Turning Science into Lifesaving Care
In this section, you will learn:
- Research that increases our understanding of the genetic, molecular, and cellular characteristics of cancer is continuing to spur advances in the treatment of cancer.
- Advances are being made across all five pillars of cancer care: surgery, radiotherapy, cytotoxic chemotherapy, molecularly targeted therapy, and immunotherapy.
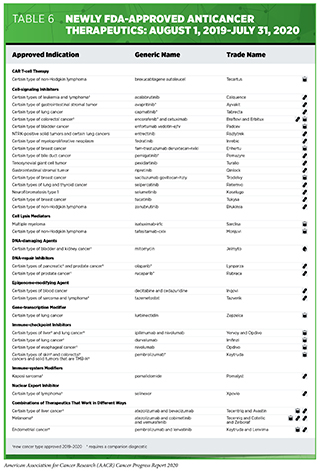
- From August 1, 2019 to July 31, 2020, the FDA approved 20 new therapeutics for treating patients with certain types of cancer.
- During the same period, the uses of 15 previously approved anticancer therapeutics were expanded by the FDA to include the treatment of additional types of cancer.
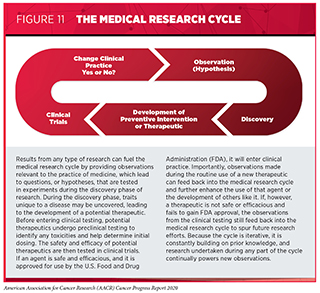
Progress across the continuum of clinical cancer care improves survival and quality of life for people around the world. The progress is driven by the dedicated efforts of individuals working throughout the cycle of medical research (see Figure 11).
Medical Research
Medical research is an iterative process that is set in motion when a discovery with the potential to affect the practice of medicine or public health is made in any area of research or clinical practice (see Figure 11). One way that researchers build on a discovery is by asking questions that can be tested through experiments in a wide range of models that mimic healthy and diseased conditions. Results from these experiments can lead to the identification of a potential preventive intervention or therapeutic target, or to the identification of a potential predictive or prognostic biomarker. They also can feed backward in the cycle by providing new discoveries that lead to more questions or hypotheses.
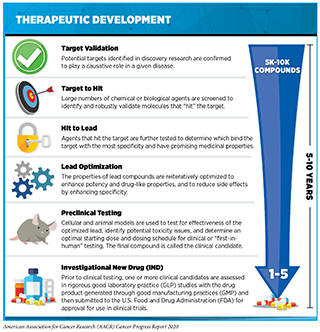
If a potential therapeutic target is identified, it takes many more years of preclinical research before a candidate therapeutic is developed and ready for testing in clinical trials (see sidebar on Therapeutic Development). During this time, several candidates are rigorously tested to identify any potential toxicity and to determine the appropriate doses and dosing schedules for testing in the first clinical trial.
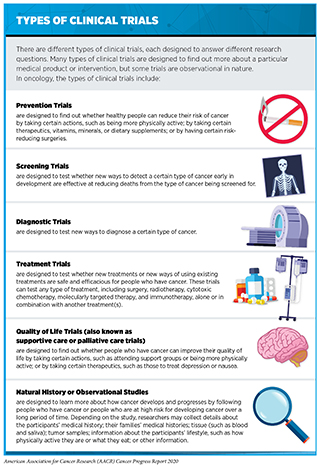
There are many types of clinical trials, each designed to answer different research questions (see sidebar on Types of Clinical Trials). All clinical trials are reviewed and approved by institutional review boards before they can begin and are monitored throughout their duration.
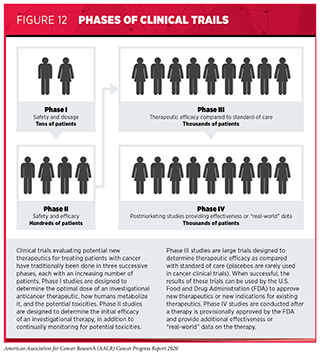
Clinical trials testing the safety and efficacy of candidate anticancer therapeutics have traditionally been done in three successive phases (see Figure 12). However, this traditional approach requires a very large number of patients and takes many years to complete, making it extremely costly and one of the major barriers to rapid translation of scientific knowledge into clinical advances. Recent analyses have estimated that the median research and development cost for a new anticancer therapeutic or immune-system modulating therapeutic is $2.77 billion and that despite efforts to reduce the overall time, it still takes about eight years for an anticancer therapeutic to progress through clinical development and approval (313)Wouters OJ, McKee M, Luyten J. Estimated Research and Development Investment Needed to Bring a New Medicine to Market, 2009-2018. JAMA [Internet]. American Medical Association; 2020 [cited 2020 Jul 1];323:844.(314)Cancer drug approvals grew from 4% of U.S. total in the 1980s to 27% in 2010-18 – Tufts CSDD Impact Report September/October Vol. 21 No. 5. 2019;21:2019..
