Understanding the COVID-19 Pandemic
In this section you will learn:
- The global health crisis caused by the rapid spread of COVID-19 was declared a pandemic by the World Health Organization (WHO) on March 11, 2020.
- As of January 01, 2022, nearly 55 million people in the United States had been diagnosed with COVID-19, and 825,816 people had died from the disease.
- COVID-19 is caused by infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
- Older adults, males, and individuals of any age with certain underlying medical conditions, including cancer, are at an increased risk for severe COVID-19 illness.
- Individuals from racial and ethnic minorities have been disproportionately impacted by COVID-19 for many of the same reasons that they shoulder a disproportionate burden of cancer.
- Viruses constantly change through alterations in their genetic material, giving rise to new variants. Many variants of SARS-CoV-2 have emerged since the onset of the outbreak.
- COVID-19 vaccines are currently widely available for all individuals age 5 years and older. The vaccines that have been approved or authorized for use in the United States are safe and effective and reduce the risk of severe illness from COVID-19.
- The FDA has approved or authorized several treatments for the management of patients with COVID-19, and there are numerous additional agents that are being evaluated in clinical studies.
The years 2020 and 2021 will be inextricably linked to COVID-19, the disease caused by the virus SARS-CoV-2, which has taken more than 5.4 million lives worldwide and caused unimaginable damage across the globe.
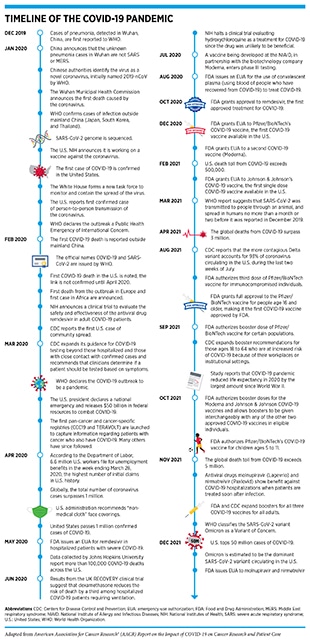
According to the World Health Organization (WHO), a disease presenting as pneumonia with unknown origins was identified at the end of 2019 in Wuhan, a city in the Hubei Province of China. In early January 2020, the Chinese Center for Disease Control and Prevention identified the underlying cause to be a novel coronavirus, the genetic sequence of which was published shortly thereafter. The International Committee on Taxonomy of Viruses termed the virus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and in February 2020, WHO designated the disease as Coronavirus Disease 2019, or COVID-19 (1)Ge H, Wang X, Yuan X, Xiao G, Wang C, Deng T, et al. The epidemiology and clinical information about COVID-19. Eur J Clin Microbiol Infect Dis 2020;39:1011–9.. Within months of its onset, COVID-19 spread around the world, and on March 11, 2020, WHO declared the ensuing global health crisis a pandemic (see sidebar on Timeline of the COVID-19 Pandemic). It is now established that COVID-19 predominantly spreads through close contact from person to person when an infected person coughs, sneezes, or talks, releasing droplets that contain the virus into the air. These droplets can enter mouths or noses of people who are nearby, or can possibly be inhaled into the lungs. Although best known as a disease of the lungs, COVID-19 can affect many organs of the body in addition to the lungs (Figure 1). In severe cases, COVID-19 causes pneumonia and acute respiratory distress syndrome (ARDS), which are associated with difficulty in breathing and low blood oxygen levels. If unchecked, ARDS can progress to respiratory failure, which is the cause of death in many fatal cases.
As of January 1, 2022, more than 289 million people worldwide have been diagnosed with COVID-19 and more than 5.4 million people have died from the disease. Beyond the personal toll, the COVID-19 pandemic has overwhelmed health care systems, devastated societal norms, and disrupted the U.S. and global economies (7)Kaye AD, Okeagu CN, Pham AD, Silva RA, Hurley JJ, Arron BL, et al. Economic impact of COVID-19 pandemic on healthcare facilities and systems: international perspectives. Best Pract Res Clin Anaesthesiol 2021;35:293–306.. The pandemic has taken an especially heavy toll on medical research and health care, including cancer research and patient care, the world over. According to a poll conducted during the early phase of the pandemic, 48 percent of U.S. adults or their family members missed medical care due to the outbreak (8)Kaiser Family Foundation. KFF Health Tracking Poll – May 2020 – Health and Economic Impacts. [updated 2020 May 27; cited 2021 Nov 27].. At the onset of the pandemic, any basic and translational research not directly related to COVID-19 was halted, while cancer researchers found ways to lend their expertise to address the pandemic. Clinical studies that are key to bringing lifesaving anticancer therapeutics to patients were also adversely affected (see Impact on Discovery Science and Clinical Studies). Although some cancer clinical trials were able to continue during the worse episodes of the pandemic and many others have resumed since then with new adaptations, the full impact of COVID-19 on cancer drug development is yet to be realized.
The AACR Report on the Impact of COVID-19 on Cancer Research and Patient Care will provide a brief overview of the underlying biology of COVID-19 and cancer, present the current evidence on the burden of COVID-19 among patients with cancer, describe the disruptions caused by the pandemic on the cancer research and care continuum including the workforce, and highlight the opportunities ahead for the cancer community that were brought into focus because of the COVID-19 pandemic.
Understanding the Biology of COVID-19 and Cancer
Evidence suggests that patients with cancer are more likely to develop severe COVID-19 and die from the disease (see Burden of COVID-19 in Patients with Cancer). In contrast, certain anticancer therapeutics have shown a protective role in patients with severe COVID-19 (see Repurposing Anticancer Agents to Treat COVID-19). This clinical overlap between COVID-19 and cancer calls for an examination of the biological mechanisms underpinning the development of these diseases. In the following sections we discuss the biology of the SARS-CoV-2 infection, COVID-19, and cancer development, and highlight the parallels between COVID-19 and cancer.
SARS-CoV-2 Infection and COVID-19
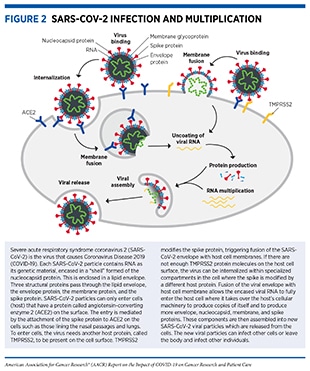
Viruses are simple microorganisms that infect cells and may cause disease. They are composed primarily of genetic material, either DNA or RNA (see sidebar on The Basis of Genetics) encased in a protein “shell” called a capsid or nucleocapsid. The capsid may or may not be enclosed in a lipid membrane called the envelope; most viruses that infect animals, including SARS-CoV-2, have this envelope. Viruses can multiply only inside infected cells. To multiply, a virus must bind to and enter an appropriate host cell, where it hijacks the host’s cellular machinery to produce additional copies of the viral genetic materials (DNA or RNA), as well as capsid and envelope proteins, which are encoded in its genetic material. The newly formed capsids are assembled around new genetic materials and transported to the host cell surface where they consolidate with new envelope proteins and exit the host cell in a process called budding (see Figure 2).
Named because of their resemblance to the solar corona, coronaviruses constitute a family of hundreds of viruses that are mostly found in birds and small mammals (e.g., bats, rodents) but have occasionally led to disease-causing infections in humans. There are seven coronaviruses, including SARS-CoV-2, that are known to infect humans; four of them cause cold-like illnesses while the other three have been responsible for the deadly global outbreaks of the respiratory illnesses severe acute respiratory syndrome (SARS) in 2002-2004, Middle East respiratory syndrome (MERS) in 2012, and COVID-19.
SARS-CoV-2 uses RNA as its genetic material and has four major structural proteins: the spike (S), nucleocapsid (N), membrane (M), and envelope (E) proteins, each of which serves multiple functions (see Figure 2) (12)Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, White KM, et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020;583:459–68.. To infect a human host, the spike protein of SARS-CoV-2 attaches to a receptor called the angiotensin-converting enzyme 2 (ACE2), which is a protein found on the surface of certain human cells in the nasal passages, lungs, and gastrointestinal tract, among others (13)Bakouny Z, Hawley JE, Choueiri TK, Peters S, Rini BI, Warner JL, et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell 2020;38:629–46.. Notably, prior to entering the host cell, the spike protein must be cleaved by an enzyme, called transmembrane serine protease 2 (TMPRSS2), which is located on the surface of the host cell (13)Bakouny Z, Hawley JE, Choueiri TK, Peters S, Rini BI, Warner JL, et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell 2020;38:629–46..
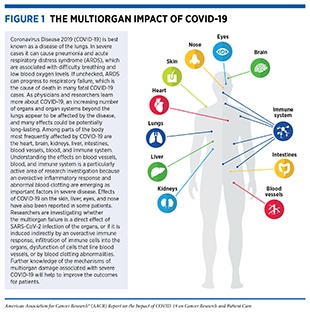
Once SARS-CoV-2 infects human cells, it makes millions of copies of itself, which can then be breathed or coughed out to infect others. Individuals can begin shedding and transmitting virus particles within two to three days of infection, often before experiencing disease symptoms (15)Moghadas SM, Fitzpatrick MC, Sah P, Pandey A, Shoukat A, Singer BH, et al. The implications of silent transmission for the control of COVID-19 outbreaks. Proc Natl Acad Sci U S A 2020;117:17513–5.(16)Furukawa NW, Brooks JT, Sobel J. Evidence supporting transmission of severe acute respiratory syndrome coronavirus 2 while presymptomatic or asymptomatic. Emerg Infect Dis 2020;26:e201595.. For most individuals, it takes about four to five days after infection with SARS-CoV-2 for symptoms to appear, but for others it may take up to two weeks. Among the most reported symptoms of COVID-19 are fever and a dry cough, fatigue, muscle pain/body aches, difficulty breathing, and loss of taste and/or smell. As the disease progresses, moving from the upper to lower respiratory tract and throughout the body, there are reports of damage to nearly every organ and system in the body (see Figure 1) (4)Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med 2020;26:1017–32.(5)Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID-19 and multiorgan failure: a narrative review on potential mechanisms. J Mol Histol 2020;51:613–28.(17)Wadman M, Couzin-Frankel J, Kaiser J, Matacic C. A rampage through the body. Science 2020;368:356–60.. Any organ that expresses the ACE2 receptor protein, such as heart muscles, kidneys, blood vessels, liver, and the central nervous system, is vulnerable to attack. This may explain the vast array of symptoms that are experienced by COVID-19 patients. An area of ongoing research is to identify the exact mechanisms by which SARS-CoV-2 gets around inside the body; it is not yet clear whether it travels through blood or through infection of the cells that form the blood vessels. Researchers are also investigating whether the multiorgan effects of SARS-CoV-2 are induced indirectly, because of increased levels of inflammatory chemicals in the blood, endothelial cell dysfunction, blood clotting abnormalities, or infiltration of inflammatory immune cells into the organs (5)Mokhtari T, Hassani F, Ghaffari N, Ebrahimi B, Yarahmadi A, Hassanzadeh G. COVID-19 and multiorgan failure: a narrative review on potential mechanisms. J Mol Histol 2020;51:613–28.. Notably, many COVID-19 patients experience long-term adverse health effects, a condition referred to as long COVID, six months or more after diagnosis, including neurocognitive, gastrointestinal, and cardiovascular symptoms, among others (18)Taquet M, Dercon Q, Luciano S, Geddes JR, Husain M, Harrison PJ. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med 2021;18:e1003773.(19)Groff D, Sun A, Ssentongo AE, Ba DM, Parsons N, Poudel GR, et al. Short-term and long-term rates of postacute sequelae of SARS-CoV-2 infection: a systematic review. JAMA Netw Open 2021;4:e2128568..
