- Investing in a Healthier Future Through Research
- Sustaining the U.S. Economy
- Supporting a Vibrant and Diverse Cancer Research Workforce
- Advancing Regulatory Science to Ensure the Safety and Efficacy of Medical Products
- Expanding Policy Opportunities in Cancer Prevention and Treatment
- Accelerating Progress Against Pediatric Cancer
- Addressing Cancer Health Disparities
- Informing Policy Through Patient Advocacy
- Supporting Patients with Cancer During the COVID-19 Pandemic
Combating Cancer Through Science-based, Patient-centered Policies
In this section you will learn:
- Federal funding for medical research, specifically through NIH and NCI, has a significant impact on our nation’s health and the United States economy.
- Regulatory science initiatives at FDA are vital to accelerating progress against cancer and require robust federal funding to support the development of safe and effective therapies.
- Policies and federally funded public health programs, many of which are supported by CDC, ensure that individuals have access to preventive services, screening, and coverage for cancer treatment.
- Tobacco control policies improve public health and reduce cancer risk.
- Newly passed legislation aims to improve outcomes for children and adolescents who are diagnosed with cancer.
- Patient advocates play a vital role in educating patients with cancer, serving on many of the advisory boards and committees related to cancer research, and raising funds for cancer research.
The mission of NIH is to seek fundamental knowledge about the nature and behavior of living systems and to apply that knowledge to enhance health, lengthen life, and reduce illness and disability. A key goal of the agency is to develop, maintain, and renew scientific human and physical resources to ensure a continued high return on the public investment in research. NIH leadership and peer-review processes exemplify and promote the highest level of scientific integrity, public accountability, and social responsibility in the conduct of biomedical research. In realizing these goals, NIH improves the health of the nation by conducting and supporting research to address the greatest health challenges facing our society (647)Natinal Institutes of Health. The NIH mission – it’s about life. [updated 2021 Apr 16; cited 2021 Aug 14]..
NCI, under the NIH umbrella, leads, conducts, and supports research across the nation to advance scientific knowledge and drive progress against cancer, a collection of more than 200 devastating diseases that impact nearly every family.
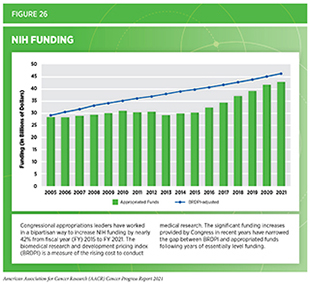
The collective progress made against cancer during the 12 months covered in this report was built on decades of publicly funded science through NIH and NCI. Robust and sustained funding for medical research is critical to continuing this progress as discussed by Congresswoman Jaime Herrera Beutler). From fiscal year (FY) 2015 to FY 2021, Congress has worked in a bipartisan fashion to increase the overall NIH appropriations by nearly $13 billion or 42 percent (see Figure 26). This remarkable investment is fueling the next wave of discoveries, thanks in large part to the leadership of Chair Rosa DeLauro (D-CT), Ranking Member Tom Cole (R-OK), Chair Patty Murray (D-WA), and Ranking Member Roy Blunt (R-MO) in their respective roles on the Labor-HHS-Education Appropriations Subcommittees in the House and Senate.
The mission of the Oncology Center of Excellence (OCE) at FDA is to achieve patient-centered regulatory decision-making through innovation and collaboration. The agency plays a critical role in reviewing new breakthrough treatments to ensure that they are safe and effective for patients with cancer.
CDC is another equally important federal partner in fueling progress against cancer, as it brings science-driven public health interventions, including cancer screening and prevention programs, to communities across the country. CDC’s Division of Cancer Prevention and Control works with state health agencies, territories, tribes and tribal organizations, and other key organizations to develop, implement, and promote effective cancer prevention and control practices.
The COVID-19 pandemic has had a profoundly negative impact on cancer research and care. For months, many research laboratories were closed or had to significantly alter their operations, while hundreds of promising clinical trials were put on hold and dozens permanently terminated (648)Carlisle BG. Clinical trials stopped by COVID-19. [updated 2021 Jan 29; cited 2021 Jul 9].(649)Upadhaya S, Yu JX, Hodge J, Campbell J. COVID-19 impact on oncology clinical trials: a 1-year analysis. Nat Rev Drug Discov. 2021;20(6):415.. Resources were shifted to respond to the immediate threat of the pandemic, and many researchers lent their expertise and supplies to addressing the public health emergency. While most research operations have returned to some level of normalcy, the impact of these disruptions will be felt for years. NIH Director Francis Collins, MD, PhD, has estimated that more than $16 billion worth of biomedical research productivity has been lost since the pandemic began (650)Bauman J. Pandemic cost NIH $16 billion in delayed, lost medical research. Bloomberg Law 2021 Mar 19.. The consequences have been especially severe for early-career researchers, women, and underrepresented minorities (651)Garrido P, Adjei AA, Bajpai J, et al. Has COVID-19 had a greater impact on female than male oncologists? Results of the ESMO Women for Oncology (W4O) Survey. ESMO open. 2021;6(3).(652)Langin K. Pandemic hits scientist parents hard. Science (80- ). 2020;369(6504).(653)Gewin V. The career cost of COVID-19 to female researchers, and how science should respond. Nature. 2020;583(7818)..
Robust annual funding increases will be essential for NIH, NCI, FDA, CDC, and other agencies to continue their vital work against cancer. Supplemental research funding for NIH will also be necessary to respond to the losses incurred by the medical research enterprise because of the pandemic. Meanwhile, new legislation, as well as policies and programs carried out by federal agencies, will play a key role in furthering our progress against cancer.
Investing in a Healthier Future Through Research
The 27 Institutes and Centers that comprise NIH represent the core of the U.S. biomedical research infrastructure by providing the majority of annual research grant and contract opportunities. NIH- and NCI-funded research grants and contracts have led to new discoveries across the broad field of cancer science, laying the groundwork for innovative new therapies, screening and diagnostic tools, and prevention modalities described in this report.
In addition to its extraordinary support for NIH and NCI, Congress has continued to appropriate full funding for the Cancer Moonshot (see sidebar on The National Cancer Moonshot Initiative), an initiative led by NCI with the goal of accelerating the pace of progress against cancer through prevention, screening, scientific discovery, collaboration, and data sharing. The 21st Century Cures Act, which was signed into law in December 2016, authorized $1.8 billion to fund the Cancer Moonshot over a 7-year period.
Despite the bipartisan congressional support for medical research, NCI is facing significant funding challenges to support investigators seeking research grants and contracts. Remarkable advances in cancer research have stimulated an unprecedented 50 percent increase in the number of research project grant (RPG) applications to NCI since FY 2013. NCI funding, however, has not increased at the same pace, resulting in low payline and declining success rates for investigator-initiated RPGs (654)NIH, National Cancer Institute. NCI director’s message: fiscal year 2022 annual plan & budget proposal. 2020 Aug 31. [cited 2021 July 9].. As a result, NCI is currently only able to fund approximately one of every eight proposals submitted, thus leaving a significant amount of potentially lifesaving cancer science and medicine unexplored. Notably, the 12.8 percent success rate at NCI is significantly lower than the nearly 21 percent NIH-wide success rate for RPGs (655)NIH, RePORT. Success rates. [cited 2021 Jul 9].. Furthermore, a fundamental challenge is that women and minority scientists are underrepresented in the biomedical research workforce and are funded at a lower rate (656)Stevens KR, Masters KS, Imoukhuede PI, et al. Fund Black scientists. Cell. 2021;184(3):561-565.(657)Taffe MA, Gilpin NW. Equity, diversity and inclusion racial inequity in grant funding from the us national institutes of health. Elife. 2021;10:1-11.(659)Oliveira DFM, Ma Y, Woodruff TK, Uzzi B. Comparison of National Institutes of Health Grant Amounts to First-Time Male and Female Principal Investigators. JAMA – J Am Med Assoc. 2019;321(9).(660)Carr PL, Raj A, Kaplan SE, Terrin N, Breeze JL, Freund KM. Gender differences in academic medicine: Retention, rank, and leadership comparisons from the national faculty survey. Acad Med. 2018;93(11).(661)Wadman M. NIH ‘high risk, high reward’ awardees skew male—again. Science (80- ). 2019;366(6463)..