Over the past few decades, the FDA has implemented several changes that have altered how clinical trials can be conducted and reviewed in an effort to reduce the length of time it takes to obtain a clear result from a clinical trial, including developing four evidence-based strategies to expedite assessment of therapeutics for life-threatening diseases such as cancer. New anticancer therapeutics are far more likely to undergo regulatory assessment using these expedited strategies than new therapeutics being tested in other fields of medicine, and this is associated with a 48 percent shorter regulatory review time for anticancer therapeutics (314)Cancer drug approvals grew from 4% of U.S. total in the 1980s to 27% in 2010-18 – Tufts CSDD Impact Report September/October Vol. 21 No. 5. 2019;21:2019..
In addition, advances in our understanding of cancer biology have enabled researchers, regulators, and the pharmaceutical industry to develop new ways to design and conduct clinical trials. The new designs, including adaptive, seamless, and master protocol designs, aim to streamline the clinical development of new anticancer therapeutics by matching the right therapeutics with the right patients earlier, reducing the number of patients who need to be enrolled in the trial before it is determined whether the anticancer therapeutic being evaluated is safe and efficacious, and/or decreasing the length of time it takes for a new anticancer therapeutic to be tested and made available to patients if the trial shows it is safe and efficacious (315)Bhatt DL, Mehta C. Adaptive Designs for Clinical Trials. N Engl J Med [Internet]. Massachusetts Medical Society; 2016;375:65–74.(316)Prowell TM, Theoret MR, Pazdur R. Seamless Oncology-Drug Development. N Engl J Med [Internet]. Massachusetts Medical Society; 2016;374:2001–3.(317)Woodcock J, LaVange LM. Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both. N Engl J Med [Internet]. 2017;377:62–70..
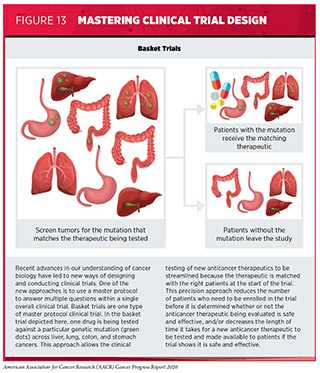
Master protocol design clinical trials aim to answer multiple questions within a single overall clinical trial (317)Woodcock J, LaVange LM. Master Protocols to Study Multiple Therapies, Multiple Diseases, or Both. N Engl J Med [Internet]. 2017;377:62–70.. The emergence of this clinical trial design has largely been driven by our increased understanding of the genetic mutations that promote cancer initiation and growth. “Basket” trials are one example of genetic mutation–based master protocol design clinical trials (see Figure 13). These trials allow researchers to test one anticancer therapeutic on a group of patients who all have the same type of genetic mutation, regardless of the anatomic site of the original cancer, as highlighted in Targeting an Array of Cancers That Share the Same Genetic Alteration).
Even though we have new ways of designing, conducting, and reviewing clinical trials that are yielding advances in patient care, there are still opportunities to improve the clinical trial enterprise. Some of the most pressing challenges that need to be overcome are low participation in clinical trials, in particular among individuals living in rural areas and adolescents and young adults, and a lack of representation from all populations among individuals of all ages who do participate (318)Kim S-H, Tanner A, Friedman DB, Foster C, Bergeron CD. Barriers to clinical trial participation: a comparison of rural and urban communities in South Carolina. J Community Health [Internet]. J Community Health; 2014 [cited 2020 Aug 3];39:562–71.(319)(320)(321)(322) (see sidebar on Disparities in Cancer Clinical Trial Participation). Overcoming barriers to clinical trial participation for all segments of the population will require all stakeholders in the cancer community to come together to develop a multifaceted approach that includes the development and implementation of new, more effective education and policy initiatives.
Progress across the Spectrum of Cancer Treatment
Research discoveries that have been made as a result of innovative cancer science are continually being translated to new medical products for cancer prevention, detection, diagnosis, treatment, and survivorship. The approval of new medical products is not the end of a linear research process. Rather, it is an integral part of the medical research cycle because observations made during the routine use of new medical products can be used to accelerate the pace at which similar products are developed and to stimulate the development of new, more effective products.
The following discussion focuses primarily on medical products approved by the FDA in the 12 months spanning this report, August 1, 2019, to July 31, 2020. In particular, it focuses on the 20 new anticancer therapeutics approved by the FDA during this period (see Table 6). Also highlighted are the 15 previously approved anticancer therapeutics that were approved by the FDA for treating additional types of cancer during that time. Not discussed are FDA approvals related to expanding the use of an anticancer therapeutic previously approved for a given type of cancer to include additional dosing regimens or additional uses during the treatment of the same cancer type; for example, an expansion to include treatment of the same type of cancer at a less advanced stage of disease.
