediction or early detection of cancer. These approvals underscore the potential of AI and minimally invasive screening tests to detect cancers early. However, large prospective studies are required to establish that these approaches will improve early detection of cancers without increasing harm for individuals and/or further exacerbating existing inequities in the receipt of cancer screening and follow-up care.
Inspiring Science. Fueling Progress. Revolutionizing Care.
The dedicated efforts of researchers working across the continuum of cancer science and medicine power breakthroughs in clinical care that are improving survival and quality of life for patients in the United States and around the world. Clinical trials are a vital part of medical research because they establish whether new cancer treatments are safe and effective. Therefore, it is imperative that participants in clinical trials represent the full spectrum of the patient population who may use these treatments if they are approved. Unfortunately, participation in cancer clinical trials is low, and there is a significant lack of sociodemographic diversity among those who do participate. It is imperative that researchers and policymakers work together to address the many barriers to clinical trial participation. Enhancing the availability of clinical studies, particularly in community settings, can be transformative for patients, as reflected in the personal story of Dr. Humberto M. Guiot.
Surgery, radiotherapy, and cytotoxic chemotherapy constitute three of the five main pillars of cancer treatment. However, these therapies can have long-term adverse effects on patients. Through ongoing clinical studies, researchers are evaluating whether less aggressive surgery, radiotherapy, and cytotoxic chemotherapy can be appropriate for some patients with cancer, allowing these patients to experience improved quality of life.
Among the advances made between July 1, 2023, and June 30, 2024, are the 15 new anticancer therapeutics approved for use by FDA. During the same period, FDA also approved a new imaging agent to aid breast cancer surgery and expanded the use of 15 previously approved anticancer therapeutics to treat additional cancer types.
Included in the FDA approvals are the first tumor-infiltrating lymphocyte-based cellular immunotherapy that is benefiting patients with advanced melanoma such as Jennifer Ficko, a new T cell–engaging bispecific antibody against a novel target for patients with small cell lung cancer, the first AKT-targeted therapeutic for patients with breast cancer such as Julia K. Levine, the first KRAS-targeted therapy for certain patients with colorectal cancer, and several new molecularly targeted therapeutics and immunotherapeutics for the treatment of patients with an array of blood cancers, such as Vicki W. Jones who is receiving a new molecularly targeted therapeutic to treat her multiple myeloma. While these exciting new advances have the potential to transform clinical care, much work is needed to ensure equitable access to these treatments for all patient populations.
Supporting Cancer Patients and Survivors
According to NCI, a person is considered a cancer survivor from the time of cancer diagnosis through the balance of their life. As of January 2022, the most recent year for which such data are available, there were more than 18 million people living in the United States with a history of a cancer diagnosis, which equates to about 5 percent of the US population. This is a significant improvement from 50 years ago when cancer survivors constituted only 1.4 percent of the US population. Understanding and addressing the short- and long-term challenges faced by cancer survivors, supporting their quality of life, and ensuring that care is accessible and equitable are important priorities in cancer survivorship research.
Each person diagnosed with cancer has a unique experience ranging from successful treatment and living cancer free for the remainder of life to living a high-quality life through successful management of metastatic cancer to experiencing varying degrees of side effects to a subsequent cancer diagnosis with the same or a different type of cancer. Survivors often face physical, psychosocial, and financial challenges, both during and after the conclusion of treatment.
Cancer survivors should adhere to a healthy diet, engage in physical activity, reduce or eliminate the consumption of alcohol, and stop smoking, all of which help mitigate the physical challenges associated with a diagnosis of cancer. Researchers are also using other evidence-based strategies, including palliative care, pyscho-oncology, patient-reported outcomes, and patient navigation, to help reduce the adverse impact of a cancer diagnosis on the physical, mental, and financial health of cancer survivors. Understanding the survivorship challenges, as well as how to reduce or eliminate them, is an active area of research that is continually evolving as new therapies are introduced in the clinic.
Challenges experienced by patients and survivors of cancer also extend to friends and family members who often act as informal caregivers. There are an estimated four million caregivers who are caring for an adult cancer patient in the United States. These caregivers support cancer survivors in multiple ways, such as by arranging transportation for clinical appointments, helping with day-to-day activities, assisting in medical care or other clinical tasks, coordinating care, and providing emotional support. Caregiving often leads to burnout, which negatively impacts caregivers’ psychological and emotional well-being. More evidence of the challenges faced by caregivers is emerging through ongoing research, which also highlights the many opportunities to assist this vulnerable population.