Immune Response to COVID-19
The two arms of the immune system—innate and adaptive—that make up our body’s natural defense against infection as well as cancer play a critical role in defending us from viral and other pathogenic infections. Immediately after infection, mediators of innate immune response, for example, chemicals known as interferons, are activated to limit viral multiplication and to signal the adaptive arm of immunity through mobilization of white blood cells, including B and T cells (see sidebar on Key Cells in the Immune System). T cells are crucial in controlling primary infection by killing virus-infected cells. Activated B cells release antibodies that can attach to specific proteins on viruses, bacteria, and other disease-causing pathogens and prevent further infection of healthy cells. Among the different antibodies that are produced in response to a pathogen such as SARS-CoV-2, neutralizing antibodies are especially important since they bind to the virus and interfere with its ability to infect a cell. Notably, for an effective antiviral immune response, it is essential that B and T cells work in concert to destroy the virus-infected cells and neutralize the circulating viral particles.
Research has shown that once SARS-CoV-2 infects the cells of the airway in the host’s lungs, it may cause massive destruction of the affected tissues. This occurs because the replication and release of SARS-CoV-2 triggers the host cells to undergo a form of cell death called pyroptosis. During pyroptosis, the damaged or dying cell releases the viral RNA and other intracellular debris, which triggers neighboring cells to produce inflammatory chemicals called cytokines and chemokines that are released into the blood of the afflicted patients. Secretion of cytokines and chemokines attracts immune cells from the blood to the site of infection (21)Bhardwaj A, Sapra L, Saini C, Azam Z, Mishra PK, Verma B, et al. COVID-19: Immunology, immunopathogenesis and potential therapies. Int Rev Immunol 2021 Feb 27 [Epub ahead of print].. In most patients, this initial immune response is enough to kill the virus and clear the infection in the lungs. However, some COVID-19 patients respond with an abnormal immune response, leading to a phenomenon known as the cytokine release syndrome (CRS) (22)Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020;20:363–74.. CRS is characterized by high levels of inflammatory chemicals such as interleukin (IL)-6, tumor necrosis factor (TNF)-α, and others in the blood and causes severe systemic inflammation (23)Zong Z, Wei Y, Ren J, Zhang L, Zhou F. The intersection of COVID-19 and cancer: signaling pathways and treatment implications. Mol Cancer 2021;20:76.(24)Iovino L, Thur LA, Gnjatic S, Chapuis A, Milano F, Hill JA. Shared inflammatory pathways and therapeutic strategies in COVID-19 and cancer immunotherapy. J Immunother Cancer 2021;9:e002392.. Inflammation of the lungs can progress to acute respiratory distress syndrome (ARDS), causing difficulty breathing and low blood oxygen levels. If unchecked, ARDS can progress to respiratory failure, which is the cause of death in many fatal COVID-19 cases (22)Tay MZ, Poh CM, Renia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol 2020;20:363–74.. In addition, uncontrolled CRS can lead to failure of other organs, most notably the heart, liver, and kidneys. Another explanation for the widespread organ damage from severe COVID-19 is abnormal blood clots frequently observed in some patients (25)Douillet D, Riou J, Penaloza A, Moumneh T, Soulie C, Savary D, et al. Risk of symptomatic venous thromboembolism in mild and moderate COVID-19: a comparison of two prospective European cohorts. Thromb Res 2021;208:4–10.. This may arise from SARS-CoV-2 infection and damage of the endothelial cells—cells lining the blood vessels—and/or the inflammatory response due to the abnormal activation of the immune system (26)Hampton T. Autoantibodies may drive COVID-19 blood clots. JAMA 2021;325:425.. Beyond abnormal blood clotting events, COVID-19 is also associated with the formation of new and abnormal blood vessels, a phenomenon called pathological angiogenesis (27)Norooznezhad AH, Mansouri K. Endothelial cell dysfunction, coagulation, and angiogenesis in coronavirus disease 2019 (COVID-19). Microvasc Res 2021;137:104188.(28)Smadja DM, Mentzer SJ, Fontenay M, Laffan MA, Ackermann M, Helms J, et al. COVID-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects. Angiogenesis 2021;24:755–88..
Since the onset of COVID-19, many research studies have characterized the abnormal immune activation that plays a major role in the widespread damage in patients with severe COVID-19 (29)Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020;369:eabc8511.(30)Kuri-Cervantes L, Pampena MB, Meng W, Rosenfeld AM, Ittner CAG, Weisman AR, et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol 2020;5:eabd7114.. These reports highlight significant dysregulation in multiple components of the immune system. For instance, multiple reports have linked the severity of COVID-19 to functional errors in interferons—chemicals that, as part of the innate immune system, are released by virus-infected cells, and have the capability of limiting viral infection (31)Carvalho T, Krammer F, Iwasaki A. The first 12 months of COVID-19: a timeline of immunological insights. Nat Rev Immunol 2021;21:245–56.. There are indications that high levels of viral multiplication during the initial phase of COVID-19 can impair an individual’s ability to mount a coordinated and adequate immune response. In fact, patients with severe COVID-19 display significant reduction in the numbers of many types of immune cells (e.g., CD4+ and CD8+ T lymphocytes and dendritic cells) in the blood (see sidebar on Key Cells in the Immune System)(32)Winheim E, Rinke L, Lutz K, Reischer A, Leutbecher A, Wolfram L, et al. Impaired function and delayed regeneration of dendritic cells in COVID-19. PLoS Pathog 2021;17:e1009742.. Additionally, researchers have noted abnormal activation of T cells as well as expression of cell-surface markers that are indicative of T cell dysfunction in patients with severe COVID-19 (REF). There are also striking anomalies in the activation pattern of B cells in patients with severe disease (29)Mathew D, Giles JR, Baxter AE, Oldridge DA, Greenplate AR, Wu JE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science 2020;369:eabc8511.(33)Kaneko N, Kuo HH, Boucau J, Farmer JR, Allard-Chamard H, Mahajan VS, et al. Loss of Bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell 2020;183:143–57.(34)Woodruff MC, Ramonell RP, Nguyen DC, Cashman KS, Saini AS, Haddad NS, et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol 2020;21:1506–16..
While most children do not exhibit serious symptoms from COVID-19, some may develop an inflammatory syndrome following infection with SARS-CoV-2. Multisystem inflammatory syndrome of children, or MIS-C, as the condition is termed, is marked by severe inflammation of one or more parts of the body, including the heart, lungs, kidneys, brain, skin, eyes, and gastrointestinal system, and can be life-threatening (31)Carvalho T, Krammer F, Iwasaki A. The first 12 months of COVID-19: a timeline of immunological insights. Nat Rev Immunol 2021;21:245–56.. Ongoing research is investigating the immunological underpinnings of MIS-C as well as the potential long-term impacts on health of the infected children (35)Vella LA, Giles JR, Baxter AE, Oldridge DA, Diorio C, Kuri-Cervantes L, et al. Deep immune profiling of MIS-C demonstrates marked but transient immune activation compared to adult and pediatric COVID-19. Sci Immunol 2021;6:eabf7570.(36)Anderson EM, Diorio C, Goodwin EC, McNerney KO, Weirick ME, Gouma S, et al. Severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) antibody responses in children with multisystem inflammatory syndrome in children (MIS-C) and mild and severe coronavi(37)NIH. NIH effort seeks to understand MIS-C, range of SARS-CoV-2 effects on children. [updated 2021 Mar 2; cited 2021 Nov 28]..
Biology of Cancer Development
Cancer is a collection of diseases that arise when the processes that control normal cell growth, division, and life span go awry. As a result, cells start to multiply uncontrollably, fail to die, acquire unique ways to obtain nutrients for survival, and begin to accumulate. In body organs and tissues, the accumulating cancer cells form masses called tumors, whereas in the blood or bone marrow they crowd out normal cells. Notably, cancer cells have unique mechanisms to escape the immune system, which normally eliminates damaged or abnormal cells. In fact, some cancer cells convince immune cells to protect the tumor instead of attacking it. Over time, certain tumor cells may invade distant tissues, a process termed metastasis, by entering the bloodstream or the lymphatic network, and form secondary tumors at remote sites.
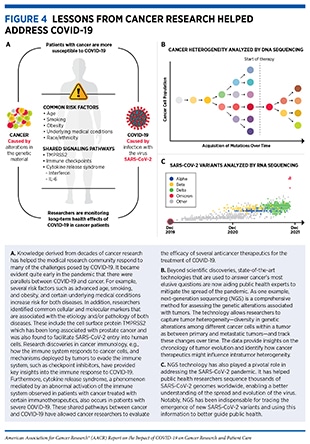
Alterations in the normal DNA sequence, referred to as mutations, can disrupt normal protein function, and are the leading cause of cancer development (see sidebar on Genetic Mutations). Each person’s cancer has a unique combination of mutations, and as cancer cells divide, new mutations arise in the daughter cells. Thus, a tumor is made up of a collection of cancer cells with a wide range of genetic abnormalities. This variation in cell types, known as heterogeneity (see Figure 4), is an important part of a cancer’s characteristics and fuels the cancer’s ability to grow faster, escape therapy, evade the immune system, and metastasize to other organs.