Discrepancies in funding will have far-reaching consequences for the cancer research community and the ability to recruit, train, and retain the next generation of cancer scientists. If the success rate for NCI-funded RPGs continues at the current low level, young scientists will be discouraged from choosing careers in cancer research and will likely seek opportunities in other fields. These trends can result in fewer women and underrepresented minorities choosing careers in cancer research. Consequently, the United States may lose its position as the global leader in cancer research.
Congressional leaders have acted to address this issue in both FY 2020 and FY 2021 by providing increases for NCI and specifically including funds to increase the number of research grants funded each year. As a result, NCI raised the payline for R01 grants from eight percent in FY 2019 to 11 percent in FY 2021. Additionally, NCI Director Norman E. Sharpless, MD, asked for $7.6 billion for NCI in FY 2022 as part of his professional judgment budget request. This level of funding would allow NCI to raise its payline for RPGs to the 12th percentile and would set the institute on track to achieving a 15th percentile payline by FY 2025 (654)NIH, National Cancer Institute. NCI director’s message: fiscal year 2022 annual plan & budget proposal. 2020 Aug 31. [cited 2021 July 9]..
Sustaining the U.S. Economy
More than 80 percent of the funds appropriated to NIH by Congress are awarded to scientists in all 50 states and the District of Columbia through a competitive review process. Investments in NIH and NCI also extend well beyond the laboratory and the clinic. As the single largest public funder of medical research in the world, NIH-funded research supported over 536,000 jobs in communities across the U.S. and generated more than $91 billion in economic activity in FY 2020 (662)United for Medical Research. NIH’s role in sustaining the U.S. economy. [updated 2021 Mar 24; cited 2021 Jul 9]..
The bipartisan commitment to providing steady funding increases for medical research benefits all Americans through new discoveries, while also boosting local economies and creating jobs. With all of the opportunities before us to make advances against cancer, it is vitally important to maintain the momentum. Therefore, policy makers must continue to prioritize robust, sustained, and predictable increases for medical research funding.
Supporting a Vibrant and Diverse Cancer Research Workforce
Continued progress against cancer requires investment in recruiting, training, and supporting the next generation of cancer researchers at every stage of their careers. Within the workforce, early-career researchers are key to ensuring a strong pipeline, bringing in fresh ideas, and addressing innovative questions in cancer research. To realize the full potential of our medical research enterprise, we must also proactively recruit and support a cancer research workforce that reflects the diversity of our society, including diversity in race, ethnicity, gender, and geography. NIH and NCI play an important role in supporting young researchers who will become the scientific and clinical leaders of the future.
Introducing children to science and other educational programs early in life greatly enhances the likelihood that they will go on to earn higher degrees (663)Reynolds AJ, Ou S-R, Temple JA. A Multicomponent, Preschool to Third Grade Preventive Intervention and Educational Attainment at 35 Years of Age. JAMA Pediatr. 2018;172(3):247-256.. The NIH Center to Reduce Cancer Health Disparities offers funding support for underrepresented minorities from middle school through the junior tenure-track faculty level through the Continuing Umbrella of Research Experiences (CURE) program. Between 2001 and 2012, CURE supported more than 3,000 early-career researchers, who generated greater than 1,700 peer-reviewed publications (664)National Cancer Institute, National Institutes of Health, Center to Reduce Cancer Health Disparities. Continuing umbrella of research experiences (CURE). [cited 2021 Jul 11].. Additionally, NIH sponsors the Science, Education, Partnership Awards (SEPA) Program, which facilitates collaborations between medical researchers and preK-12th grade teachers (665)Science Education Partnership Award. [cited 2021 Jul 9].. Of the 351 participants in Q-Cubed, a University of Arizona’s SEPA-sponsored high school program, 82 percent went on to attend college (666)The University of Arizona, Q-Cubed. Statistics & evaluation. [cited 2021 Jul 9].. These awards provide valuable early exposures to the world of medical research and showcase how rewarding a career in research can be.
Graduate students and postdoctoral fellows comprise the largest share of the academic research workforce. In addition to their advisors’ grants, trainees are supported by a variety of institutional “T” awards, as well as individual “F” and “K” awards. These awards cover stipend and research costs of promising pre- and postdoctoral scientists, which enables them to take on more ambitious research. Some of these awards are targeted toward underrepresented minorities, while others, like the K99/R00 award, are designed to help bridge the gap between postdoctoral research and the establishment of a new independent laboratory. A study of 1,846 physician scientists (those with the dual MD-PhD degree) found that 63.8 percent of those who received an F30 or F31 grant during their training had a full-time faculty appointment within eight years of graduating, compared to 51.6 percent of those who did not receive an F30 or F31 grant (667)Andriole DA, Jeffe DB. Predictors of full-time faculty appointment among MD–PhD program graduates: a national cohort study. https://doi.org/103402/meo.v2130941. 2016;21(1).. Another study found that 30.2–48.4 percent of post-doctoral fellows who received a K01, K08, K23, or K99 award scored an R01 independent research grant within seven years (668)Conte ML, Omary MB. NIH Career Development Awards: conversion to research grants and regional distribution. J Clin Invest. 2018;128(12):5187-5190.. These data suggest that identifying and supporting promising early-career researchers facilitate a successful transition to independent scientists.
NCI has also taken steps to support junior tenure-track research faculty. For example, NCI has created several programs and policies to help establish independent laboratories, including setting a payline in the 15th percentile for “early-stage investigators” (researchers within 10 years of completing their terminal degree), compared to 10 percent for the general applicant pool. In addition, the most meritorious NCI R01 applications from early-stage investigators can be converted to Method to Extend Research in Time (MERIT) R37 awards (669)NIH, National Cancer Institute. MERIT award (R37). [updated 2020 Nov 5; cited 2021 Jul 9].. This program, which began in 2018, extends grants up to seven years instead of five, allowing more time for new faculty to establish their laboratories before submitting renewal applications. Furthermore, the NIH Faculty Institutional Recruitment for Sustainable Transformation (FIRST) program supports innovative recruitment strategies at institutions with the goal of developing a critical mass of early-career faculty who have shown a demonstrated commitment to building a diverse research workforce (670)National Institutes of Health. Faculty institutional recruitment for sustainable transformation (FIRST). [updated 2021 Aug 17; cited 2021 Jul 9]..