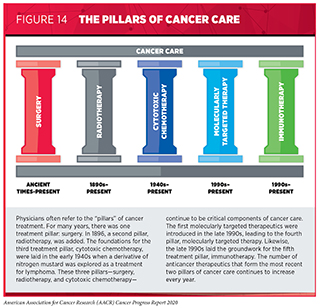
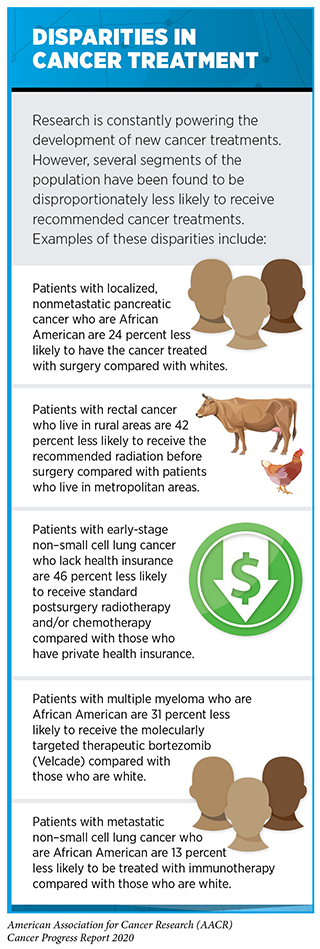
New FDA-approved medical products are usually used alongside treatments already in use, including surgery, radiotherapy, and cytotoxic chemotherapy, which continue to be vital pillars of clinical cancer care (see Figure 14) [see Supplemental Table 2 (Part 1 and Part 2) and Supplemental Table 3. Despite the continual progress in cancer treatment, not all patients receive the care recommended for the type and stage of cancer with which they have been diagnosed (sidebar on Disparities in Cancer Treatment). It is imperative that all stakeholders committed to accelerating the pace at which we make breakthroughs against cancer work together to address the challenge of disparities in cancer treatment because these can be associated with adverse differences in survival. In fact, recent research shown that disparities in multiple myeloma and prostate cancer survival for African Americans compared with whites were eliminated if they had equivalent access to care and to standard treatments (323)Riviere P, Luterstein E, Kumar A, Vitzthum LK, Deka R, Sarkar RR, et al. Survival of African American and non‐Hispanic white men with prostate cancer in an equal‐access health care system. Cancer [Internet]. John Wiley & Sons, Ltd; 2020 [cited 2020 Jan 27];cncr.32666.(324)Fillmore NR, Yellapragada S V, Ifeorah C, Mehta A, Cirstea D, White PS, et al. With equal access, African American patients have superior survival compared to white patients with multiple myeloma: a VA study. Blood [Internet]. 2019 [cited 2019 Jun 20];133:2615–8..
Treatment with Surgery
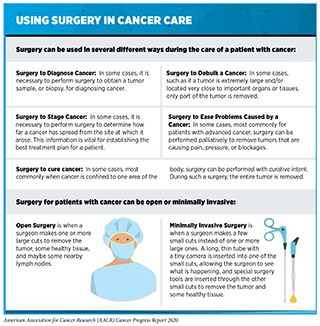
Until the late 19th century, surgery was the only approach to treating patients with cancer (see Figure 14). Today, it remains the foundation of treatment for many patients (330)National Academies of Sciences E and M. Appropriate Use of Advanced Technologies for Radiation Therapy and Surgery in Oncology [Internet]. Nass SJ, Patlak M, editors. Washington, D.C.: National Academies Press; 2016 [cited 2020 Jul 1]. (see sidebar on Using Surgery in Cancer Care).
Adding Therapy before Surgery
For some patients with cancer, surgery alone may be the best treatment option. However, other patients are treated with radiotherapy, cytotoxic chemotherapy, molecularly targeted therapy, and/or immunotherapy before surgery, a treatment approach called neoadjuvant therapy. Neoadjuvant therapy can be used to shrink a large tumor so that the surgery performed is less invasive, less complicated, and/or more likely to be curative. It can also be used to shrink a tumor that cannot be removed surgically because it is too large or too close to an important organ or tissue so that it can be removed surgically.
Researchers are continually investigating ways to increase the number of patients who benefit from neoadjuvant therapy and, thereby, hone the use of surgery in cancer treatment. For example, recent data showed that neoadjuvant cytotoxic chemotherapy has the potential to increase the number of patients with pancreatic cancer who are eligible for surgery: In two early-stage clinical trials, this treatment led to a significant proportion of patients with locally advanced pancreatic cancer that could not be removed by surgery going on to have the cancer fully removed surgically, and this was associated with improved survival (331)JE M, JY W, DP R, JW C, W J, BY Y, et al. Total Neoadjuvant Therapy With FOLFIRINOX in Combination With Losartan Followed by Chemoradiotherapy for Locally Advanced Pancreatic Cancer: A Phase 2 Clinical Trial. JAMA Oncol [Internet]. JAMA Oncol; 2019 [cited 2020 Jul 1];5.(332)G G, VP G, AB B, DA L, L Z, AK N, et al. Survival in Locally Advanced Pancreatic Cancer After Neoadjuvant Therapy and Surgical Resection. Ann Surg [Internet]. Ann Surg; 2019 [cited 2020 Aug 14];270:340–7..