Inherited genetic mutations play a role in about 10 percent of all cancer cases; however, most mutations are acquired over an individual’s lifetime due to errors arising during normal cell multiplication or because of environmental exposures, lifestyle factors, or underlying health conditions (38)Sengupta R, Zaidi SK. AACR Cancer Progress Report 2021: discovery science driving clinical breakthroughs. Clin Cancer Res 2021;27:5757–9.. While cancers are initiated due to the disruption of normal cellular functions through genetic and epigenetic changes, complex interactions between cancer cells and their surrounding environment—known as the tumor microenvironment—contribute to disease progression. The tumor microenvironment is a specialized niche surrounding the cancer cells and consists of immune cells—components of one’s natural defense mechanism—as well as other cellular and molecular elements. Bidirectional communications between cancer cells and their microenvironment affect tumor growth and metastasis.
Intersection of COVID-19 and Cancer Biology
It is well documented that patients with cancer are more susceptible to SARS-CoV-2 infection and have a higher probability of severe disease, including mortality, from COVID-19 (see Burden of COVID-19 in Patients with Cancer). This may be due to the fact that certain cancer patients have a compromised immune system attributable to their disease and/or the treatments they receive (see sidebar on Why Are Cancer Patients at an Increased Risk of Infection?). The clinical association between the two diseases has led researchers to investigate the biological links between SARS-CoV-2 infection/COVID-19 and cancer, and multiple lines of evidence have emerged thus far (13)Bakouny Z, Hawley JE, Choueiri TK, Peters S, Rini BI, Warner JL, et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell 2020;38:629–46.(23)Zong Z, Wei Y, Ren J, Zhang L, Zhou F. The intersection of COVID-19 and cancer: signaling pathways and treatment implications. Mol Cancer 2021;20:76..
Studies have shown that the expression of ACE2 protein— the receptor for SARS-CoV-2—in human lung tissue increases with age. Since cancer diagnosis is most common among those age 65 and older, it is possible that the abundance of lung ACE2 contributes to severe COVID-19 among patients with cancer (39)Sinha S, Kundu CN. Cancer and COVID-19: why are cancer patients more susceptible to COVID-19? Med Oncol 2021;38:101.. In addition, ACE2 levels are also shown to be elevated in the lungs of individuals who are regular smokers, including those who suffer from smoking-related lung diseases such as chronic obstructive pulmonary disease (COPD) (13)Bakouny Z, Hawley JE, Choueiri TK, Peters S, Rini BI, Warner JL, et al. COVID-19 and cancer: current challenges and perspectives. Cancer Cell 2020;38:629–46.(39)Sinha S, Kundu CN. Cancer and COVID-19: why are cancer patients more susceptible to COVID-19? Med Oncol 2021;38:101.. This may explain why smokers, or patients with smoking-related COPD or lung cancer, are especially prone to adverse outcomes from COVID-19.
Insights into a second biological link between COVID-19 and cancer have been derived from research in prostate cancer. The enzyme TMPRSS2 that cleaves SARS-CoV-2 spike protein and facilitates viral entry in human cells has been studied extensively in the context of prostate cancer. TMPRSS2 is one of the most frequently altered genes in prostate cancer (40)Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID-19: serendipity or opportunity for intervention? Cancer Discov 2020;10:779–82.. TMPRSS2 protein is present in high levels in prostate cancer cells. Interestingly, the level of TMPRSS2 in the prostate is regulated by androgen, a hormone that regulates the development and maintenance of male characteristics. Ongoing research is investigating whether the level of TMPRSS2 in the lungs is also regulated by androgen, and if so, whether that explains the higher burden of COVID-19 severity in men (40)Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID-19: serendipity or opportunity for intervention? Cancer Discov 2020;10:779–82.. Clinical researchers are evaluating whether therapeutics that work by lowering TMPRSS2 levels or by inhibiting its function can mitigate the symptoms of COVID-19 (23)Zong Z, Wei Y, Ren J, Zhang L, Zhou F. The intersection of COVID-19 and cancer: signaling pathways and treatment implications. Mol Cancer 2021;20:76.(41)Chakravarty D, Nair SS, Hammouda N, Ratnani P, Gharib Y, Wagaskar V, et al. Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer. Commun Biol 2020;3:374..
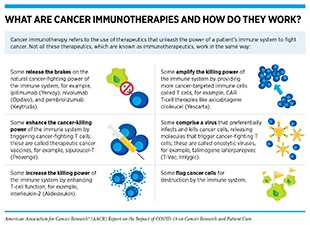
Important insights into the shared molecular mechanisms between cancer and COVID-19 have also been obtained from studies that have investigated the role of the immune system in the pathogenesis of both diseases. Both SARS-CoV-2-infected cells and cancer cells express proteins that can mark them for destruction by the immune system. Unfortunately, as research shows, both cancer cells and SARS-CoV-2 can evade the immune system by suppressing the host’s defense mechanisms, e.g., by lowering levels of major histocompatibility complex (MHC)—proteins that are critical for flagging virus-infected cells or cancer cells for recognition and elimination by immune cells (23,24,42). Another mechanism of immune suppression utilized by both cancer and viral infection is “T-cell exhaustion”, a phenomenon which leads to T-cell inactivation. This can occur during persistent stimulation of T cells due to chronic inflammation (often associated with cancer) or long-term infection. T-cell exhaustion is linked to adverse outcomes for patients with cancer or COVID-19. In addition, cytokine release syndrome (CRS)—the uncontrolled production of inflammatory cytokines and chemokines—a serious life-threating condition seen in patients with severe COVID-19 is also a known adverse event experienced by certain cancer patients treated with chimeric antigen receptor (CAR) T-cell therapy (see sidebar on What Are Cancer Immunotherapies and How Do They Work?)(43). In fact, researchers have identified common biomarkers, e.g., IL-6, associated with both COVID-19 and CAR T-cell therapy-induced CRS (see Figure 4) (23,24,42). These parallels between the mechanisms of CRS in cancer and COVID-19 have also prompted researchers to evaluate immunotherapy-inspired agents to mitigate the cytokine release in COVID-19. Anticancer agents that target IL-6 or its downstream effector Janus kinase (JAK) are being tested in numerous clinical studies for their ability to reduce the CRS and ARDS associated with COVID-19 (23)Zong Z, Wei Y, Ren J, Zhang L, Zhou F. The intersection of COVID-19 and cancer: signaling pathways and treatment implications. Mol Cancer 2021;20:76.(24)Iovino L, Thur LA, Gnjatic S, Chapuis A, Milano F, Hill JA. Shared inflammatory pathways and therapeutic strategies in COVID-19 and cancer immunotherapy. J Immunother Cancer 2021;9:e002392.(42)Derosa L, Melenotte C, Griscelli F, Gachot B, Marabelle A, Kroemer G, et al. The immuno-oncological challenge of COVID-19. Nat Cancer 2020;1:946–64..
COVID-19 is associated with the formation of new blood vessels in the patient’s lungs, a phenomenon known as pathological angiogenesis that is a hallmark of developing tumors. In fact, recent findings suggest that several proteins that are associated with tumor angiogenesis, such as VEGF, HIF, etc., may be also elevated in the plasma or lungs of patients with COVID-19 (27)Norooznezhad AH, Mansouri K. Endothelial cell dysfunction, coagulation, and angiogenesis in coronavirus disease 2019 (COVID-19). Microvasc Res 2021;137:104188.(28)Smadja DM, Mentzer SJ, Fontenay M, Laffan MA, Ackermann M, Helms J, et al. COVID-19 is a systemic vascular hemopathy: insight for mechanistic and clinical aspects. Angiogenesis 2021;24:755–88.. These data have led to the ongoing evaluation of antiangiogenic cancer therapeutics in the treatment of COVID-19.
State of the COVID-19 Pandemic
As of January 01, 2022, 289,225,595 people worldwide have been diagnosed with COVID-19, and 5,440,035 people have died from the disease (44)Johns Hopkins Coronavirus Resource Center. COVID-19 Map. [updated 2022 Jan 13; cited 2021 Dec 15].. The United States accounts for 19 percent of the recorded cases and more than 15 percent of recorded deaths from COVID-19 (44)Johns Hopkins Coronavirus Resource Center. COVID-19 Map. [updated 2022 Jan 13; cited 2021 Dec 15].. Since the onset of the pandemic, the United States has experienced several surges and ebbs in the outbreak of infections and deaths. The pandemic has caused unprecedented disruptions to the society, economy, and public health, worldwide. According to a recent analysis, COVID-19 deaths have led to a decline in life expectancy at birth—a widely used metric of population health and longevity—between 2019 to 2020, in 27 out of 29 countries included in the study (45)Aburto JM, Scholey J, Kashnitsky I, Zhang L, Rahal C, Missov TI, et al. Quantifying impacts of the COVID-19 pandemic through life-expectancy losses: a population-level study of 29 countries. Int J Epidemiol 2021 Sep 26 [Epub ahead of print].; according to these data, U.S. males experienced the largest losses (2.2 years) in life expectancy at birth during 2020. As with the burden of cancer, racial and ethnic minorities and other underserved populations have shouldered a disproportionate burden of COVID-19 (see Disparities in the Burden of COVID-19 Among Certain U.S. Populations). In fact, it is estimated that the reductions of life expectancy for Black and Hispanic populations are 3 to 4 times higher than the reductions for whites (46)Andrasfay T, Goldman N. Reductions in 2020 US life expectancy due to COVID-19 and the disproportionate impact on the Black and Latino populations. Proc Natl Acad Sci U S A 2021;118:e2014746118..