As with other aspects of life, the COVID-19 pandemic has created enormous challenges for early-career researchers, including laboratories closing for months; long-term experiments being cancelled before they yielded results; and capacity limits reducing the availability of mentors and collaborators. Additionally, many universities halted the hiring of new junior tenure-track faculty due to financial constraints, creating a bottleneck in the research workforce pipeline with potentially devastating consequences. Early-career female scientists, especially those with young children, have been particularly impacted by the pandemic, highlighting the importance of focusing additional support for women in science (671)Krukowski RA, Jagsi R, Cardel MI. Academic Productivity Differences by Gender and Child Age in Science, Technology, Engineering, Mathematics, and Medicine Faculty During the COVID-19 Pandemic. https://home.liebertpub.com/jwh. 2021;30(3):341-347.(672)Cardel MI, Dhurandhar E, Yarar-Fisher C, et al. Turning Chutes into Ladders for Women Faculty: A Review and Roadmap for Equity in Academia. https://home.liebertpub.com/jwh. 2020;29(5):721-733.(673)National Academies of Sciences, Engineering and M. Impact of COVID-19 on the Careers of Women in Academic Sciences, Engineering, and Medicine. National Academies Press; 2021.. The influx of innovative ideas from young scientists is critical for future breakthroughs against cancer and other deadly diseases. As Congress considers both annual appropriations and supplemental funding, it will be vital to invest in additional resources to support early-career researchers.
Advancing Regulatory Science to Ensure the Safety and Efficacy of Medical Products
The role of FDA in ensuring the safety and efficacy of anticancer therapeutics is critical for medical research. As cutting-edge advances in research expand our arsenal against cancer, the agency must keep pace with innovation, while ensuring regulatory oversight over the growing number of therapeutics. Although user fee agreements are an essential source of support, congressionally appropriated funds are essential to the agency’s mission. Discretionary funds support crucial regulatory science programs that generate evidence for the development of regulatory policies to accelerate the delivery of safe and effective anticancer therapeutics into the hands of patients.
FDA OCE was established in 2017 under the 21st Century Cures Act to facilitate the development of anticancer treatments and improve regulatory efficiency. OCE promotes collaborations among other FDA staff members with oncology expertise from the Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation (CBER), and Center for Devices and Radiological Health (CDRH).
OCE has also made important strides in accelerating regulatory review of anticancer therapeutics. The center’s Real-Time Oncology Review (RTOR) Program was initiated in 2018 to jumpstart the review process of oncology products by facilitating earlier submission of data. During its first two years, RTOR supported the application submission and review of 20 oncology products, of which nine received breakthrough therapy designation and all received priority review (674)Angelo de Claro R, Gao JJ, Kim T, et al. U.S. Food and drug administration: initial experience with the real-time oncology review program. Clin Cancer Res. 2021;27(1).. In 2019, Project Orbis was established for concurrent submission of drug approval applications for review by multiple international regulatory agencies to facilitate faster global adoption of new anticancer therapies. Over the course of its first year, OCE approved 38 of the 60 marketing applications it received, and soon afterward many were approved abroad by foreign agencies (675)de Claro RA, Spillman D, Hotaki LT, et al. Project Orbis: Global Collaborative Review Program. Clin Cancer Res. 2020;26(24):6412-6416..
Despite the unprecedented adversities caused by the COVID-19 pandemic, OCE has continued its critical work of reviewing anticancer therapeutics. This is demonstrated by the approval of 19 new anticancer therapeutics by the Center in 2020. In early 2021, OCE started Project Post COVIDity to develop partnerships with external experts and to study outcomes of patients with cancer infected with SARS-CoV-2 using real-world evidence generated from electronic health records, insurance claims data, and wearable health devices. In addition, Project Post COVIDity will analyze the impact of COVID-19 on patients with cancer, including effects on treatment delays, long-term COVID-19 symptoms, and therapeutic regimen selection (676)U.S. Food & Drug Administration. 2020 Annual Report: oncology center of excellence. [cited Aug 13].. Project Post COVIDity also provides an important opportunity to inform clinical trial design and conduct for oncology drugs. Furthermore, Project Post COVIDity continues ongoing efforts of FDA to explore potential uses for real-world evidence in regulatory decision-making, as mandated by legislation including the 21st Century Cures Act.
Reducing Barriers to Clinical Trial Participation
Participation in clinical trials drives improvements in overall survival for the represented diseases and demographics (677)Bleyer A, Tai E, Siegel S. Role of clinical trials in survival progress of American adolescents and young adults with cancer—and lack thereof. Pediatr Blood Cancer. 2018;65(8).(678)Hunger SP, Lu X, Devidas M, et al. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: A report from the children’s oncology group. J Clin Oncol. 2012;30(14).(679)Valecha G, Thumallapally N, El Bitar S, et al. Clinical trial awareness in oncology patients of diverse ethnic background: A single-institution analysis. J Clin Oncol. 2020;38(15_suppl)., and often results in better clinical outcomes for participants compared to nonparticipants (680)Han JJ, Kim JW, Suh KJ, et al. Clinical characteristics and outcomes of patients enrolled in clinical trials compared with those of patients outside clinical trials in advanced gastric cancer. Asia Pac J Clin Oncol. 2019;15(3).(681)Koo KC, Lee JS, Kim JW, et al. Impact of clinical trial participation on survival in patients with castration-resistant prostate cancer: A multi-center analysis. BMC Cancer. 2018;18(1).(682)Narui K, Ohno S, Mukai H, et al. Overall survival of participants compared to non-participants in a randomized-controlled trial (SELECT BC): A prospective cohort study. https://doi.org/101200/JCO20163415_suppl2527. 2016;34(15_suppl):2527-2527.. When adult patients with cancer are offered to join a trial, 55 percent accept (683)Unger JM, Hershman DL, Till C, et al. “When Offered to Participate”: A Systematic Review and Meta-Analysis of Patient Agreement to Participate in Cancer Clinical Trials. J Natl Cancer Inst. 2021;113(3).. However, only 8 percent of adult patients with cancer (684)Unger JM, Vaidya R, Hershman DL, Minasian LM, Fleury ME. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst. 2019;111(3)., and 19.9 percent of pediatric and adolescent patients with cancer (685)Faulk KE, Anderson-Mellies A, Cockburn M, Green AL. Assessment of enrollment characteristics for Children’s Oncology Group (COG) upfront therapeutic clinical trials 2004-2015. PLoS One. 2020;15(4)., enroll in clinical trials in the United States. While participation rates tend to be significantly higher at academic medical centers (684)Unger JM, Vaidya R, Hershman DL, Minasian LM, Fleury ME. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst. 2019;111(3).(685)Faulk KE, Anderson-Mellies A, Cockburn M, Green AL. Assessment of enrollment characteristics for Children’s Oncology Group (COG) upfront therapeutic clinical trials 2004-2015. PLoS One. 2020;15(4).(686)Aristizabal P, Singer J, Cooper R, et al. Participation in pediatric oncology research protocols: Racial/ethnic, language and age-based disparities. Pediatr Blood Cancer. 2015;62(8)., the vast majority of patients with cancer never participate in trials. This is, in part, because more than 75 percent of patients with cancer are unable to join trials; either a trial is unavailable for their cancer type or they do not meet the eligibility criteria due to previous treatments or comorbidities (684)Unger JM, Vaidya R, Hershman DL, Minasian LM, Fleury ME. Systematic review and meta-analysis of the magnitude of structural, clinical, and physician and patient barriers to cancer clinical trial participation. J Natl Cancer Inst. 2019;111(3).. Additional barriers to participation include the lack of health care facilities in underserved areas, mistrust in the health care system, failure of physicians to offer clinical trials to patients, childcare needs, and the time and costs associated with traveling to study sites (687)HT B, L Z, A S, E C, CJ R. At What Cost to Clinical Trial Enrollment? A Retrospective Study of Patient Travel Burden in Cancer Clinical Trials. Oncologist. 2018;23(10):1242-1249.(688)Largent EA, Lynch HF. Addressing Financial Barriers to Enrollment in Clinical Trials. JAMA Oncol. 2018;4(7):913-914.(689)Nipp RD, Lee H, Gorton E, et al. Addressing the Financial Burden of Cancer Clinical Trial Participation: Longitudinal Effects of an Equity Intervention. Oncologist. 2019;24(8):1048-1055.(690)Lungevity Foundation. LUNGevity barriers to clinical trial participation as perceived by lung cancer patients and caregivers.(691)Unger JM, Cook E, Tai E, Bleyer A. The Role of Clinical Trial Participation in Cancer Research: Barriers, Evidence, and Strategies. Am Soc Clin Oncol Educ B. 2016;(36)..