Many patients with stage III melanoma are treated with surgery. Unfortunately, even if the surgery is successful, these individuals are at high risk of the melanoma recurring (333)GV L, RPM S, S L, OE N, KF S, M G, et al. Neoadjuvant Dabrafenib Combined With Trametinib for Resectable, Stage IIIB-C, BRAF V600 Mutation-Positive Melanoma (NeoCombi): A Single-Arm, Open-Label, Single-Centre, Phase 2 Trial. Lancet Oncol [Internet]. Lancet Oncol; 2019 [cited 2020 Jul 1];20.(334)EA R, AM M, ACJ van A, C A, C B, BA van de W, et al. Identification of the Optimal Combination Dosing Schedule of Neoadjuvant Ipilimumab Plus Nivolumab in Macroscopic Stage III Melanoma (OpACIN-neo): A Multicentre, Phase 2, Randomised, Controlled Trial. Lancet Oncol [Internet]. Lancet Oncol; 2019 [cited 2020 Jul 1];20.. Therefore, researchers are investigating whether neoadjuvant therapy can improve the chances of surgery being curative for stage III melanoma. In one study, neoadjuvant therapy with a combination of the molecularly targeted therapeutics dabrafenib (Tafinlar) and trametinib (Mekinist) was shown to make it easier for the tumor and surrounding tissue to be surgically removed in almost half of patients (333)GV L, RPM S, S L, OE N, KF S, M G, et al. Neoadjuvant Dabrafenib Combined With Trametinib for Resectable, Stage IIIB-C, BRAF V600 Mutation-Positive Melanoma (NeoCombi): A Single-Arm, Open-Label, Single-Centre, Phase 2 Trial. Lancet Oncol [Internet]. Lancet Oncol; 2019 [cited 2020 Jul 1];20.. It also shrank tumors for most patients, as did a combination of immunotherapeutics—ipilimumab (Yervoy) and nivolumab (Opdivo)—in another clinical trial (334)EA R, AM M, ACJ van A, C A, C B, BA van de W, et al. Identification of the Optimal Combination Dosing Schedule of Neoadjuvant Ipilimumab Plus Nivolumab in Macroscopic Stage III Melanoma (OpACIN-neo): A Multicentre, Phase 2, Randomised, Controlled Trial. Lancet Oncol [Internet]. Lancet Oncol; 2019 [cited 2020 Jul 1];20.. Longer follow-up of the patients in both trials is needed to determine whether these neoadjuvant therapies ultimately reduce risk of relapse and improve cure rates.
Reducing the Need for a Second Surgery
Despite the immense benefits of surgery, complications can occur and can negatively affect a patient’s quality of life. One recent advance may help women with breast cancer who choose to have breast-conserving surgery avoid the challenge of needing a second surgery to provide the best chance of a cure (335)A K, C R, KP M, W S, SM D, S A, et al. Evaluation of 2014 Margin Guidelines on Re-Excision and Recurrence Rates After Breast Conserving Surgery: A Multi-Institution Retrospective Study. Breast [Internet]. Breast; 2020 [cited 2020 Jul 1];51.. It is estimated that about 50 percent of women who are diagnosed with breast cancer have breast-conserving surgery—surgery to remove a breast tumor and a small amount of normal tissue around it that leaves most of the breast skin and tissue in place. However, more than 20 percent of patients require a second surgery, known as re-excision, because post-surgery analysis of the removed tumor shows an inadequate margin of normal tissue around the tumor, leaving open the possibility that not all the tumor was removed. A recent study showed that using 3-dimensional breast tomosynthesis in the operating room to guide the surgery reduced the rate of re-excision by more than 50 percent compared with using standard 2-dimensional breast imaging (336).
Using Surgery to Treat Metastatic Cancer
Traditionally, the main use of surgery in the treatment of patients with metastatic cancer has been to reduce or control problems caused by the cancer, such as pain, pressure, and blockages. However, recent research suggests that surgery can benefit patients with some types of cancer who have metastatic tumors at a limited number of sites and are said to have oligometastatic disease (337)JE G-O, NP W, DS D, JD C, DG K. Multidisciplinary Management of Oligometastatic Soft Tissue Sarcoma. Am Soc Clin Oncol Educ book Am Soc Clin Oncol Annu Meet [Internet]. Am Soc Clin Oncol Educ Book; 2018 [cited 2020 Jul 1];38.(338)Fernandez RAS, Lau RWH, Ho JYK, Yu PSY, Chow SCY, Wan IYP, et al. Evidence for surgical resections in oligometastatic lung cancer. J Thorac Dis [Internet]. AME Publications; 2019 [cited 2020 Jul 1];11:S969.. For example, although soft tissue sarcoma metastases often recur after surgical removal, it is possible to perform serial surgeries that allow patients who have oligometastases to live disease free for periods of time (337)JE G-O, NP W, DS D, JD C, DG K. Multidisciplinary Management of Oligometastatic Soft Tissue Sarcoma. Am Soc Clin Oncol Educ book Am Soc Clin Oncol Annu Meet [Internet]. Am Soc Clin Oncol Educ Book; 2018 [cited 2020 Jul 1];38.. For patients with lung cancer who have oligometastases, surgical removal of the initial lung tumor and oligometastases combined with other approaches to treatment can improve survival, and for patients with colorectal cancer who have a limited number of metastases in the liver, radiofrequency ablation combined with surgery can improve survival (338)Fernandez RAS, Lau RWH, Ho JYK, Yu PSY, Chow SCY, Wan IYP, et al. Evidence for surgical resections in oligometastatic lung cancer. J Thorac Dis [Internet]. AME Publications; 2019 [cited 2020 Jul 1];11:S969.(339)T R, F VC, CJ P, JE P, I B-R, JA L, et al. Local Treatment of Unresectable Colorectal Liver Metastases: Results of a Randomized Phase II Trial. J Natl Cancer Inst [Internet]. J Natl Cancer Inst; 2017 [cited 2020 Jul 1];109..
