Understanding How Cancer Develops
In this section, you will learn:
- Research provides our understanding of the biology of cancer, which is not one disease, but a collection of diseases characterized by the uncontrolled growth of cells.
- Genetic mutations underpin cancer initiation and development in most cases; the mutations are inherited in about 10 percent of cancer cases.
- Cancer initiation, development, and progression are strongly influenced by interactions among cancer cells and numerous factors in their environment.
- The more we know about the interplay among the individual factors influencing cancer in all populations, the more precisely and effectively we can prevent and treat cancer.
Over the past few decades, we have made tremendous progress in preventing, detecting, diagnosing, and treating cancer. This progress is epitomized by the declining overall cancer death rate and the rising number of cancer survivors (see Research: Driving Progress against Cancer). It has been possible because of discoveries across the breadth of medical research, from basic science to translational and clinical research and population research, which have deepened our understanding of how cancer develops (see sidebar on What Is Basic Research and How Does It Drive Progress against Cancer?).
We now understand that cancer is a collection of diseases that arise when the processes that control normal cell growth, division, and life span go awry. As a result, cells start multiplying uncontrollably, fail to die, acquire blood vessels to obtain nutrients that support their altered cell biology, and begin to accumulate. In body organs and tissues, the accumulating cancer cells form masses called tumors, whereas in the blood or bone marrow they crowd out normal cells. Over time, some cancer cells may invade distant tissues, a process termed metastasis, by entering the bloodstream or lymphatic network, and form secondary tumors at remote sites.
Cancer Development: Influences inside the Cell
The normal behavior of each cell in the human body is controlled by its genetic material, or genome. The genetic material comprises chains of deoxyribonucleic acid (DNA) units arranged in a certain order and packaged into condensed structures called chromosomes, which are contained within the cell’s nucleus (see sidebar on Genetic and Epigenetic Control of Cell Function). The order of the DNA units and how the DNA chains are packaged dictate which proteins and how much of them are made by each cell.
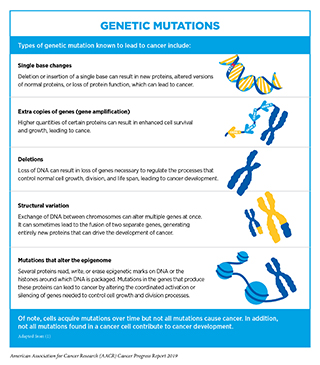
Alterations in the DNA sequence, referred to as mutations, can disrupt normal protein function and are the leading cause of cancer development (see sidebar on Genetic Mutations). Each person’s cancer has a unique combination of mutations, and as a cancer progresses, additional mutations accumulate. The number of cells within a growing tumor that carry a given mutation depends on when the mutation was acquired during tumor growth. Therefore, even within the same tumor, different cancer cells often have different combinations of genetic mutations. This variation, or heterogeneity, within a tumor or between a primary and metastatic tumor, is a leading cause of resistance to treatment.
While inherited genetic mutations play a role in about 10 percent of all cancer cases (see Table 2), most mutations are acquired over an individual’s lifetime due to errors arising during normal cell multiplication or because of environmental exposures, lifestyle factors, or coexisting health conditions that fuel chronic inflammation (see sidebar on Sources of Genetic Mutations). Scientists are actively investigating whether it is possible to identify the causative origins of a cancer by looking at the patterns of mutations in the cells that comprise it (29). Ongoing research is also uncovering the unique mutational landscapes of specific cancer types (30). For example, childhood cancers carry fewer single base changes, but more copy number variations and/or structural rearrangements, than cancers in adults (31)(32).
Not all mutations acquired by a cell lead to cancer. In fact, the genes that are mutated, and the order and speed at which a cell acquires mutations, determine whether a cancer will develop and, if a cancer does develop, the length of time it takes to happen. The progressive nature of cancer provides distinct time points for medical intervention to prevent cancer, detect and/or intercept it early, and treat progressive disease. In general, the further a cancer has progressed, the harder it is to stop the chain of events that leads to the emergence of metastatic disease, which is the cause of most deaths from solid tumors (see Screening for Early Detection).