The COVID-19 pandemic greatly exacerbated the existing hurdles for clinical trials, but also made it necessary to implement long-sought approaches to address those challenges. In March 2020, FDA issued guidance outlining flexibilities in the conduct of clinical trials to help lessen the adverse effects of COVID-19 and trial sponsors quickly adopted them (692)U.S. Food & Drug Adminstration. Clinical trial conduct during the COVID-19 pandemic. [updated 2020 Jun 1; cited 2021 Aug 13].. Flexibilities included:
- Virtual visits to assess safety and clinical outcomes
- Delivering investigational products to the homes of participants
- Consenting participants remotely
- Collaborating with local physicians, laboratories, and imaging facilities
Such flexibilities have been recommended by FDA in the past, are popular with patients, decrease costs to participants, and may increase trial participation in the future if implemented permanently (also see sidebar on Lessons from COVID-19 to Streamline Oncology Clinical Trials). FDA has ongoing engagement efforts with stakeholders to determine the path forward on increasing clinical trial accessibility and ease of participating while maintaining standards for patient safety and data integrity (390)Flaherty KT, Doroshow JH, Galbraith S, et al. Rethinking Cancer Clinical Trial Conduct Induced by COVID-19: An Academic Center, Industry, Government, and Regulatory Agency Perspective. Cancer Discov. Published online 2021.. In addition to these enhancements to clinical trials, many patients, clinicians, and advocacy groups recommend increased use of patient navigators to help connect patients with cancer to clinical trials (693)Sharpe K, Scheid K. The benefits of patient navigation. J Oncol Nav Surv 2021;9.(694)Natale-Pereira A, Enard KR, Nevarez L, Jones LA. The role of patient navigators in eliminating health disparities. Cancer. 2011;117(SUPPL. 15).(695)Freeman HP, Rodriguez RL. History and principles of patient navigation. Cancer. 2011;117(SUPPL. 15).. However, patient navigation often lacks sustainable payment models or insurance coverage (696)Osundina F, Garfield K, Downer S. Patient navigation in cancer care review of payment models for a sustainable future. American Cancer Society, Inc. No. 08051..
FDA has also prioritized improving representation of racial, ethnic, and gender minorities in oncology clinical trials. Project Equity, launched by OCE in 2020, aims to improve evidence generation for underrepresented populations in trials by issuing guidance to industry to facilitate the accrual of diverse populations, fostering collaboration among stakeholders, and characterizing outcomes among underrepresented groups. Furthermore, CDER and CBER released voluntary guidance in November 2020 to encourage trial sponsors to implement strategies that would increase representation of racial and ethnic minorities (697)U.S. Food & Drug Adminstration. Enhancing the diversity of clinical trial populations – eligibility criteria, enrollment practices, and trial designs guidance for industry, November 2020. [updated 2020 Nov 13; cited 2021 Jul 9]., including:
- Broadening eligibility criteria for late-stage efficacy trials when more patients with comorbidities can be safely included;
- Detailing strategies to ensure trial participants reflect the diversity of the intended patient population of an investigational therapeutic or device;
- Encouraging trials or follow-up studies to include representation of racial and ethnic minorities, when possible, to definitively determine differences in safety and efficacy;
- Conducting trials at decentralized local health facilities while maintaining data integrity and patient safety; and
- Advancing the appropriate use of real-world evidence to fill evidence gaps where randomized clinical trials may not be feasible.
Recently, FDA has also taken actions to improve the availability of anticancer therapeutics for patients across all ages. In 2020, the agency initiated enforcement of key provisions in the Research to Accelerate Cures and Equity (RACE) for Children Act requiring certain targeted cancer therapies developed for adult patients to be studied in pediatric patients. In addition, FDA’s Project Silver represents a global regulatory effort to highlight drug development programs with indications particularly impacting older patients (75 and older) and promotes increased enrollment of geriatric patients in clinical trials for anticancer therapeutics.
Expanding Policy Opportunities in Cancer Prevention and Treatment
It is estimated that about 40 percent of cancer cases in the United States are attributable to preventable causes, such as tobacco use, HPV infection, and UV exposure, among others (see Preventing Cancer: Identifying Risk Factors). Furthermore, screening for early detection makes it more likely that cancer can be intercepted, and patient treated successfully (see Screening for Early Detection). A key hurdle to receiving preventive interventions and cancer screenings is coverage by insurance. A provision in the Affordable Care Act requires full insurance coverage of any preventive service recommended by U.S. Preventive Services Task Force (USPSTF). In May 2021, USPSTF updated its recommendation for Americans who should be screened for lung cancer. Previously, current and former smokers between the ages of 55 and 80, who smoked a pack a day for 30 years, were included in the recommendation. Under the new guidelines, smokers as young as 50 years old and those who smoked a pack a day for 20 years are recommended to receive annual screening free of charge through insurance. This expansion is expected to nearly double the number of smokers who should be screened, and it is particularly beneficial for women and African American smokers who tend to smoke fewer cigarettes on average compared to white men and yet have an elevated risk of developing lung cancer.
In May 2021, USPSTF addressed the sharp increase in colorectal cancer incidence among individuals under the age of 50 by lowering its age recommendation from 50 to 45 for initiating colorectal cancer screening for individuals at average risk for the disease. The new guidelines are expected to help identify more patients with colorectal cancer earlier when it is easier to treat. Additionally, USPSTF is currently reviewing the breast cancer screening guidelines (698)U.S. Preventive Services Task Force. Screening for breast cancer. [updated 2021 Apr 29; cited 2021 Jul 9]..