Since the onset of the SARS-CoV-2 outbreak, researchers across the globe have been tracking the progression of the pandemic in terms of disease epidemiology in populations as well as the evolution of the virus with time. Through genomic sequencing and epidemiological analysis, they have observed that, like other viruses, SARS-CoV-2 has been changing constantly through mutations in its genetic material (see sidebar on Genetic Mutations). Of note, genetic mutations happen frequently during the replication of the virus, but only sometimes change the functional characteristics of the virus. Over time, mutations that do affect viral characteristics lead to the emergence of new forms or “variants” of the virus, often with different transmissibility. Scientists use a technology known as genomic sequencing to characterize SARS-CoV-2 genetic materials, monitor how it changes over time giving rise to new variants, understand how these changes may affect the properties of the virus, and use this information to predict how these new variants might impact public health.
Variants with distinct genetic alterations have emerged across the globe throughout the pandemic that were first noticed in the U.K. (Alpha variant), South Africa (Beta and Omicron variants), Brazil (Gamma variant), and India (Delta variant) (see sidebar on SARS-CoV-2 Variants). While these variants shared some of the same genetic mutations, they all emerged independently. The Delta variant, which was first detected in India ignited new surges of COVID-19 infections across the globe including in the United States (47)World Health Organization. COVID-19 weekly epidemiological update [updated 2022 Jan 11; cited 2021 Nov 27].. The Delta variant was found to be far more transmissible than any other variant of SARS-CoV-2 identified thus far and was the most dominant strain in the United States until mid-December 2021 (48)Zhang J, Xiao T, Cai Y, Lavine CL, Peng H, Zhu H, et al. Membrane fusion and immune evasion by the spike protein of SARS-CoV-2 Delta variant. Science 2021;374:1353–60.(49)Centers for Disease Control and Prevention. COVID data tracker [updated 2020 Mar 28; cited 2021 Nov 27].. Despite the higher transmissibility, recent data are somewhat encouraging and show that the rates of hospitalization and severe disease were not different during the time period when the Delta variant was the predominant strain in the U.S. compared to the earlier periods in the pandemic (50)Taylor CA, Patel K, Pham H, Whitaker M, Anglin O, Kambhampati AK, et al. Severity of disease among adults hospitalized with laboratory-confirmed COVID-19 before and during the period of SARS-CoV-2 B.1.617.2 (Delta) predominance – COVID-NET, 14 States, Jan.
In November 2021, a new variant associated with a concerning rise of COVID-19 cases was first detected in Botswana followed by South Africa and reported to the WHO by public health officials in South Africa (51)Callaway E. Heavily mutated Omicron variant puts scientists on alert. Nature 2021;600:21.(52)World Health Organization. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern [updated 2021 Nov 26; cited 2021 Dec 19].(53)Centers for Disease Control and Prevention. Omicron variant: what you need to know. [updated 2021 Dec 19; cited 2021 Dec 19].. The variant, named Omicron, has since been detected in several additional countries and as of January 1, 2022 is the predominant strain in the United States (54)Centers for Disease Control and Prevention. SARS-CoV-2 B.1.1.529 (Omicron) variant — United States, December 1–8, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1731–4.. Omicron has a variety of mutations, including over 30 in the SARS-CoV-2 spike protein alone, and has raised concerns among scientists since some of these mutations are known to be associated with reduced response to available therapeutics (55)Centers for Disease Control and Prevention. Science brief: Omicron (B.1.1.529) variant [updated 2021 Dec 7; cited 2021 Dec 15].. While early studies indicate that Omicron may spread more easily but produce less severe disease than previous variants, researchers are gathering evidence to answer definitively whether Omicron is more transmissible than Delta and other variants that were prevalent earlier during the pandemic, whether the combination of mutations allows Omicron to evade immune protection, including the protection generated by prior infection or vaccines, and whether available therapeutics are effective against patients with COVID-19 who are infected with Omicron (51)Callaway E. Heavily mutated Omicron variant puts scientists on alert. Nature 2021;600:21.(56)Cathcart AL, Havenar-Daughton C, Lempp FA, Ma D, Schmid MA, Agostini ML, et al. The dual function monoclonal antibodies VIR-7831 and VIR-7832 demonstrate potent in vitro and in vivo activity against SARS-CoV-2. bioRxiv 2021 Dec 15 [Epub ahead of print].(57)Dejnirattisai W, Shaw RH, Supasa P, Liu C, Stuart AS, Pollard AJ, et al. Reduced neutralisation of SARS-COV-2 Omicron-B.1.1.529 variant by post-immunisation serum. Lancet 2021;399:234–36.(58)Wilhelm A, Widera M, Grikscheit K, Toptan T, Schenk B, Pallas C, et al. Reduced neutralization of SARS-CoV-2 Omicron variant by vaccine sera and monoclonal antibodies. Cell 2021;184:2201–11.(59)Pulliam JRC, van Schalkwyk C, Govender N, von Gottberg A, Cohen C, Groome MJ, et al. Increased risk of SARS-CoV-2 reinfection associated with emergence of the Omicron variant in South Africa. medRxiv 2021 Dec 2 [Epub ahead of print].(60)Christie B. Covid-19: early studies give hope Omicron is milder than other variants. BMJ 2021;375:n3144.(61)Abdullah F, Myers J, Basu D, Tintinger G, Ueckermann V, Mathebula M, et al. Decreased severity of disease during the first global Omicron variant covid-19 outbreak in a large hospital in Tshwane, South Africa. Int J Infect Dis 2021;116:38–42.(62)Centers for Disease Control and Prevention. Omicron variant: what you need to know [updated 2022 Jan 5; cited 2022 Jan 1]..
Public health experts at the CDC and worldwide are studying each SARS-CoV-2 variant to understand its differences, to monitor or predict whether a variant is more dangerous than others, and to track the spread of a variant. Experts at the CDC classify certain variants as Variants Being Monitored (VBM), Variants of Concern (VOC), Variants of Interest (VOI), or Variants of High Consequence (VOHC), based on how easily they spread, how severe their symptoms are, and how they can be treated (63)Centers for Disease Control and Prevention. SARS-CoV-2 variant classifications and definitions [updated 2021 Nov 16; cited 2021 Nov 27]. (see sidebar on SARS-CoV-2 Variants).
Disparities in the Burden of COVID-19 Among Certain U.S. Populations
In the United States, which accounts for one out of five of all recorded cases of COVID-19 globally (44)Johns Hopkins Coronavirus Resource Center. COVID-19 Map. [updated 2022 Jan 13; cited 2021 Dec 15]., a disproportionate burden of the disease has fallen on racial and ethnic minorities (see sidebar on U.S. Racial and Ethnic Population Groups and Inequities in the Burden of COVID-19 in the United States) and other medically underserved populations (65)Sengupta R, Honey K. AACR Cancer Disparities Progress Report 2020: achieving the bold vision of health equity for racial and ethnic minorities and other underserved populations. Cancer Epidemiol Biomarkers Prev 2020;29:1843..
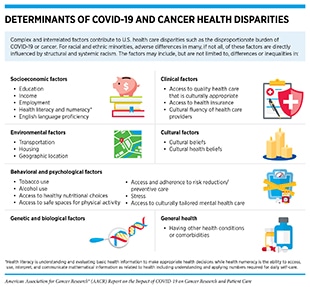
Researchers have been actively investigating the factors that have contributed to the disproportionate burden of COVID-19 among racial and ethnic minorities and other underserved populations. Most evidence suggests that there are several complex and interrelated causes, many of which overlap with the factors that contribute to cancer health disparities (65)Sengupta R, Honey K. AACR Cancer Disparities Progress Report 2020: achieving the bold vision of health equity for racial and ethnic minorities and other underserved populations. Cancer Epidemiol Biomarkers Prev 2020;29:1843. (see sidebar on Determinants of COVID-19 and Cancer Health Disparities).
Social determinants of health, defined by NCI as the conditions in which people are born, grow, live, work, and age, play a significant role in driving cancer health disparities and have emerged as some of the most important factors contributing to the stark inequities in the burden of COVID-19 (62)Centers for Disease Control and Prevention. Omicron variant: what you need to know [updated 2022 Jan 5; cited 2022 Jan 1].(72)Centers for Disease Control and Prevention. Health equity considerations and racial and ethnic minority groups [updated 2021 Aug 20; cited 2021 Nov 27].(73)Carethers JM. Insights into disparities observed with COVID-19. J Intern Med 2021;289:463–73.(74)Miller S, Wherry LR, Mazumder B. Estimated mortality increases during the COVID-19 pandemic by socioeconomic status, race, and ethnicity. Health Aff (Millwood) 2021;40:1252–60.. For U.S. racial and ethnic minorities, adverse differences in many, if not all, of these determinants are directly influenced by decades of structural and systemic racism. During the past two years of the pandemic, these inequities have drawn the renewed attention of public health and policy experts. For instance, it was clear that individuals who belong to racial and ethnic minorities were more likely to live in conditions that posed challenges for social distancing, which was one of the main preventive strategies for reducing infection with SARS-CoV-2 at the initial phase of the pandemic. For economic reasons, they are more likely to live in lower-income apartment complexes, with higher numbers of occupants per unit, and more likely to live in multigenerational family units. The same population groups are also more likely to work in occupations considered essential for society to function—such as staffing grocery stores, hospitals, nursing homes, building maintenance, forms of transportation, and delivery services—which increases their chances of being exposed to SARS-CoV-2 because they are unable to shelter at home (65)Sengupta R, Honey K. AACR Cancer Disparities Progress Report 2020: achieving the bold vision of health equity for racial and ethnic minorities and other underserved populations. Cancer Epidemiol Biomarkers Prev 2020;29:1843..