Treatment with Radiotherapy
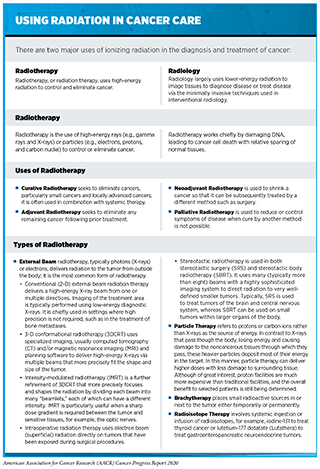
Radiotherapy became the second pillar of cancer treatment in 1896 (see Figure 14). Today, about 50 percent of patients receive radiotherapy to shrink or eliminate tumors or to prevent local recurrence (330)National Academies of Sciences E and M. Appropriate Use of Advanced Technologies for Radiation Therapy and Surgery in Oncology [Internet]. Nass SJ, Patlak M, editors. Washington, D.C.: National Academies Press; 2016 [cited 2020 Jul 1]. (see sidebar on Using Radiation in Cancer Care).
Traditionally, the main use of radiotherapy in the treatment of patients with metastatic cancer was to reduce or control symptoms of disease. This is beginning to change as a result of advances in radiotherapy and emerging evidence that up to 50 percent of patients diagnosed with metastatic cancer have oligometastatic disease, meaning there are metastatic tumors at a limited number of sites. Stereotactic ablative radiotherapy is an advanced approach to radiotherapy that can more precisely target radiation to tumors than conventional forms of external beam radiotherapy. The high degree of precision means that higher doses of radiation can be used and that healthy tissues surrounding a tumor are spared from damage caused by the radiation, which can reduce the long-term adverse effects of radiotherapy. Recent clinical trials have shown that stereotactic ablative radiotherapy targeted to oligometastatic tumors can reduce the chances of disease progression and increase survival for patients who have solid tumors, such as prostate cancer, lung cancer, and gastrointestinal tumors (340)Palma DA, Olson R, Harrow S, Gaede S, Louie A V, Haasbeek C, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet [Internet]. Elsevier; 2019 [cited 2020 Jul 1];393:2051–8.(341)(342)(343). In other clinical trials, adding prostate-targeted radiotherapy to standard treatment for metastatic prostate cancer significantly increased survival for patients who had limited metastatic disease (344)Parker CC, James ND, Brawley CD, Clarke NW, Hoyle AP, Ali A, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet (London, England) [Internet]. Elsevier; 2018 [cited 2019 Jun 20];392:2353–66.(345)Boevé LMS, Hulshof MCCM, Vis AN, Zwinderman AH, Twisk JWR, Witjes WPJ, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol [Internet]. 2019 [cited 2019 Jun 20];75:410–8.. These data, together with the data on using surgery to treat oligometastatic disease (see Using Surgery to Treat Metastatic Cancer, highlight how research is improving outcomes for patients with limited metastatic disease.)
Despite the immense benefits of radiotherapy, it can have long-term adverse effects that negatively impact patient quality of life. Intensity-modulated radiotherapy (IMRT) is an advanced approach to radiotherapy in which specialized imaging, usually computed tomography (CT) or MRI, and planning software are used to deliver high-energy beams of radiation. There are multiple beams of radiation divided into many “beamlets,” each of which can have a different intensity. Given that IMRT delivers radiation in a way that more precisely fits the shape and size of the tumor compared with conventional radiotherapy, healthy tissues surrounding a tumor are spared from damage caused by the radiation, which can reduce the adverse effects of radiotherapy. This benefit of using an advanced approach to radiotherapy was highlighted recently by the demonstration that patients with cervical or endometrial cancer who received IMRT after surgery rather than conventional radiotherapy reported a reduction in adverse events from the treatment, including a reduction in adverse gastrointestinal events such as diarrhea and fecal incontinence (346).
Treatment with Cytotoxic Chemotherapy
Cytotoxic chemotherapy was the third type of treatment to become a pillar of cancer care (see Figure 14). It remains the backbone of treatment for many patients with cancer to this day, although its use is constantly evolving as researchers develop new cytotoxic chemotherapeutics and identify new ways to use existing cytotoxic chemotherapeutics to improve survival and quality of life for patients.
Increasing Options for Patients with Small Cell Lung Cancer
In June 2020, the FDA approved a new cytotoxic chemotherapeutic called lurbinectedin (Zepzelca) for treating adults who have small cell lung cancer (SCLC) that has progressed despite treatment with a platinum-based cytotoxic chemotherapeutic such as cisplatin or carboplatin.