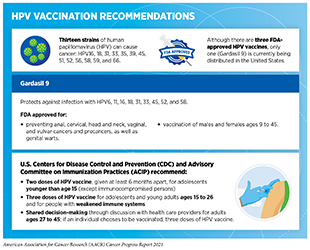
Public health policies and programs play an important role in supporting equitable access to effective cancer prevention methods such as screening, early treatment, and HPV vaccinations. Every year in the United States, HPV infection accounts for about 35,900 cases of cancer, including almost all cases of cervical cancer (699)Centers for Disease Control and Prevention. How many cancers are linked with HPV each year? [updated 2020 Sep 3; cited 2021 Jul 11].. HPV vaccination is highly effective at preventing cancer (309)Lei J, Ploner A, Elfström KM, et al. HPV Vaccination and the Risk of Invasive Cervical Cancer. 2020;383(14):1340-1348. and is recommended for girls and boys age 11 or 12 years (See sidebar on HPV Vaccination Recommendations). Unfortunately, HPV vaccination rates among U.S. adolescents have risen slowly in recent years; only 58.6 percent of eligible U.S. teens were fully vaccinated against HPV in 2020 (311)Pingali C. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years — United States, 2020. MMWR Morb Mortal Wkly Rep. 2021;70(35):1183-1190., which is significantly below the national goal of 80 percent set by U.S. Department of Health and Human Services in Healthy People 2020 (700)Elam-Evans LD. National, Regional, State, and Selected Local Area Vaccination Coverage Among Adolescents Aged 13–17 Years — United States, 2019. MMWR Morb Mortal Wkly Rep. 2020;69(33):1109-1116.. Therefore, continued funding for screening programs such as CDC’s National Breast and Cervical Cancer Early Detection Program is essential. The elimination of HPV-related cancers in the United States will only be possible through concerted efforts by all stakeholders to enhance public awareness of the importance of vaccination and to improve screening and treatment of precancerous HPV-related lesions.
The cancer advocacy and scientific communities continue to work with members of Congress, NCI, CDC, and other federal agencies to support and accelerate the elimination of HPV-related cancers in the United States and globally through public policy. State- and local-level vaccination mandates to attend public schools have greatly reduced the incidence of diseases like measles, mumps, and pertussis. However, only Hawaii, Rhode Island, Virginia, Puerto Rico, and Washington, DC, require HPV vaccination for school attendance. The states of Connecticut and New York have been pursuing similar bills to mandate HPV vaccines since 2020, but further efforts are needed to achieve the goal of an 80 percent vaccination rate in the United States.
Reducing Tobacco-related Illness Through Public Health Policy
Tobacco use in the United States is at a historic low due to effective tobacco control policies and smoking awareness campaigns since the 1960s. In 2019, 20.8 percent of U.S. adults regularly used any tobacco product (23)Cornelius ME, Wang TW, Jamal A, Loretan CG, Neff LJ. Tobacco Product Use Among Adults — United States, 2019. MMWR Morb Mortal Wkly Rep. Published online 2020., reflecting the fact that the majority of adult smokers have successfully quit smoking; 20.9 percent of all adults in 2018 were former smokers (200)United States Public Health Services Office of the Surgeon General; National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. Smoking cessation: a report of the Surgeon General. Washington (DC); US Department o. Unfortunately, 23.6 percent of high school students used tobacco products in 2020, the vast majority of whom used flavored e-cigarettes (701)Truth Initiative. E-cigarettes drive overall youth tobacco use to highest rate in decades. 2019 Dec 9. [cited 2021 Jul 9].(702)U.S. Food & Drug Administration. Youth tobacco use: results from the National Youth Tobacco Survey. [updated 2020 Dec 22; cited 2021 Jul 9].. This epidemic of nicotine dependence among youth threatens to reverse the progress made against tobacco-related illnesses. Furthermore, despite successes in reducing adult tobacco use, tobacco remains the number one preventable cause of cancer, highlighting the importance of additional tobacco control policies to prevent and cure all tobacco-related cancers.
In February 2020, FDA implemented a ban on all flavored pod and cartridge-based e-cigarettes, except for tobacco and menthol flavors. Open-tank and single-use e-cigarettes were also exempted from any flavor restrictions, leaving thousands of appealing flavors on the market. As a result, there was a more than 1,000 percent increase from 2019 to 2020 (2.4 vs. 26.6 percent) in the number of high school students who vape using disposable e-cigarettes (216)Gentzke AS, Wang TW, Neff LJ, et al. E-cigarette Use Among Middle and High School Students — United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(37):1310-1312.. With more than 80 percent of youth e-cigarette users vaping flavored products, loopholes that allow flavored tobacco products should be eliminated. Additionally, manufacturers of e-cigarettes, cigars, and other deemed tobacco products that were on the market as of August 8, 2016, were required to submit Premarket Tobacco Product Applications (PMTA) to FDA by September 9, 2020. FDA received more than 6 million PMTAs by the deadline. FDA will determine which products comply with regulations and whether scientific evidence submitted by manufacturers proves that their products meet the statutory level of being “appropriate for the protection of public health.”
In April 2021, the Biden administration announced its intent to ban menthol cigarettes, as well as menthol and other flavors in mass-produced cigars. This development was welcomed by public health organizations, including AACR, that have advocated for this policy for nearly 10 years. FDA plans to develop rules and regulations on menthol cigarettes and flavored cigars by the end of 2021. Numerous studies have shown that menthol flavoring makes it easier for smokers to get addicted to cigarettes, results in greater nicotine exposure, and makes it harder to quit smoking than non-flavored combustible cigarettes (703)Lee YO, Glantz SA. Menthol: putting the pieces together. Tob Control. 2011;20(Suppl 2):ii1-ii7.(704)Okuyemi KS, Faseru B, Cox LS, Bronars CA, Ahluwalia JS. Relationship between menthol cigarettes and smoking cessation among African American light smokers. Addiction. 2007;102(12):1979-1986.(705)Ross KC, Dempsey DA, Helen G St., Delucchi K, Benowitz NL. The Influence of Puff Characteristics, Nicotine Dependence, and Rate of Nicotine Metabolism on Daily Nicotine Exposure in African American Smokers. Cancer Epidemiol Prev Biomarkers. 2016;25(6):936(706)Villanti AC, Collins LK, Niaura RS, Gagosian SY, Abrams DB. Menthol cigarettes and the public health standard: a systematic review. BMC Public Heal 2017 171. 2017;17(1):1-13.(707)Brody AL, Mukhin AG, La Charite J, et al. Up-regulation of nicotinic acetylcholine receptors in menthol cigarette smokers. Int J Neuropsychopharmacol. 2013;16(5):957-966.(708)EC L, T B, DR S, JP P. Effects of menthol use and transitions in use on short-term and long-term cessation from cigarettes among US smokers. Tob Control. Published online July 2021:tobaccocontrol-2021-056596.. For years, the tobacco industry has targeted and advertised menthol cigarettes to communities of color with devastating results that have driven tobacco-related health disparities (709)Campaign for Tobacco-Free Kids. Stopping menthol, saving lives: ending big tobacco’s predatory marketing to black communities. [updated 2021 Feb 24].. Menthol also contributes to youth initiation of tobacco products, and about half of all high school smokers use menthol, according to a new study (216)Gentzke AS, Wang TW, Neff LJ, et al. E-cigarette Use Among Middle and High School Students — United States, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(37):1310-1312.. A recent report estimates that banning menthol would prevent 630,000 tobacco-related deaths over the next 40 years, of which more than one third will be among African Americans (710)U.S. Food & Drug Administration. FDA commits to evidence-based actions aimed at saving lives and preventing future generations of smokers. [updated 2021 Apr 29; cited 2021 Jul 9]..
The Biden administration is also considering issuing a product standard to restrict the nicotine content in cigarettes to less addictive levels (711)Maloney J. Biden administration considering rule to cut nicotine in cigarettes. [updated 2021 Apr 19; cited 2021 Jul 9]. Wall Street Journal.. Modeling data suggest that lowering nicotine to minimally addictive levels could result in 5 million smokers quitting within a year, and 13 million smokers quitting within five years; the projected smoking rate among U.S. adults would decrease to 1.4 percent by 2060 as a result (712)Apelberg BJ, Feirman SP, Salazar E, et al. Potential Public Health Effects of Reducing Nicotine Levels in Cigarettes in the United States. https://doi.org/101056/NEJMsr1714617. 2018;378(18):1725-1733..