Another important factor contributing to racial and ethnic COVID-19 disparities is that many people from racial and ethnic minority groups are more likely to have one or more of the health conditions associated with an increase in a person’s chance of severe COVID-19 compared with whites (see Increasing Risk for COVID-19). Inequity in access to quality health care is another key factor contributing to the higher levels of underlying health conditions that increase the risk of severe COVID-19 among people in racial and ethnic minority groups and have contributed to the higher COVID-19 burden among these populations (75)Magesh S, John D, Li WT, Li Y, Mattingly-App A, Jain S, et al. Disparities in COVID-19 outcomes by race, ethnicity, and socioeconomic status: a systematic-review and meta-analysis. JAMA Netw Open 2021;4:e2134147.. Notably, according to a recent study, Blacks and Hispanics even from the least socioeconomically disadvantaged groups experienced higher all-cause mortality increases due to the COVID-19 pandemic compared to whites (74)Miller S, Wherry LR, Mazumder B. Estimated mortality increases during the COVID-19 pandemic by socioeconomic status, race, and ethnicity. Health Aff (Millwood) 2021;40:1252–60.. Researchers are actively investigating whether there are biological factors contributing to racial and ethnic COVID-19 disparities (76)Saini G, Swahn MH, Aneja R. Disentangling the coronavirus disease 2019 health disparities in African Americans: biological, environmental, and social factors. Open Forum Infect Dis 2021;8:ofab064.(77)Webb Hooper M, Napoles AM, Perez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA 2020;323:2466–7.. There is deep concern that undocumented immigrants have also experienced adverse differences in COVID-19 measures, in large part because of a lack of access to quality health care (78)Horner KM, Wrigley-Field E, Leider JP. A first look: disparities in COVID-19 mortality among US-born and foreign-born Minnesota residents. Popul Res Policy Rev 2021 Aug 2 [Epub ahead of print].(79)IDSA and HIVMA. COVID-19 policy brief: disparities among immigrant populations in the United States [updated 2020 Sep 10; cited 2021 Nov 27]..
Measuring the true impact of COVID-19 on racial and ethnic minorities and other medically underserved populations will require collection and reporting of high-quality, accurate information on race, ethnicity, and the relevant social determinants of health. Unfortunately, early in the pandemic, many state health departments experienced challenges in collecting complete or accurately classified race and ethnicity data (80)Ossom-Williamson P, Williams IM, Kim K, Kindratt TB. Reporting and availability of COVID-19 demographic data by US Health Departments (April to October 2020): observational study. JMIR Public Health Surveill 2021;7:e24288.. Granularity in demographic data on COVID-19 testing, cases, vaccination, hospitalizations, and deaths at a county and zip code level will allow better assessment of the impact of COVID-19, enable effective health care interventions in underserved communities, and drive public health policy. In this regard, the NIH has initiated the Rapid Acceleration of Diagnostics-Underserved Populations (RADx-UP) program to understand the factors associated with disparities in COVID-19 morbidity and mortality and to lay the foundation to reduce disparities for those underserved populations who are disproportionately affected by the COVID-19 pandemic (81)NIH. RADx programs [updated 2021 Nov 16; cited 2021 Nov 27]..
Increasing Risk for COVID-19
The presentation of disease experienced by individuals who are infected with SARS-CoV-2 covers a wide spectrum, from no symptoms to mild disease, to severe disease, to critical disease and even death. Advanced age (65 and older), sex (male), and having certain chronic health conditions, such as cancer; chronic kidney disease; chronic liver disease; chronic lung diseases, e.g., chronic obstructive pulmonary disease (COPD); dementia or other neurological conditions; diabetes; Down syndrome; heart conditions; HIV infection; immunocompromised state; mental health conditions; sickle cell disease or thalassemia; stroke or cerebrovascular disease; substance use disorders; and tuberculosis increase a person’s risk of severe COVID-19 (82)Centers for Disease Control and Prevention. People with certain medical conditions [updated 2021 Nov 24; cited 2021 Nov 27].. Solid organ transplant or blood stem cell transplant and pregnancy have also been linked to an increased risk of severe COVID-19 (82)Centers for Disease Control and Prevention. People with certain medical conditions [updated 2021 Nov 24; cited 2021 Nov 27]..
According to a study published early in the pandemic, patients with COVID-19 who had underlying chronic health conditions are six times more likely to be hospitalized and 12 times more likely to die compared to those who had no underlying chronic health conditions (83)Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, et al. Coronavirus disease 2019 case surveillance – United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:759–65.. However, as the pandemic has progressed and newer and more transmissible variants of SARS-CoV-2 have emerged, it has become clear that healthy patients of any age and sex can develop severe disease (49)Centers for Disease Control and Prevention. COVID data tracker [updated 2020 Mar 28; cited 2021 Nov 27].. It should be noted that many of the conditions that increase an individual’s risk for severe COVID-19 are also causally linked with cancer. For example, the U.S. population group that accounts for 55 percent of new cancer diagnoses—individuals age 65 and older— is also at high risk for severe COVID-19 and has the highest death rate compared to its proportion in the general population (49)Centers for Disease Control and Prevention. COVID data tracker [updated 2020 Mar 28; cited 2021 Nov 27].(84)NCI. Surveillance, Epidemiology, and End Results Program [updated 2022 Jan 8; cited 2021 Nov 29]..
Beyond underlying health conditions, modifiable risk factors such as obesity and smoking, which are also linked to cancer development, have been shown to increase the risk for severe COVID-19 (82)Centers for Disease Control and Prevention. People with certain medical conditions [updated 2021 Nov 24; cited 2021 Nov 27].. Therefore, it is concerning that during the COVID-19 pandemic (between 2019 and 2020), prevalence of obesity increased among youth and adults (85)Wu AJ, Aris IM, Hivert MF, Rocchio C, Cocoros NM, Klompas M, et al. Association of changes in obesity prevalence with the COVID-19 pandemic in youth in Massachusetts. JAMA Pediatr 2021 Dec 13 [Epub ahead of print].(86)Centers for Disease Control and Prevention. Obesity, race/ethnicity, and COVID-19 [updated 2021 Nov 12; cited 2021 Nov 27]. and, according to the most recent Federal Trade Commission Cigarette Report, so did the annual cigarette sales for the first time in 20 years (87)Federal Trade Commission. Cigarette report for 2020 [updated 2021 Jan 30; cited 2021 Nov 27].. These worrying trends underscore the urgent need for new strategies to enhance the dissemination and implementation of our current knowledge of healthy living, disease prevention, and modifiable behavioral risk factors.
Researchers are also investigating the role of genetic factors that are associated with a person’s chances of becoming severely ill with COVID-19 (91)Callaway E. The quest to find genes that drive severe COVID. Nature 2021;595:346–8.. Concerted efforts from academic laboratories and the private sector have provided valuable insights into the genetic underpinnings of infection and severe disease (92)Zeberg H, Paabo S. The major genetic risk factor for severe COVID-19 is inherited from Neanderthals. Nature 2020;587:610–2.(93)COVID-19 Host Genetics Initiative. Mapping the human genetic architecture of COVID-19. Nature 2021;600:472–7.(94)Carapito R, Li R, Helms J, Carapito C, Gujja S, Rolli V, et al. Identification of driver genes for critical forms of COVID-19 in a deeply phenotyped young patient cohort. Sci Transl Med 2021;14:eabj7521(95)Wickenhagen A, Sugrue E, Lytras S, Kuchi S, Noerenberg M, Turnbull ML, et al. A prenylated dsRNA sensor protects against severe COVID-19. Science 2021;374:eabj3624. These studies have uncovered several genetic alterations associated with severe COVID-19. Among the genes identified are those that boost our defense against viral infections, such as members of the interferon pathway, as well as novel genes of currently unknown significance (96)Kaiser J. Found: genes that sway the course of the coronavirus. Science 2020;370:275–6.(97)Downes DJ, Cross AR, Hua P, Roberts N, Schwessinger R, Cutler AJ, et al. Identification of LZTFL1 as a candidate effector gene at a COVID-19 risk locus. Nat Genet 2021;53:1606–15.(98)Severe Covid-19 GWAS Group, Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med 2020;383:1522–34.. As researchers dive deeper into the genetic, environmental, social, and other determinants of susceptibility to infection and severe illness from COVID-19, they may uncover additional factors that confer risk. These studies will also provide definitive answers to some of the pandemic’s most elusive questions, such as factors that may be protective from SARS-CoV-2 infection and COVID-19. In this regard, early in the pandemic, some reports suggested that individuals with certain blood types were less susceptible to COVID-19 compared to those with other blood types (98)Severe Covid-19 GWAS Group, Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, et al. Genomewide association study of severe Covid-19 with respiratory failure. N Engl J Med 2020;383:1522–34.(99)Li J, Wang X, Chen J, Cai Y, Deng A, Yang M. Association between ABO blood groups and risk of SARS-CoV-2 pneumonia. Br J Haematol 2020;190:24–7.(100)Barnkob MB, Pottegard A, Stovring H, Haunstrup TM, Homburg K, Larsen R, et al. Reduced prevalence of SARS-CoV-2 infection in ABO blood group O. Blood Adv 2020;4:4990–3.. However, more recent research, including a review of more than 100,000 patients across three U.S. state health networks, found no link between blood type and COVID-19 susceptibility or severity (101)Dzik S, Eliason K, Morris EB, Kaufman RM, North CM. COVID-19 and ABO blood groups. Transfusion 2020;60:1883–4.(102)Anderson JL, May HT, Knight S, Bair TL, Muhlestein JB, Knowlton KU, et al. Association of sociodemographic factors and blood group type with risk of COVID-19 in a US population. JAMA Netw Open 2021;4:e217429.(103)Bai Y, Yan Z, Murray EJ. Systematic review of the association between ABO blood type and COVID-19 incidence and mortality. medRxiv 2021 23 Apr [Epub ahead of print]..