SCLC accounts for about 13 percent of the lung cancer cases diagnosed each year in the United States (5)Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin [Internet]. 2020;. This translates to almost 30,000 cases of the disease in 2020. Most patients are diagnosed with metastatic disease. Even with treatment, which is commonly a combination of carboplatin or cisplatin and the cytotoxic chemotherapeutic etoposide, most patients have disease progression. In this situation, the only FDA-approved therapeutic is the cytotoxic chemotherapeutic topotecan, but it leads to tumor responses in only about 16 percent of patients and median survival is less than 8 months (347)J T, V S, B B, V M, R L, MA S, et al. Lurbinectedin as Second-Line Treatment for Patients With Small-Cell Lung Cancer: A Single-Arm, Open-Label, Phase 2 Basket Trial. Lancet Oncol [Internet]. Lancet Oncol; 2020 [cited 2020 Jul 2];21..
The approval of lurbinectedin was based on results from a phase II clinical trial that showed that 35 percent of patients had partial tumor shrinkage (347)J T, V S, B B, V M, R L, MA S, et al. Lurbinectedin as Second-Line Treatment for Patients With Small-Cell Lung Cancer: A Single-Arm, Open-Label, Phase 2 Basket Trial. Lancet Oncol [Internet]. Lancet Oncol; 2020 [cited 2020 Jul 2];21.. These responses lasted for a median of 5.3 months. Further follow-up of the patients is required to determine whether lurbinectedin will improve overall survival for patients with metastatic SCLC, but the accelerated approval of the cytotoxic chemotherapeutic provides new hope for patients with this deadly disease.
Using Cytotoxic Chemotherapy to Reduce the Need for Surgery
In April 2020, the FDA approved a novel formulation of the cytotoxic chemotherapeutic mitomycin for treating patients with low-grade upper tract urothelial cancer. The new mitomycin formulation is called Jelmyto.
Urothelial cancer arises in cells called urothelial cells. These cells line the urethra, bladder, ureters, renal pelvis, and some other organs. Most urothelial cancers occur in the bladder, but some occur in the ureters, which are the tubes that connect the kidneys to the bladder, and the renal pelvis, which is the very top part of the ureters. These cancers are collectively referred to as upper tract urothelial cancers.
One of the main factors affecting outcomes for patients with upper tract urothelial cancer is the grade of the tumor. Low-grade upper tract urothelial cancer is usually not invasive and does not spread outside the ureter or kidney. It is a rare type of cancer; there are just 6,000 to 8,000 new patients diagnosed with the disease each year in the United States.
Most patients who have low-grade upper tract urothelial cancer are treated through surgery. In many cases, the surgery has to be very extensive, involving the complete removal of the affected kidney, ureter, and bladder cuff.
The cytotoxic chemotherapy gel provides a new, minimally invasive approach to treating low-grade upper tract urothelial cancer. The mitomycin is delivered to the cancer in a reverse thermal hydrogel that is given to patients using a ureteral catheter or nephrostomy tube. The cytotoxic chemotherapy gel is chilled at the time of administration and slowly becomes liquid as it warms up over the course of several hours before being excreted in the urine. Its approval was based on results from a phase III clinical trial that showed that 58 percent of patients had complete tumor shrinkage following six treatments of the gel administered weekly. Among these patients, 19 continued to have complete tumor shrinkage after 12 months, nine had not yet been followed for 12 months, and the rest had disease recurrence.
Mitomycin has been previously approved by the FDA in alterative formulations for treating certain patients with either stomach cancer or pancreatic cancer. By developing a new formulation of the cytotoxic chemotherapeutic, researchers are building on prior knowledge generated in the cycle of medical research to drive progress for patients with cancer.
Treatment with Molecularly Targeted Therapy
Extraordinary advances in our understanding of the biology of cancer, including the identification of numerous genetic mutations that fuel tumor growth in certain patients, set the stage for the new era of precision medicine. In this era, the standard of care for many patients is changing from a one-size-fits-all approach to one in which greater understanding of the individual patient and the characteristics of his or her cancer dictates the best treatment option for the patient (see Understanding Cancer Development.)
Therapeutics directed to the molecules influencing cancer cell multiplication and survival target the cells within a tumor more precisely than cytotoxic chemotherapeutics, which target all rapidly dividing cells, thereby limiting damage to healthy tissues. The greater precision of these molecularly targeted therapeutics tends to make them more effective and less toxic than cytotoxic chemotherapeutics. As a result, they are not only saving the lives of patients with cancer, but also allowing these individuals to have a higher quality of life than many who came before them.
In the 12 months spanning August 1, 2019 to July 31, 2020, the FDA approved 16 new molecularly targeted anticancer therapeutics (see Table 6). During this period, they also approved nine previously approved molecularly targeted anticancer therapeutics for treating additional types of cancer. For example, in April 2020, the FDA approved the molecularly targeted therapeutic encorafenib (Braftovi) for use in combination with another molecularly targeted therapeutic called cetuximab (Erbitux) for treating adults who have metastatic colorectal cancer fueled by a BRAF V600E mutation that has progressed despite prior treatments. This approval followed the approval of encorafenib for treating melanoma, which was highlighted in the AACR Cancer Progress Report 2018 (212).