Other potential actions to protect public health include prohibiting the sale of all flavored tobacco products, such as disposable and open-tank e-cigarettes commonly used by youth; supporting strong action by FDA’s Center for Tobacco Products to regulate the manufacturing, distribution, and marketing of tobacco products; and increasing funding for the prevention and cessation activities that are supported by CDC’s Office on Smoking and Health.
Accelerating Progress Against Pediatric Cancer
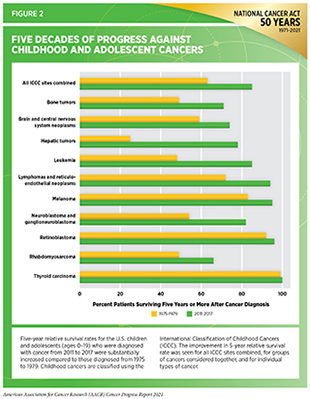
Cancer is the leading cause of disease-related deaths among children ages 1-14, and the second leading cause of death overall. Thanks to advances in cancer treatments over the last few decades, the 5-year survival rate for childhood cancer has increased to over 84 percent (713)NIH, National Cancer Institute. Cancer in children and adolescents. [updated 2021 Apr 20; cited 2021 Jul 9].(see Figure 2). However, there are many types of childhood cancers with significantly poorer outcomes and for which there are no effective treatments. Additionally, children who survive cancer face long-term side effects from their treatment, as well as life-threatening secondary effects of childhood cancer (see Challenges Faced by Cancer Survivors). Policies that encourage the development of new treatments for childhood cancers—and those that support survivors of childhood cancers—are critical to ensuring the best outcomes for every child impacted by cancer.
In 2018, Congress passed the Childhood Cancer Survivorship, Treatment, Access, and Research (STAR) Act, the most comprehensive childhood cancer legislation to date. This law contains numerous provisions to improve data collection, tracking, and survivorship support related to childhood cancers, and many of these items are being implemented, including:
- Grants awarded by NCI to support and expand the collection of biospecimens from children, adolescents, and young adults diagnosed with cancer;
- The expansion of childhood cancer surveillance programs at CDC, made possible by the development of a new cloud-based data reporting system;
- NCI-supported research on childhood cancer survivorship, including an emphasis on late effects of pediatric cancer treatment, disparities in outcomes for pediatric patients, and barriers to follow-up care;
- A report from the Government Accountability Office (GAO) entitled Survivors of Childhood Cancer: Factors Affecting Access to Follow-up Care (released July 2020);
- A series of reports from the Agency for Healthcare Research and Quality (AHRQ), including Disparities and Barriers to Pediatric Cancer Survivorship Care (released March 2021) and Models of Care That Include Primary Care for Adult Survivors of Childhood Cancer: A Realist Review (released May 2021); and
- A mandate to include at least one pediatric oncologist on the National Cancer Advisory Board.
Congress has consistently appropriated $30 million per year in funding as authorized by the STAR Act. Continued full appropriations will be essential to realizing the potential of this landmark childhood cancer law. Additionally, Congress will need to reauthorize the STAR Act before its expiration at the end of FY 2023 to continue NCI-supported research and further development of biorepositories, as well as to implement recommendations highlighted in GAO and AHRQ reports.
The Childhood Cancer Data Initiative (CCDI) is another program designed to improve the collection and sharing of data related to pediatric cancers, with the goal to better understand cancer biology specific to children and ultimately to improve prevention, treatment, quality of life, and survivorship. CCDI is complementary to the work that NCI is leading on biorepositories under the STAR Act. CCDI funding is proposed for a total of 10 years from FY 2020 to FY 2029, with $50 million to be allocated each year, and Congress fully funded the initiative in both FY 2020 and FY 2021. NCI has granted CCDI funds for childhood cancers and research activities and has engaged the entire childhood cancer community in the implementation of the initiative.
Molecularly targeted therapies have shown remarkable success for the treatment of adults with specific mutations that fuel cancer development. Many pediatric cancers exhibit the same mutations as adult cancers. However, it is challenging to establish clinical trials only for pediatric cancers with specific mutations, because all pediatric cancers are rare. The low availability of molecularly targeted trials for pediatric patients means that targeted drugs approved to treat adult forms of cancer often do not get approved for children even when there is strong potential of benefit. To address this issue, Congress passed key provisions of the Research to Accelerate Cures and Equity (RACE) for Children Act as part of the FDA Reauthorization Act of 2017. The RACE Act requires that drug manufacturers study molecularly targeted therapeutics developed for adult cancer patients in pediatric populations with the same mutations. In response to these provisions, FDA has developed a Pediatric Molecular Target List to provide guidance to companies as they plan for new drug and biologic submissions (714)Reaman G, Karres D, Ligas F, et al. Accelerating the Global Development of Pediatr Cancer Drugs: A Call to Coordinate the Submissions of Pediatric Investigation Plans and Pediatric Study Plans to the European Medicines Agency and US Food and Drug Administ. As of August 18, 2020, applications submitted to FDA for therapies that meet the RACE Act criteria must have agency-approved pediatric study plans.
The Gabriella Miller Kids First Pediatric Research Program (Kids First) at NIH is supporting new discoveries in understanding the biology of childhood cancers and their links to birth defects. Funding for this program was established in the Gabriella Miller Kids First Research Act, passed by Congress in 2014. Since then, over $75 million has been invested in pediatric research through the program. The bipartisan Gabriella Miller Kids First Research Act 2.0 was introduced in the House in January 2021 by Reps. Jennifer Wexton (D-VA), Tom Cole (R-OK), Peter Welch (D-VT), and Gus Bilirakis (R-FL), and a companion bill was introduced in the Senate by Sens. Tim Kaine (D-VA), Jerry Moran (R-KS), Mark Warner (D-VA), and Bill Cassidy (R-LA). This legislation would redirect penalties against pharmaceutical, cosmetic, supplement, and medical device companies for specified violations to the Kids First program, which is part of the NIH Common Fund. NIH would make allocations from this fund to support lifesaving pediatric research that does not duplicate existing activities.
Children with cancer are among those most impacted by drug shortages, which are largely driven by economic factors and occur primarily in the United States. This issue was brought into focus in the summer of 2019 when a shortage of vincristine—a chemotherapeutic agent that is an essential component of treatments for many childhood cancers—caused delays in treatment and forced pediatric oncologists to consider rationing the short supply of the drug that was available (715)Oncology Drug Shortages Persist. Cancer Discov. 2020;10(1).. Even though the vincristine shortage was eventually resolved, it highlighted the fragile nature of the supply chain for many drugs, and particularly those for the treatment of childhood cancers. In October 2019, FDA issued a report titled Drug Shortages: Root Causes and Potential Solutions (716)U.S. Food & Drug Administration. Drug shortages: root causes and potential solutions. [updated 2021 Mar 11; cited 2021 Aug 13]., which includes the following recommendations:
- Creating a shared understanding of the impact of drug shortages on patients and the contracting practices that may contribute to shortages;
- Developing a rating system to incentivize drug manufacturers to invest in quality management maturity for their facilities; and
- Promoting sustainable private sector contracts (e.g., with payers, purchasers, and group purchasing organizations) to make sure there is a reliable supply of medically important drugs.