Studies have unequivocally shown that patients with cancer such as Julie Campbell are at an increased risk for COVID-19 (see Burden of COVID-19 in Patients with Cancer). Patients who have blood cancer are at the greatest risk (see Patients with Hematopoietic Cancers). Research is underway to understand whether the higher risk of COVID-19 infection and death among patients with cancer are a result of the cancer itself, the cancer treatments, or other factors such as smoking or additional coexisting chronic illnesses. One possibility that scientists have considered is that certain patients with cancer have a compromised immune system due to their disease or the treatments, making them more vulnerable to infection (see sidebar on Why Are Cancer Patients at an Increased Risk of Infection?). Notably, researchers have shown that SARS-CoV-2 infection can be persistent in immunocompromised individuals such as certain patients with cancer, with reports of individuals who shed the virus for 70 days or those who stayed infected for nearly one year (104)Couzin-Frankel J. A cancer survivor had the longest documented COVID-19 infection. Here’s what scientists learned. Science. 2021 Oct 19.(105)Avanzato VA, Matson MJ, Seifert SN, Pryce R, Williamson BN, Anzick SL, et al. Case study: prolonged infectious SARS-CoV-2 shedding from an asymptomatic immunocompromised individual with cancer. Cell 2020;183:1901–12.. These findings raise the concern that the virus may develop mutations and give rise to new variants in immunocompromised individuals who suffer from long-term SARS-CoV-2 infection (106)Siqueira JD, Goes LR, Alves BM, de Carvalho PS, Cicala C, Arthos J, et al. SARS-CoV-2 genomic analyses in cancer patients reveal elevated intrahost genetic diversity. Virus Evol 2021;7:veab013.. Public health experts are especially worried about the possibility that persistent SARS-CoV-2 infection may generate more transmissible or more pathogenic SARS-CoV-2 variants (107)Corey L, Beyrer C, Cohen MS, Michael NL, Bedford T, Rolland M. SARS-CoV-2 variants in patients with immunosuppression. N Engl J Med 2021;385:562–6.. Therefore, it is critical to prevent immunocompromised patients with cancer from contracting COVID-19 through prioritized vaccination or other preventive measures, not only to protect them from the risk of severe disease and its long-term sequelae, but also to reduce the likelihood of new viral variants. It is equally important to ensure that patients with cancer are adequately represented in clinical trials assessing the safety and effectiveness of vaccines and treatment for COVID-19. Without such research, we cannot ensure that preventive and therapeutic agents will benefit this particularly vulnerable population.
Detection, Prevention, and Clinical Management of COVID-19
Timely testing to identify those who are or have been infected with SARS-CoV-2 is a crucial step in understanding and reducing the spread of COVID-19. Some viral tests look for current infection (see sidebar on How Can We Test for SARS-CoV-2?). The knowledge gathered from these tests is critical for the implementation of appropriate measures to prevent further spread of the virus and to understand when such measures can be eased. Additionally, certain tests can identify whether an individual had a past infection, although the utility of these tests has been highly debated (108)Abbasi J. The flawed science of antibody testing for SARS-CoV-2 immunity. JAMA 2021;326:1781–2.. SARS-CoV-2 tests can be performed at a health care facility, a designated testing site, or at home using a self-test kit.
Since the onset of the COVID-19 outbreak, the U.S. Food and Drug Administration (FDA) has worked with test developers from the private and academic sectors to streamline the testing process and make more tests available to all citizens. To address the urgency of SARS-CoV-2 detection in limiting spread and controlling the pandemic, the FDA has allowed for three independent pathways for the development of coronavirus tests (see sidebar on How Can We Test for SARS-CoV-2?) (109)U.S. Food and Drug Administration. Coronavirus testing basics [updated 2021 Dec 27; cited 2021 Nov 27].. It is important to note that no test is 100 percent accurate all the time, and there are some individuals who may receive a false-positive or a false-negative result. Individuals who think that they may have COVID-19 and need a test should immediately talk to their health care providers about getting tested. It is also important to discuss the type of test they received and the outcomes to understand what their results mean and to determine the next steps. CDC provides detailed guidelines on who should be tested, which test should be used, how to get a SARS-CoV-2 test, and what to do in case a positive test result is received (110)Centers for Disease Control and Prevention. Testing for COVID-19 [updated 2021 Nov 16; cited 2021 Nov 27]..
In addition to diagnostic testing, CDC has recommended the identification of symptomatic and asymptomatic infected individuals through the process of contact tracing, to control the spread of COVID-19 (112)Centers for Disease Control and Prevention. COVID-19 contact tracing [updated 2021 Oct 21; cited 2021 Nov 27].. Contact tracing is key to slowing the spread of infectious diseases such as COVID-19 when implemented with necessary additional measures such as isolation or quarantine (113)Centers for Disease Control and Prevention. COVID-19 quarantine and isolation [updated 2021 Nov 26; cited 2021 Nov 27]., and treatment, and helps protect infected individuals, their families, and their communities (114)Thomas Craig KJ, Rizvi R, Willis VC, Kassler WJ, Jackson GP. Effectiveness of contact tracing for viral disease mitigation and suppression: evidence-based review. JMIR Public Health Surveill 2021;7:e32468.. In the United States, individual state and local Departments of Health have been primarily responsible for contact tracing, with some state-level collaborations with community partners (115)Chengane S, Cheney A, Garth S, Medcalf S. The COVID-19 response in Nebraska: how students answered the call. Prev Chronic Dis 2020;17:E81.(116)Koetter P, Pelton M, Gonzalo J, Du P, Exten C, Bogale K, et al. Implementation and process of a COVID-19 contact tracing initiative: leveraging health professional students to extend the workforce during a pandemic. Am J Infect Control 2020;48:1451–6.. Digital technologies in the form of symptom and contact tracing apps for smartphones have also been used to track new COVID-19 cases and identify “hot spots” of infection in real time (117)Servick K. COVID-19 contact tracing apps are coming to a phone near you. How will we know whether they work? | Science. 2020 May 20.(118)Wacksman J. Digitalization of contact tracing: balancing data privacy with public health benefit. Ethics Inf Technol 2021 Jun 10 [Epub ahead of print].(119)Elkhodr M, Mubin O, Iftikhar Z, Masood M, Alsinglawi B, Shahid S, et al. Technology, privacy, and user opinions of COVID-19 mobile apps for contact tracing: systematic search and content analysis. J Med Internet Res 2021;23:e23467.(120)Almalki M, Giannicchi A. Health apps for combating COVID-19: descriptive review and taxonomy. JMIR Publications 2021;9:e24322.. This information can potentially allow local hospitals and health care systems to better prepare for surges in new cases. Large sets of data collected through contact tracing apps can also be used by researchers to deepen scientific understanding of SARS-CoV-2 and the symptoms related to COVID-19, as well as to identify potential risk factors and disparities related to infection and disease severity (121)Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med 2020;26:1037–40.(122)Drew DA, Nguyen LH, Steves CJ, Menni C, Freydin M, Varsavsky T, et al. Rapid implementation of mobile technology for real-time epidemiology of COVID-19. Science 2020;368:1362–7.. However, ongoing research is needed to determine ways to overcome the technical and ethical challenges that have emerged as the primary obstacles toward the widespread public acceptance of these tools.
Because cancer patients are more vulnerable to infection (see sidebar on Why Are Cancer Patients at an Increased Risk of Infection?), and could be at a high risk for severe disease (see Burden of COVID-19 in Patients with Cancer), preventing infections in this vulnerable population has been a priority for health care providers since the onset of the pandemic. Depending on the location (e.g., areas with high rates of ongoing community transmission), the availability of COVID-19 diagnostic tests, and/or the type of cancer, many health care institutions implemented either universal testing of patients with cancer or testing of asymptomatic patients who were scheduled to receive certain anticancer therapeutics (123)Shah MA, Mayer S, Emlen F, Sholle E, Christos P, Cushing M, et al. Clinical screening for COVID-19 in asymptomatic patients with cancer. JAMA Netw Open 2020;3:e2023121.(124)Haradaa G, Antonacio FF, Gongora AB, Behar MH, Capareli FC, Bastos DA, et al. SARS-CoV-2 testing for asymptomatic adult cancer patients before initiating systemic treatments: a systematic review. Ecancermedicalscience 2020;14:1100.(125)Yekeduz E, Utkan G, Urun Y. Commentary: should all cancer patients be tested for COVID-19 before each chemotherapy cycle? J Oncol Pharm Pract 2021;27:450–2.(126)Madariaga A, McMullen M, Sheikh S, Kumar R, Liu FF, Zimmermann C, et al. COVID-19 testing in patients with cancer: does one size fit all? Clin Cancer Res 2020;26:4737–42.(127)Messing I, Rao YJ, Scully D, Ojong-Ntui M, Goyal S, Huynh-Le MP. COVID-19 Testing trends: preradiation and throughout cancer care. Int J Radiat Oncol Biol Phys 2021;111:e359.. Health care professionals strongly recommend that patients with cancer speak with their health care teams to decide when and if a COVID-19 detection test is needed for them.
Prevention of COVID-19: Vaccines
As our knowledge of the spread of SARS-CoV-2 accumulated, the CDC recommended several prevention strategies: washing hands frequently; avoiding close contact with people who don’t live in the same household by staying six feet apart; covering mouth and nose with a cloth face cover when around other people; covering mouth and nose when coughing and sneezing; cleaning and disinfecting frequently touched surfaces daily; and monitoring health daily. Prior to the authorization of the SARS-CoV-2 vaccines, these prevention strategies were the only ways to limit infection with the virus and, therefore, minimize the morbidity and mortality of COVID-19. It should be noted that these strategies are still recommended for individuals who are not fully vaccinated or those who have a weak immune system (128)Centers for Disease Control and Prevention. Use masks to help slow spread [updated 2021 Sep 14; cited 2021 Nov 27]..
Vaccines are compounds that trigger the immune system to respond to a pathogen, such as a bacterium, virus, or to a tumor. A vaccine can help the body recognize and destroy cancer cells or microorganisms. When viruses such as SARS-CoV-2 infect our bodies, the immune system responds with cellular and molecular tools to fight off the infectious pathogen (see sidebar on Key Cells in the Immune System). Once a person is infected, it takes several days or weeks for the immune system to generate all the cellular and molecular tools needed to eradicate the infection. After the infection is cleared, the individual’s immune system remembers what it learned about protecting the body against that pathogen (130)Goel RR, Painter MM, Apostolidis SA, Mathew D, Meng W, Rosenfeld AM, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021;374:abm0829.. COVID-19 vaccines are the only known safe and effective way to help our bodies develop immunity to SARS-CoV-2 without having to get the illness.
Vaccines that prevent SARS-CoV-2 infection are considered the most promising approach to control the COVID-19 pandemic. Soon after the onset of the COVID-19 outbreak all stakeholders in the global research community came together in an unprecedented manner and worked collaboratively to generate safe and effective vaccines against COVID-19. In the United States, significant investments and accelerated regulatory policies provided by federal agencies including the NIH, FDA, and CDC, as well as public–private partnerships such as Operation Warp Speed, brought tremendous resources to tackle this challenge, as discussed by Senator Roy Blunt. As a result, in less than a year after the identification of SARS-CoV-2 as the causative agent of COVID-19, researchers delivered multiple vaccines capable of eliciting immunologic protection and helping limit the spread of COVID-19 (see sidebar on SARS-CoV-2 Vaccination Recommendations) (131)Wherry EJ, Jaffee EM, Warren N, D’Souza G, Ribas A, AACR COVID-19 and Cancer Task Force. How did we get a COVID-19 vaccine in less than 1 year? Clin Cancer Res 2021;27:2136–8..