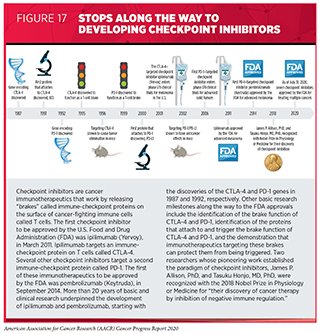
Targeting an Array of Cancers That Share the Same Genetic Alteration
One of the most significant precision medicine advances in the 12 months spanning this report was the FDA approval of a second molecularly targeted therapeutic to treat cancer based on the presence of a specific genetic biomarker in the tumor irrespective of the site at which the tumor originated. The therapeutic, entrectinib (Rozlytrek), was approved by the FDA in August 2019 for treating children and adults who have solid tumors that test positive for the NTRK gene fusion biomarker and who have no other options for treatment.
Entrectinib targets three related proteins called TRKA, TRKB, and TRKC. The genes NTRK1, NTRK2, and NTRK3 provide the code that cells use to make these proteins. Entrectinib also targets two other proteins, ROS1 and ALK.
Research has shown that genetic alterations known as chromosomal translocations that involve the three NTRK genes and lead to the production of TRK fusion proteins drive the growth of up to 1 percent of all solid tumors (348)Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, et al. Efficacy of Larotrectinib in TRK Fusion–Positive Cancers in Adults and Children. N Engl J Med [Internet]. Massachusetts Medical Society; 2018 [cited 2019 Jun 20];378:731–9.. These solid tumors encompass a wide array of cancer types that occur in adults and children, including many rare cancers, such as mammary analogue secretory carcinoma of the salivary gland, infantile fibrosarcoma, and cholangiocarcinoma.
Entrectinib was approved based on combined data from several phase I and phase II basket trials (349)RC D, A D, L P-A, S S, AT S, AF F, et al. Entrectinib in Patients With Advanced or Metastatic NTRK Fusion-Positive Solid Tumours: Integrated Analysis of Three Phase 1-2 Trials. Lancet Oncol [Internet]. Lancet Oncol; 2020 [cited 2020 Jul 2];21.(see Figure 13). The data showed that 57 percent of patients treated with the molecularly targeted therapeutic had complete or partial tumor shrinkage. Tumor shrinkage was seen across a range of cancer types, including NSCLC, mammary analogue secretory carcinoma of the salivary gland, breast cancer, colorectal cancer, thyroid cancer, pancreatic cancer, and sarcomas.
Providing New Treatments for Patients with Lung Cancer
Lung cancer is the second most commonly diagnosed cancer in the United States, with about 228,820 new cases expected to be diagnosed in 2020 (5)Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin [Internet]. 2020;. About 84 percent of lung cancers diagnosed in the United States are classified as NSCLC.
In the past decade, researchers have significantly increased our understanding of the genetic changes that fuel NSCLC growth in certain patients and developed therapeutics that target some of these changes (350)(351)Gainor JF, Shaw AT. Novel Targets in Non-Small Cell Lung Cancer: ROS1 and RET Fusions. Oncologist [Internet]. Wiley-Blackwell; 2013 [cited 2020 Jul 2];18:865.. Despite the emergence of molecularly targeted therapy as a groundbreaking new treatment for patients with NSCLC, a recent international survey of physicians who treat such patients found that 61 percent of all respondents believe that fewer than 50 percent of patients with lung cancer in their country have their tumors tested for the genetic mutations that determine whether the patient is eligible for these treatments (352)Smeltzer MP, Wynes MW, Lantuejoul S, Soo R, Ramalingam SS, Varella-Garcia M, et al. The International Association for the Study of Lung Cancer (IASLC) Global Survey on Molecular Testing in Lung Cancer. J Thorac Oncol [Internet]. Elsevier; [cited 2020 Jul 2];0.. Even among physicians in the United States and Canada, 51 percent thought this was the case. The results of this survey highlight the need for efforts to increase awareness and use of molecular testing of patients with lung cancer around the world.
About one percent of NSCLC cases are fueled by chromosomal translocations that involve the ROS1 gene and lead to the production of ROS1 fusion proteins (353)Shaw AT, Ou S-HI, Bang Y-J, Camidge DR, Solomon BJ, Salgia R, et al. Crizotinib in ROS1 -Rearranged Non–Small-Cell Lung Cancer. N Engl J Med [Internet]. Massachusetts Medical Society; 2014 [cited 2020 Jul 2];371:1963–71.. The ROS1-targeted therapeutic entrectinib was approved by the FDA in August 2019 as an initial treatment for patients with metastatic NSCLC that tests positive for chromosomal translocations involving the ROS1 gene. The approval was based on results from three phase I and phase II basket trials that showed that 78 percent of patients who received entrectinib had partial or complete tumor shrinkage (354)A D, S S, R D, F B, MG K, AT S, et al. Entrectinib in ROS1 Fusion-Positive Non-Small-Cell Lung Cancer: Integrated Analysis of Three Phase 1-2 Trials. Lancet Oncol [Internet]. Lancet Oncol; 2020 [cited 2020 Jul 2];21.. Entrectinib is also being tested in clinical trials as a potential new treatment for patients with other types of cancer fueled by ROS1 fusion proteins, including diffuse intrinsic pontine glioma, a type of pediatric brain cancer that Camden Green was diagnosed with in 2018.