Addressing Cancer Health Disparities
Medically underserved populations experience poorer health outcomes due to systemic disadvantages. Centuries of policies that restrict housing, educational, and employment opportunities for racial and ethnic minorities have led to lower health insurance rates, lower utilization of preventive health services, and poor nutrition. Additionally, an under-representation of high-quality health care facilities in low-income neighborhoods and rural communities results in a lower quality of care even for those who can afford it. Reducing cancer health disparities will require a long-term, multipronged approach that supports individuals, communities, health care centers, and federal agencies, as well as local, tribal, and state governments. Over the past year, policy developments related to cancer screening, clinical trial participation, nutrition, and health insurance have demonstrated that progress in addressing cancer health disparities is possible.
Unequal access to cancer screening contributes to cancer health disparities. In the past, USPSTF had received criticism for underrepresentation of racial and ethnic minorities in the clinical studies that the taskforce used to develop cancer screening guidelines for average risk individuals (717)Doubeni C, Simon M, Alex H. Krist. Addressing Systemic Racism Through Clinical Preventive Service Recommendations From the US Preventive Services Task Force. JAMA. Published online 2021.. Consequently, the existing guidelines were more likely to identify cancers in white patients while missing many cases in underserved racial and ethnic minorities. In an effort to address disparities in cancer screening, USPSTF recently revisited its guidelines for lung and colorectal cancers (see Cancer Screening Guidelines), as well as for Hepatitis B infection, which can cause liver cancer (26)Jonas DE, Reuland DS, Reddy SM, et al. Screening for Lung Cancer With Low-Dose Computed Tomography: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2021;325(10):971-987.(718)R C, I B, C B, et al. Screening for Hepatitis B Virus Infection in Nonpregnant Adolescents and Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2020;324(23):2423-2436.(719)Lin JS, Perdue LA, Henrikson NB, Bean SI, Blasi PR. Screening for Colorectal Cancer: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. 2021;325(19):1978-1997.. The new guidelines greatly expand the number and diversity of people who can now receive these cancer screening tests at no cost. Furthermore, CDC’s National Breast and Cervical Cancer Early Detection Program provides 139,000 low-income and uninsured women with annual access to screening, diagnostic, and treatment services for breast and cervical cancer (720)National Breast and Cervical Cancer Early Detection Program | CDC. Accessed January 27, 2020..
Food insecurity is a key driver of health disparities and contributes to worse cancer outcomes as well as to obesity, a major risk factor for cancer, due to limited access to healthy food options (721)Patel KG, Borno HT, Seligman HK. Food insecurity screening: A missing piece in cancer management. Cancer. 2019;125(20):3494-3501.. With widespread job losses during the COVID-19 pandemic, there was a fear that the prevalence of food insecurity would dramatically increase. Therefore, Congress included increased unemployment and nutritional support benefits in COVID-19 relief bills in March 2020, December 2020, and March 2021 (722)Moss K, Dawson L, Long M, Kates J, Musumeci MB, Cubanski J, et al. The Families First Coronavirus Response Act: summary of key provisions. KFF 2020 Mar 23.(723)Center on Budget and Policy Priorities. States are using much-needed temporary flexibility in SNAP to respond to COVID-19 challenges. [updated 2021 Aug 6; cited 2021 July 9].. Increases to nutrition benefits included the Supplemental Nutrition Assistance Program (SNAP); Special Supplemental Nutrition Program for Women, Infants, and Children (WIC); The Emergency Food Assistance Program (TEFAP); and support to provide free meals to low-income children who would qualify for free school lunches. Increases to SNAP benefits alone provided roughly an extra $27 per month per beneficiary through September 2021, helping feed families during the pandemic. CDC programs like the Racial and Ethnic Approaches to Community Health also fund local public health efforts, such as promoting exercise and ensuring underserved communities have access to fresh fruit and vegetables (724)Centers for Disease Control and Prevention.REACH program impact. [updated 2020 Mar 10; cited 2021 Aug 20]..
Lack of health insurance coverage is another major contributor to health disparities, as highlighted by Congresswoman Gwen Moore, with 30 million uninsured Americans in early 2020 (725)Finegold K, Conmy A, Chu RC, Bosworth A, Sommers BD. Trends in the U.S. uninsured population, 2010-2020. Department of Health and Human Services, Office of Health Policy Issue Brief, 2021 Feb 11.. The Affordable Care Act provided states the option to expand Medicaid coverage to families earning 138 percent of the federal poverty line or less. In states that have expanded Medicaid coverage, uninsured rates have decreased by nearly half compared to states that have not expanded Medicaid (726)Status of state Medicaid expansion decisions: interactive map. [cited 2021 Jul 9]. KFF 2021 Aug 10.. Medicaid expansion has been particularly beneficial for young adult cancer survivors (727)Su CT, Okullo D, Hingtgen S, Levine DA, Goold SD. Affordable Care Act and Cancer Survivors’ Financial Barriers to Care: Analysis of the National Health Interview Survey, 2009-2018. JCO Oncol Pract. Published online 2021., who have seen dramatic increases in the ability to afford health care and are therefore less likely to skip medications or delay refills. Over the past year, Nebraska and Oklahoma joined 35 other states in expanding Medicaid. Additionally, Missouri voters approved Medicaid expansion via ballot referendum in August 2020, but implementation is on hold while the expansion faces legal challenges (728)Guth M, Garfield R, Rudowitz R, Damico A. The status of Medicaid expansion in Missouri and implications for coverage and cost. [cited 2021 Jul 9]. KFF 2021 Jun 30.. Medicaid has been a crucial safety net during the COVID-19 pandemic as unemployed Americans lost workplace health insurance plans. The number of Medicaid beneficiaries grew by 7.6 million between February 2020 and November 2020 (729)Corallo B, Rudowitz R. Analysis of recent national trends in Medicaid and CHIP enrollment. [cited 2021 Jul 9]. KFF 2021 Aug 16.. Furthermore, Congress passed the CLINICAL TREATMENT Act as part of the fiscal year 2021 federal spending bill, which requires Medicaid to cover the routine medical costs of patients enrolled in clinical trials. The CLINICAL TREATMENT Act greatly enhances the ability of Medicaid beneficiaries to enroll in trials.
Several programs run by NIH, NCI, the National Institute on Minority Health and Health Disparities (NIMHD), and CDC are designed to address cancer health disparities. For example, NIH’s All of Us program, funded by the 21st Century Cures Act, aims to improve precision medical research by increasing representation of racial and ethnic minorities. NIMHD is NIH’s core institute to support research on the many factors that cause disparate health outcomes, including socioeconomics, politics, discrimination, culture, and environment. Unfortunately, NIMHD has an even lower RPG success rate than NCI, funding fewer than 8 percent of investigator-initiated grants (655)NIH, RePORT. Success rates. [cited 2021 Jul 9].. The NCI Community Oncology Research Program is a national network that helps connect community health facilities to clinical trials at NCI-designated cancer centers. Additionally, the NCI Center to Reduce Cancer Health Disparities supports disparities research within NCI and reinforces training a diverse cancer research workforce. CDC’s National Program of Cancer Registries is essential for understanding the scope of cancer disparities by tracking cancer rates all over the United States.