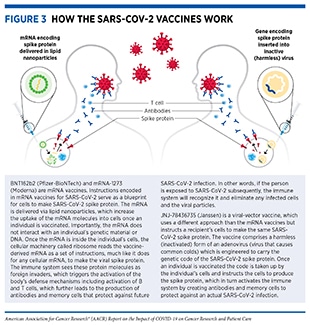
Currently, there are three COVID-19 vaccines (produced by three different biopharmaceutical companies) that are authorized for use in the United States; BNT162b2 or Comirnaty (Pfizer-BioNTech), mRNA-1273 (Moderna), and JNJ-78436735 (Janssen). While these vaccines work in different ways, they are all safe and effective against COVID-19 infection and severe disease (134)Centers for Disease Control and Prevention. Understanding how COVID-19 vaccines work [updated 2021 Nov 24; cited 2021 Nov 29].. BNT162b2 and mRNA-1273 vaccines contain fragments of a type of nucleic acid called mRNA which when injected into the body instructs certain immune cells to produce a harmless version of the SARS-CoV-2 spike protein and display fragments of the protein on the cell surface (Figure 3). These fragments are recognized by the body’s immune system leading to the production of antibodies and the activation of a wide array of protective immune cells (130)Goel RR, Painter MM, Apostolidis SA, Mathew D, Meng W, Rosenfeld AM, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021;374:abm0829.(135)Painter MM, Mathew D, Goel RR, Apostolidis SA, Pattekar A, Kuthuru O, et al. Rapid induction of antigen-specific CD4(+) T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity 2021;54:2133–42.. The process trains the immune system on how to defend against a potential future attack from the actual virus. JNJ-78436735, a viral vector vaccine, is an inactivated version of SARS-CoV-2 which, when injected, instructs the body’s cells to produce a harmless version of the spike protein leading to activation of the immune system and protection against future infection from the actual virus.
Bringing a new vaccine from the bench to the clinic involves many steps, including laboratory research, clinical trials, FDA review and approval, large-scale manufacturing, and distribution. While COVID-19 vaccines were developed rapidly, all stakeholders in the medical research community worked diligently to take the necessary steps to ensure their safety and effectiveness. It is also important to note that the foundation of the research leading to SARS-CoV-2 vaccines was laid decades ago with fundamental basic science in understanding the biology of prior coronaviruses such as SARS and MERS, mechanisms of vaccine-induced immunity, protection from viral infections, strategies to use mRNA to induce an immune response, and pathways by which the immune system generates antibody responses to parts of coronaviruses (128)Centers for Disease Control and Prevention. Use masks to help slow spread [updated 2021 Sep 14; cited 2021 Nov 27].. The vaccine delivery platforms that form the backbone of the SARS-CoV-2 vaccines had also been under development and were already tested in humans prior to COVID-19. In fact, the mRNA vaccine platform was developed and tested in humans initially as an experimental cancer vaccine (131)Wherry EJ, Jaffee EM, Warren N, D’Souza G, Ribas A, AACR COVID-19 and Cancer Task Force. How did we get a COVID-19 vaccine in less than 1 year? Clin Cancer Res 2021;27:2136–8.. The rapid development of COVID-19 vaccines can therefore be attributed to decades of robust investments across all areas of medical research and unprecedented scientific efforts from the biomedical community including key contributions from cancer researchers (see Cancer Researchers Working to Combat the COVID-19 Pandemic).
Data from the vaccine clinical trials as well as use in public settings after FDA authorization suggest that all three vaccines currently used in the U.S. are effective, especially against severe illness, hospitalization, and death from COVID-19 (137)Centers for Disease Control and Prevention. COVID-19 vaccines work [updated 2021 Nov 9; cited 2021 Nov 29].. According to the most recent data from CDC, unvaccinated individuals have a five-fold higher risk of testing positive for COVID-19 and a fourteen-fold greater risk of dying from COVID-19 compared to those who are fully vaccinated (138)Centers for Disease Control and Prevention. COVID data tracker [updated 2020 Mar 28; cited 2021 Dec 17].. The level of protection is even higher for fully vaccinated individuals who have received an additional (booster) dose (138)Centers for Disease Control and Prevention. COVID data tracker [updated 2020 Mar 28; cited 2021 Dec 17].. Fully vaccinated individuals who contract COVID-19 may also be less likely to have long COVID compared to the unvaccinated although more confirmatory data are needed (130)Goel RR, Painter MM, Apostolidis SA, Mathew D, Meng W, Rosenfeld AM, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021;374:abm0829.(139)Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol 2021;21:626–36.(140)Woldemeskel BA, Garliss CC, Blankson JN. mRNA vaccine-elicited SARS-CoV-2-specific T cells persist at 6 months and recognize the Delta variant. Clin Infect Dis 2021 Oct 25 [Epub ahead of print].(141)Grannis SJ, Rowley EA, Ong TC, Stenehjem E, Klein NP, DeSilva MB, et al. Interim estimates of COVID-19 vaccine effectiveness against COVID-19-associated emergency department or urgent care clinic encounters and hospitalizations among adults during SARS-Co. There is also evidence that the vaccines are protective against the Delta variant which was the most common variant in the United States until December 2021 (130)Goel RR, Painter MM, Apostolidis SA, Mathew D, Meng W, Rosenfeld AM, et al. mRNA vaccines induce durable immune memory to SARS-CoV-2 and variants of concern. Science 2021;374:abm0829.(139)Tregoning JS, Flight KE, Higham SL, Wang Z, Pierce BF. Progress of the COVID-19 vaccine effort: viruses, vaccines and variants versus efficacy, effectiveness and escape. Nat Rev Immunol 2021;21:626–36.(140)Woldemeskel BA, Garliss CC, Blankson JN. mRNA vaccine-elicited SARS-CoV-2-specific T cells persist at 6 months and recognize the Delta variant. Clin Infect Dis 2021 Oct 25 [Epub ahead of print].(141)Grannis SJ, Rowley EA, Ong TC, Stenehjem E, Klein NP, DeSilva MB, et al. Interim estimates of COVID-19 vaccine effectiveness against COVID-19-associated emergency department or urgent care clinic encounters and hospitalizations among adults during SARS-Co. Researchers anticipate that with time, additional SARS-CoV-2 VOC will arise. As a recent example, on November 26, 2021, a new variant first reported out of South Africa (Omicron) was classified as a VOC by the WHO (51)Callaway E. Heavily mutated Omicron variant puts scientists on alert. Nature 2021;600:21.. Omicron variant was soon detected in the U.S. and as of January 1, 2022, it was estimated to make up more than 95 percent of the COVID-19 variants circulating in the United States (138)Centers for Disease Control and Prevention. COVID data tracker [updated 2020 Mar 28; cited 2021 Dec 17].. Ongoing and future studies will need to investigate how long the vaccines are effective, the impact of the variants on vaccine effectiveness, and whether it is necessary to administer additional doses or develop new vaccines that more closely reflect the circulating virus variants at the time (142)Harvey WT, Carabelli AM, Jackson B, Gupta RK, Thomson EC, Harrison EM, et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 2021;19:409–24.(143)Cohn BA, Cirillo PM, Murphy CC, Krigbaum NY, Wallace AW. SARS-CoV-2 vaccine protection and deaths among US veterans during 2021. Science 2021 Nov 4 [Epub ahead of print].(144)Barda N, Dagan N, Cohen C, Hernan MA, Lipsitch M, Kohane IS, et al. Effectiveness of a third dose of the BNT162b2 mRNA COVID-19 vaccine for preventing severe outcomes in Israel: an observational study. Lancet 2021;398:2093–100.. Of note, there are currently 272 vaccines in various stages of development globally, of which 104 are in clinical testing (145)The Milken Institute. COVID-19 Vaccine visualization [updated 2022 Jan 5; cited 2021 Nov 27]..
Despite the knowledge that COVID-19 vaccines are safe and effective, uptake of vaccination has been suboptimal in the United States. As of January 01, 2022, only 63 percent of the U.S. population has been fully vaccinated. There are also stark disparities in the uptake of vaccination. For instance, among insured U.S. individuals age 16 and older, the receipt of one or more doses was lower among non-Hispanic Blacks (41 percent) and Hispanics (41 percent) compared to non-Hispanic whites (55 percent); coverage was highest (57 percent) among non-Hispanic Asians (146)Pingali C, Meghani M, Razzaghi H, Lamias MJ, Weintraub E, Kenigsberg TA, et al. COVID-19 vaccination coverage among insured persons aged 16 Years, by race/ethnicity and other selected characteristics – eight integrated health care organizations, United S. A study that examined data from the nine largest U.S. cities found that, in neighborhoods with the lowest vaccination rates, 25 percent of the population was Black, and 52 percent was white, while in neighborhoods with the highest vaccination rates, only six percent of the population was Black while 70 percent was white (147)Sacarny A, Daw JR. Inequities in COVID-19 vaccination rates in the 9 largest US cities. JAMA Health Forum 2021;2:e212415.. The average low-vaccination neighborhood had half the median income and over twice the poverty rate of the average high-vaccination neighborhood (149)Carethers JM. Rectifying COVID-19 disparities with treatment and vaccination. JCI Insight 2021;6:e147800.. COVID-19 vaccination coverage has also been lower in rural counties (39 percent) compared to urban counties (46 percent) (147)Sacarny A, Daw JR. Inequities in COVID-19 vaccination rates in the 9 largest US cities. JAMA Health Forum 2021;2:e212415.. These data are especially concerning because the same populations in which the uptake of COVID-19 vaccines is particularly low are also the ones that have shouldered the greatest burden of the pandemic (149)Carethers JM. Rectifying COVID-19 disparities with treatment and vaccination. JCI Insight 2021;6:e147800.. Public health interventions at local, state, and federal levels are needed to raise awareness and education around the vital importance of COVID-19 vaccination to ensure equitable uptake in communities that have been hardest hit by the pandemic.