In addition to genetic mutations, changes in the physical structure of DNA caused by chemical modifications of the DNA and/or the proteins associated with it, termed epigenetic modifications, can lead to cancer development (see sidebar on Genetic and Epigenetic Control of Cell Function). Epigenetic modifications regulate how and when our genes are turned “on” or “off” and they are made by specialized proteins that “add” or “erase” unique chemical modifications on DNA and/or histones (37). In contrast to genetic mutations, epigenetic changes are often reversible, providing an opportunity for therapeutic intervention. Our understanding of the role of epigenetics in cancer is, however, still incomplete, and continued research is needed to fulfil the real potential of the epigenome in cancer science and medicine. For example, a recent report suggested that by looking at epigenetic patterns on DNA from blood, scientists may be able to predict which individuals are at a higher risk for cancer development (38).
Comprehensive analyses of human cancer genomes, such as those carried out through The Cancer Genome Atlas, have revealed numerous cancer-causing mutations. These discoveries have led to the development of a new class of therapeutics”molecularly targeted therapeutics”which aim to rectify the cellular changes that arise due to cancer-causing mutations. While these advances have revolutionized cancer treatment, they have also brought attention to the fact that individuals of European ancestry are grossly overrepresented in most genomic investigations (39)(40). The lack of ethnic diversity in human genomic studies is limiting our understanding of cancer biology, including inherited cancer predisposition, in underrepresented populations. Rectifying this issue is an area of active research investigation.
Cancer Development: Influences outside the Cell
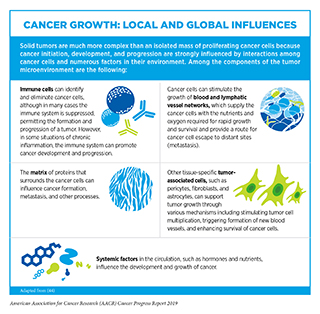
Cancer arises due to the disruption of normal cellular functions through genetic and epigenetic changes. Once cancer is initiated, complex interactions between cancer cells and their surrounding environment—known as the tumor microenvironment—contribute to disease progression.
The tumor microenvironment is a specialized niche surrounding the cancer cells in a tumor (see sidebar on Cancer Growth: Local and Global Influences). Bidirectional communications between cancer cells and their microenvironment affect tumor growth and metastasis (41)(42). Furthermore, the tumor microenvironment can shelter cancer cells from the effects of radiation, chemotherapy, and immunotherapy, thereby rendering them resistant to treatment (43). Future studies that uncover additional cellular and molecular properties of the tumor microenvironment will be vital for improving cancer diagnosis and treatment.
Cancer Development: Integrating Our Knowledge
Over the past decade, we have made significant progress in how we understand and treat the complex group of diseases we call cancer. We have learned that each person’s cancer is unique, in part because it is influenced by a patient’s biological characteristics, environmental exposures, and lifestyle. As a result, we have seen a major shift from a “one size fits all” approach to cancer prevention, screening, and treatment to a more personalized approach called precision medicine. The aim of precision medicine is to use information about a person’s genes, proteins, and environment to prevent, diagnose, and treat disease (see Figure 1).
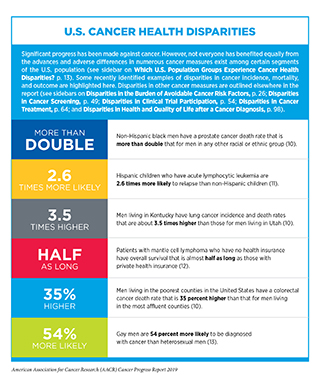
Precision medicine aims to use genetic and other information about a patient’s tumor, as well as other factors, to help diagnose, plan treatment, determine how well treatment is working, or make a prognosis, with the goal of improving clinical outcomes and minimizing unnecessary diagnostic and therapeutic interventions. Precision medicine approaches to treatment are already showing promise for certain patients with cancer (45). Nevertheless, our current knowledge of the underlying causes of cancer initiation and progression is still incomplete and ongoing research will continue to uncover additional cellular and molecular alterations that lead to cancer development. An area of primary focus is understanding the biological basis for disparities in cancer incidence and outcomes among certain segments of the U.S. population (see sidebar on U.S. Cancer Health Disparities). Concerted efforts from all stakeholders in the medical research community will be critical in order to realize the full potential of precision medicine.