Informing Policy Through Patient Advocacy
Patient advocacy organizations work tirelessly to educate, promote awareness, support, and raise funds for cancer research. Dating back to 1938, when the 75th Congress passed House Joint Resolution 468, “To dedicate the month of April in each year to a voluntary national program for the control of cancer,” awareness days and months have become valuable tools to rally the community and engage Capitol Hill. As of 2021, there are 70 officially recognized annual awareness events in support of cancer. For example, in May 2021, AACR spearheaded the effort to recognize May as National Cancer Research Month. Senator Dianne Feinstein (D-CA) and Senator Shelley Moore Capito (R-WV) sponsored S. Res. 253 to designate May as National Cancer Research Month, which recognized the importance of cancer research and acknowledged the efforts of cancer researchers (730)Feinstein D. S.Res.253 – a resolution supporting the designation of May 2021 as “National Cancer Research Month”. 117th Congress (2021-2022). [cited 2021 Aug 13].. In addition to the Senate resolution, AACR received a Presidential Message from President Biden recognizing May as National Cancer Research Month (731)Cancer Researh Catalyst staff. Letter from President Biden to the AACR recognizes National Cancer Research Month. [cited 2021 Aug 13]. American Association for Cancer Research 2021 May 25..
In addition to congressional resolutions, patient advocacy organizations bring thousands of advocates to Washington, DC, to engage Capitol Hill in advocacy “Hill Days”. Hill Days provide an organization’s membership the opportunity to meet members of Congress, share the goals and mission of their organization, and ask members of Congress to introduce or support legislation beneficial to their community of researchers, clinicians, and patient advocates. Patient advocates are essential participants as they personalize the disease and issues related to the disease and provide members of Congress with critical information to influence their policy decisions. According to congressional staffers, in-person visits from constituents are the most influential way to communicate with a senator or representative who is undecided on an issue.
In 2020, the move to virtual platforms expanded access for constituents to engage with legislators outside of Washington, DC, but also created barriers for policy makers and advocates to develop relationships. Legislators cited the lack of face-to-face interactions with patient advocates as the biggest challenge they encountered when they needed to gain information on an issue. (732)Congressional Management Foundation. Citizen-centric advocacy: the untapped power of constituent engagement, 2017.(733)Ballast Research. Washington insights review: the new advocacy environment. 2021 Jan..
Increasing Patient Advocate Engagement with FDA, NCI, and Academia
Well-organized patient advocates have a positive influence in cancer research, drug development, and legislation. Nonprofit patient advocacy groups have demonstrated the power to significantly improve research outcomes (734)English P. The power of patient advocacy in drug development. [cited 2021 Jul 9]. Advara 2021 May 27.. A meaningful patient advocacy strategy has become an important part of the mission for many cancer research organizations (735)Deverka PA, Bangs R, Kreizenbeck K, et al. A New Framework for Patient Engagement in Cancer Clinical Trials Cooperative Group Studies. JNCI J Natl Cancer Inst. 2018;110(6):553-559.. Meaningful engagement with patient advocates is necessary for clinical trial design and conduct, ensuring the relevance and prioritization of research questions, identification of opportunities and barriers to accrual, success and transparency of research activities, dissemination of findings into practice, and broader understanding of the disease.
Patient advocacy is rooted in the right of all people to be informed and have as much participation and control as possible over their health care decisions. It expanded into research and policy following the AIDS (Acquired Immune Deficiency Syndrome) activist movement; broader visibility and understanding of the disease forced pharmaceutical industries, the United States government, and regulatory agencies to open a dialogue with patient advocates, allocate funding, and expedite a response to the epidemic. Encouraged by their success, other health-related organizations have developed alliances between scientists, clinicians, and patient advocates. These highly productive dialogues are advancing scientific discourse and fueling research advocacy in both private and public sectors at local, regional, and national levels.
Today, patient advocates have a substantial role in the development and regulatory review of potentially life-changing treatments at FDA and NCI.
In addition to patient advocacy organizations, many professional societies, health systems, and organizations recognize the need to encourage greater engagement between scientists and the general public. Starting in 2012, NCI-designated Comprehensive Cancer Centers were required to include proposals for community engagement in their core grant applications, which helped create valuable new relationships with their communities (736)Paskett ED, Hiatt RA. Catchment areas and community outreach and engagement: The new mandate for NCI-designated cancer centers. Cancer Epidemiol Biomarkers Prev. 2018;27(5).. Adding to this established engagement framework, the COVID-19 pandemic created a large demand for up-to-date and easy to understand health information. In response, numerous health systems across the United States hosted regular webinars to understand the concerns of their communities and explain the latest public health guidance (737)University of Michigan Library. Research guides: COVID-19 (novel coronavirus): webinars & online learning.(738)Emory University School of Medicine, Emergency Medicine. COVID-19 response.(739)Irving Institute for Clinical and Translational Research. Health education lectures for lay community.. Professional societies and scientific journals also provided unprecedented open access to peer-reviewed articles and organized a wide range of conference sessions and forums. These heightened community engagement efforts provide effective models to continue outreach for other important public health issues following the pandemic.
Many young scientists also recognize the importance of engaging with the public and are taking the initiative to create communication resources. In the past ten years, the number of active graduate student and postdoctoral science policy and communication organizations at U.S. universities increased from just a small handful to nearly 50 in 2020 (740)National Science Policy Network. FY20 annual report, 2019-2020.. These student-run organizations are leading the way in providing training and communication and public engagement for hundreds of early-career researchers. Additionally, some universities have created courses and other opportunities for PhD students to learn from patients and clinicians and to participate in tumor boards (741)Dartmouth, Office of the Registrar. Organization, regulations, and courses 2020-21; PEMM 132 clinical management of cancer. [cited 2021 Aug 13].. The enthusiasm of early-career researchers brings promise for the future of engagement between patients and scientists.
Supporting Patients with Cancer During the COVID-19 Pandemic
Patient advocacy organizations did not slow operations during the COVID-19 pandemic. Communities collaborated to share information and combat misinformation; educational programs focusing on the coronavirus, vaccines, and clinical trials were released weekly; and financial assistance resources were created for patients with cancer. Furthermore, advocates united to support timely access to the COVID-19 vaccine, promote inclusion in clinical trials, highlight health inequities, and mitigate the adverse impact of COVID-19 on cancer screenings and care. A survey by the National Coalition for Cancer Survivorship highlighted the increased need for these programs as patients with cancer reported heightened feelings of anxiety and excessive worry due to COVID-19 (742)National Coalition for Cancer Survivorship. 2020 state of Cancer Survivorship Survey. [published 2021 Feb 1; cited 2021 Sep 11]. Available from https://canceradvocacy.org/policy/2020-state-of-cancer-survivorship-survey/. Eighty-five percent of the families surveyed had lost their jobs or had a reduction in work hours, and 25 percent experienced a delay in treatment or follow-up care. Fortunately, virtual programs were widely adopted for support groups, remote visits for care, and research, thereby expanding opportunities for outreach and engagement.
As programs increased, many walks, runs, and other cancer-specific fundraising events were cancelled or saw a decline in participation and charitable revenue. According to the Giving USA 2021 Annual Report, donations to health organizations were estimated to have declined by 3.0 percent (4.2 percent adjusted for inflation) in a year when charitable giving in the United States reached an all-time high. This funding shortage strained many patient advocacy organizations which were asked to do more with less money and fewer staff members. The long-term implications for cancer research funding are still unclear. Each year, private and nonprofit patient advocacy organizations award millions of dollars to fuel progress in cancer research. Cancer researchers rely on this steady stream of funding to advance their discoveries from the laboratory to the patient and are grateful for the continued support from the thousands of patient advocates committed to funding cancer research. In the wake of the COVID-19 pandemic, support for cancer research is even more important to ensure continued progress in discovering new and innovative treatments.