Envisioning the Future of Cancer Science and Medicine
Breakthrough discoveries and technological advances across the fields of medicine have substantially increased the understanding of cancer initiation and progression, providing the foundational knowledge for better strategies to reduce the risk of developing cancer, detect cancer at the earliest possible stage, and treat cancer effectively and more precisely. As a result, cancer deaths are declining, and survivors are living longer and fuller lives. Researchers, including AACR president (2024-2025) Patricia M. LoRusso, DO, PhD (hc), FAACR, firmly believe that the fast-paced trajectory of progress against cancer can be further accelerated through sustained and predictable funding for cancer research.
Radiotherapy, one of the pillars of cancer treatment, has experienced a wave of innovation in the past decade, including delivering radiation precisely to tumors, thus minimizing harm to the surrounding normal tissues. Radiotheranostics is another promising technique for detecting and treating cancer using radioisotopes that has shown remarkable success against multiple cancer types, marking a significant advance in radiation-based cancer treatment.
Advances in non-invasive cancer imaging are revolutionizing visualization of tumor metabolism and assessment and monitoring of treatment response inside the body, thus enabling clinicians to make informed treatment decisions in a timely manner. Another exciting advance is the emergence of cancer engineering, a powerful interdisciplinary approach that combines principles from engineering, biology, and medicine for understanding the complexities of cancer development to improve health outcomes.
Advancing Cancer Research and Patient Care Through Evidence-based Policies
Sustained investments in medical research are critical for progress against cancer, including risk reduction, early detection, and treatment. As the largest public source of funding for medical research, NIH supports a vast array of scientific and educational programs that enable breakthroughs, which benefit human health and train the next generation of researchers. Within NIH, NCI leads the National Cancer Program and is the world’s largest single supporter of cancer research and training.
Federal support is also needed to ensure that the benefits of medical research are shared by all populations. Achieving health equity requires further investments in NIH and NCI on cancer disparities research and in education and training programs to ensure that the cancer research and care workforce is broadly representative of society. Other key investments include FDA programs for improving access to and diversity of cancer clinical trials; CDC initiatives for building a robust public health infrastructure and programs to improve cancer screening and reduce the use of tobacco; and US Environmental Protection Agency (EPA) actions and environmental health policies for reducing environmental exposures to carcinogens.
In recent decades, cancer mortality rates have declined for many childhood cancers. However, further policy solutions are needed to continue to expand research on cancer in children and adolescents, improve data collection, and expand and increase access to clinical trials for children and AYA with cancer. Congress has begun considering several pieces of legislation to address these issues, but more work is needed to speed progress against childhood, AYA, and other rare cancers.
AACR Call to Action
From fiscal year FY 2016 to FY 2023, Congress increased NIH funding for eight consecutive years. These funding increases for medical research accelerated the pace of scientific progress and contributed to the longer-term decline in cancer mortality in the United States. Unfortunately, after years of growing federal budgets for medical research, Congress cut NIH funding in FY 2024. This budget reduction threatens to curtail the progress seen in recent years and stymie future advancements. AACR urges Congress to continue to support robust, sustained, and predictable funding growth for the medical research and health programs that are vital to the fight against cancer.
We call on Congress to:
- Appropriate at least $51.3 billion in FY 2025 for the base budget of NIH and at least $7.934 billion for NCI.
- Provide $3.6 billion in dedicated funding for Cancer Moonshot activities through FY 2026 in addition to other funding, consistent with the President’s FY 2025 budget.
- Appropriate at least $472.4 million in FY 2025 for the CDC Division of Cancer Prevention to support comprehensive cancer control, central cancer registries, and screening and awareness programs for specific cancers.
- Allocate $55 million in funding for the Oncology Center of Excellence at FDA in FY 2025 to provide regulators with the staff and tools necessary to conduct expedited review of cancer-related medical products.
By following these recommendations, Congress will help speed the rate of discovery and create vital pathways for young scientists to contribute to future advances in cancer research. This investment will improve our nation’s health, including the lives of the millions of people who have been affected by cancer.
Next Section: Snapshot of a Year of Progress Previous Section: Message from the AACR