Patients with cancer were not included in the original clinical trials that tested the safety and efficacy of the currently authorized COVID-19 vaccines in the U.S. However, real-world evidence gathered since the EUA and/or approval of COVID-19 vaccines has indicated that they are effective and elicit anti-SARS-CoV-2 immune responses in patients with cancer (150)Thakkar A, Gonzalez-Lugo JD, Goradia N, Gali R, Shapiro LC, Pradhan K, et al. Seroconversion rates following COVID-19 vaccination among patients with cancer. Cancer Cell 2021;39:1081–90.(151)Embi PJ, Levy ME, Naleway AL, Patel P, Gaglani M, Natarajan K, et al. Effectiveness of 2-dose vaccination with mRNA COVID-19 vaccines against COVID-19-associated hospitalizations among immunocompromised adults – nine states, January–September 2021. MMWR M (see Prevention and Treatment of COVID-19 in Patients with Cancer). However, because many patients with cancer are immunocompromised due to their disease and/or the treatments received, the immune responses generated in this vulnerable population may be weaker and may last for a shorter period of time compared to healthy immunocompetent individuals (152)Monin L, Laing AG, Munoz-Ruiz M, McKenzie DR, Del Molino Del Barrio I, Alaguthurai T, et al. Safety and immunogenicity of one versus two doses of the COVID-19 vaccine BNT162b2 for patients with cancer: interim analysis of a prospective observational study(153)Palich R, Veyri M, Marot S, Vozy A, Gligorov J, Maingon P, et al. Weak immunogenicity after a single dose of SARS-CoV-2 mRNA vaccine in treated cancer patients. Ann Oncol 2021;32:1051–3.(154)Eliakim-Raz N, Massarweh A, Stemmer A, Stemmer SM. Durability of response to SARS-CoV-2 BNT162b2 vaccination in patients on active anticancer treatment. JAMA Oncol 2021;7:1716–8.. Therefore, FDA and CDC recommend that patients with cancer receive additional doses of the vaccine for optimal protection against COVID-19 (see Prevention and Treatment of COVID-19 in Patients with Cancer).
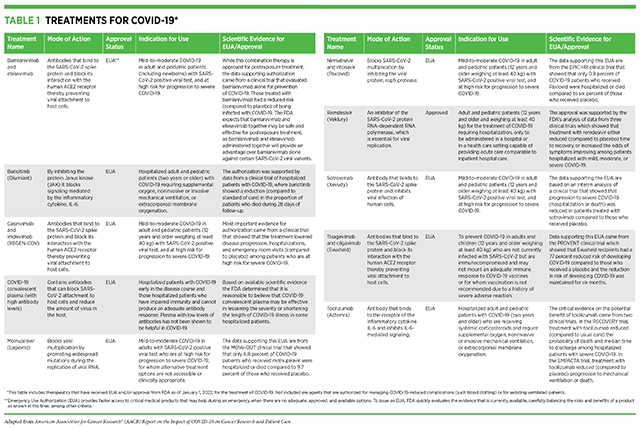
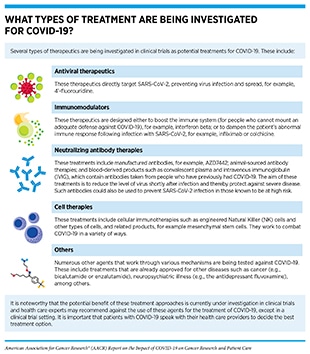
Treatments for COVID-19
Infection with SARS-CoV-2 causes a wide range of symptoms and disease severity. More than 80 percent of people who are diagnosed with COVID-19 have mild symptoms and do not require hospitalization (83)Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai Felix S, et al. Coronavirus disease 2019 case surveillance – United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep 2020;69:759–65.(155)Mehta V, Goel S, Kabarriti R, Cole D, Goldfinger M, Acuna-Villaorduna A, et al. Case fatality rate of cancer patients with COVID-19 in a New York Hospital System. Cancer Discov 2020;10:935–41.. Among those patients who require hospitalization, fewer than 30 percent have critical disease and require admission to the intensive care unit (156)Nguyen NT, Chinn J, Nahmias J, Yuen S, Kirby KA, Hohmann S, et al. Outcomes and mortality among adults hospitalized with COVID-19 at US Medical Centers. JAMA Netw Open 2021;4:e210417.. Several treatments are available for COVID-19 patients who have mild to moderate symptoms, for those who are hospitalized, and for individuals who may be asymptomatic but are at high risk for serious COVID-19 and have been exposed to someone who has tested positive for COVID-19. It should be noted that randomized, double-blind, placebo-controlled clinical trials are the most rigorous and reliable ways to find successful treatments for diseases including COVID-19. In these trials, participants are assigned randomly to receive either the drug being tested, or a placebo—a treatment that has none of the active drug in it. To remove any bias, both health care providers and participants are kept unaware (double-blind) of which intervention the participants have received until the trial is over.
The NIH has provided specific guidelines for clinical management of COVID-19 depending on the symptoms, susceptibility of a patient for severe COVID-19, and whether a patient is being cared for at home or at the hospital (157)NIH. Information on COVID-19 treatment, prevention and research [updated 2022 Jan 5; cited 2021 Nov 29].. COVID-19 patients with mild symptoms who are being cared for at home are recommended over-the-counter medicines, such as acetaminophen, to relieve their symptoms. FDA has issued EUAs for three treatments for patients who are at home with mild or moderate symptoms or are asymptomatic but are at a high risk for progressing to severe disease due to exposure to someone with COVID-19 (see Table 1) (158)US Department of Health and Human Services. Possible treatment options for COVID-19 [updated 2021 Jun 30; cited 2021 Nov 27].. These treatments comprise antibodies that attach to SARS-CoV-2 and help the immune system recognize and eliminate the virus. By reducing the amount of virus in the body the antibodies decrease the likelihood of a patient progressing to severe disease.
Additional treatment options have been approved or authorized for emergency use by the FDA for hospitalized COVID-19 patients with serious illness (see Table 1). These include antivirals—therapeutics that block viral multiplication and spread in the body —and convalescent plasma—blood plasma from patients who have recovered from COVID-19—which contain SARS-CoV-2 antibodies that help the immune system recognize and eliminate the virus. Furthermore, the FDA has issued EUAs for treatments that are aimed to mitigate the overactive immune response as well as certain other pulmonary or cardiovascular complications associated with severe COVID-19. Of note, investigations are still ongoing to yield definitive evidence of clinical benefit for some of these treatments (159)New York Times. Coronavirus drug and treatment tracker. [updated 2020 Jul 16, cited 2021 Nov 27].. Researchers are also evaluating the benefit of these therapeutics at different stages of disease beyond what has been already approved and/or authorized (160)Korley FK, Durkalski-Mauldin V, Yeatts SD, Schulman K, Davenport RD, Dumont LJ, et al. Early convalescent plasma for high-risk outpatients with Covid-19. N Engl J Med 2021;385:1951–60.,.
In addition to the treatments that received EUA and/or full approval by the FDA, there are numerous other therapeutics at various stages of clinical testing as potential treatments for COVID-19 (159)New York Times. Coronavirus drug and treatment tracker. [updated 2020 Jul 16, cited 2021 Nov 27].(161)ClinicalTrials.gov. A study of PF-07321332/ritonavir in nonhospitalized high risk adult participants with COVID-19 [updated 2022 Jan 12; cited 2021 Nov 27].(162)Mahase E. Covid-19: Molnupiravir reduces risk of hospital admission or death by 50% in patients at risk, MSD reports. BMJ 2021;375:n2422.(163)US Department of Health and Human Services. Clinical Trials [updated 2021 Jun 30; cited 2021 Nov 27].. A wide range of agents, including antivirals, anti-inflammatory drugs, and immune-system modulators, are being investigated in large, international, cross-sector, multi-institutional clinical trials such as Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV), Randomized Evaluation of COVID-19 Therapy (RECOVERY), and the WHO SOLIDARITY PLUS, among others (see sidebar on What Types of Treatment Are Being Investigated for COVID-19?) (164)NIH. Accelrating COVID-19 therapeutic interventions and vaccines (ACTIV) [updated 2020 Apr 17; cited 2021 Nov 27].(165)RECOVERYtrial.net. Randomised evaluation of COVID-19 therapy trial [updated 2022 Jan 12; cited 2021 Nov 27].(166)World Health Organization. WHO’s Solidarity clinical trial enters a new phase with three new candidate drugs [updated 2021 Aug 11; cited 2021 Nov 27].. One area of active research is the repurposing of therapeutics that are already under investigation or have been approved by the FDA for other diseases, including several anticancer agents, for use in COVID-19 because we already have information about dosage, toxicity, and adverse effects for these therapeutics which can accelerate the pace of development and approval relative to the development of novel therapeutics (167)Poh A. Repurposing cancer drugs for COVID-19. Cancer Discov 2020;10:OF2.(168)Aldea M, Michot JM, Danlos FX, Ribas A, Soria JC. Repurposing of anticancer drugs expands possibilities for antiviral and anti-inflammatory discovery in COVID-19. Cancer Discov 2021;11:1336–44..
Because patients with cancer are especially susceptible to severe COVID-19 (see Burden of COVID-19 in Patients with Cancer), it is vitally important that researchers investigate the safety and efficacy of COVID-19 treatments in this vulnerable population. The fact that the compromised immune system in many cancer patients allows for persistent infection, together with new evidence of novel mechanisms of immune evasion and SARS-CoV-2 variant emergence induced by certain treatments (171)Pommeret F, Colomba J, Bigenwald C, Laparra A, Bockel S, Bayle A, et al. Bamlanivimab + etesevimab therapy induces SARS-CoV-2 immune escape mutations and secondary clinical deterioration in COVID-19 patients with B-cell malignancies. Ann Oncol 2021;32:144, necessitates that health care providers carry out comprehensive ongoing clinical and virological follow-up of these patients.
Next Section: Cancer Researchers Working to Combat the Pandemic Previous Section: Snapshot of the Impact of the Pandemic on Cancer Research and Care