Another 3 to 4 percent of NSCLCs are fueled by mutations in the MET gene that result in the part of the gene called exon 14 being missing (355)M S, R B, WT L, M de J, TM B, A A, et al. Molecular Correlates of Response to Capmatinib in Advanced Non-Small-Cell Lung Cancer: Clinical and Biomarker Results From a Phase I Trial. Ann Oncol Off J Eur Soc Med Oncol [Internet]. Ann Oncol; 2020 [cited 2020 Jul 2];. In May 2020, the FDA approved the first MET-targeted therapeutic, capmatinib (Tabrecta). It was approved for treating patients with metastatic NSCLC that tests positive for MET exon 14 skipping mutations using a specific companion diagnostic called FoundationOne CDx (see sidebar on Companion Diagnostics). The approval was based on results from a phase II clinical trial that showed that 68 percent of patients who received capmatinib as their initial treatment had partial or complete tumor shrinkage. Among patients who received capmatinib after other treatments, 41 percent had partial or complete tumor shrinkage.
Yet another 1 to 2 percent of NSCLCs are fueled by chromosomal translocations that involve the RET gene and lead to the production of RET fusion proteins (356)Subbiah V, Gainor JF, Rahal R, Brubaker JD, Kim JL, Maynard M, et al. Precision Targeted Therapy with BLU-667 for RET -Driven Cancers. Cancer Discov [Internet]. American Association for Cancer Research; 2018 [cited 2020 Jul 2];8:836–49.. In May 2020, the FDA approved the first RET-targeted therapeutic, selpercatinib (Retevmo), for treating patients with metastatic NSCLC that tests positive for chromosomal translocations involving the RET gene. The approval was based on results from a phase I/II clinical trial that showed that 85 percent of patients who received selpercatinib as their initial treatment had partial or complete tumor shrinkage. Among patients whose NSCLC had progressed after platinum-based chemotherapy, 64 percent had partial or complete tumor shrinkage.
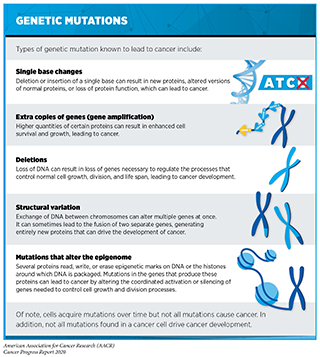
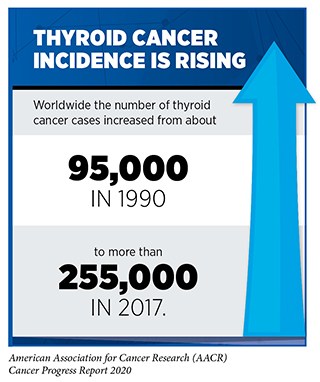
In May 2020, selpercatinib was also approved by the FDA for treating certain patients with thyroid cancer. It is projected that 52,890 new cases of the disease will be diagnosed in 2020 (5)Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin [Internet]. 2020;. Papillary thyroid cancer is the most common type of thyroid cancer. Chromosomal translocations involving the RET gene are found in up to 20 percent of these cancers (356)Subbiah V, Gainor JF, Rahal R, Brubaker JD, Kim JL, Maynard M, et al. Precision Targeted Therapy with BLU-667 for RET -Driven Cancers. Cancer Discov [Internet]. American Association for Cancer Research; 2018 [cited 2020 Jul 2];8:836–49.. For medullary thyroid cancer, more than 50 percent of cases are fueled by other types of RET gene mutation (see sidebar on Genetic Mutations). The FDA approved selpercatinib specifically for patients age 12 or older who have advanced or metastatic medullary thyroid cancer that tests positive for RET gene alterations and who require systemic therapy, and for patients age 12 and older who have advanced or metastatic thyroid cancer that tests positive for chromosomal translocations involving the RET gene, who require systemic therapy, and whose cancer is not responding to standard treatment with radioactive iodine. These two approvals were based on results from the same clinical trial that led to the approval of selpercatinib for NSCLC. Among patients with medullary thyroid cancer that tests positive for RET gene alterations, 73 percent had partial or complete tumor shrinkage. Among those with thyroid cancer that tests positive for chromosomal translocations involving the RET gene and that is not responding to radioactive iodine, 100 percent had partial or complete tumor shrinkage.
Expanding the Uses for PARP-targeted Therapeutics
The number of cancer types for which therapeutics targeting poly ADP-ribose polymerase (PARP) proteins are an FDA approved treatment doubled in the 12 months covered by this report. On August 1, 2019, there was at least one PARP-targeted therapeutic approved to treat certain patients with breast cancer or ovarian cancer. As of July 31, 2020, certain patients with pancreatic cancer or with prostate cancer were also eligible for treatment with these molecularly targeted therapeutics (see Table 6).
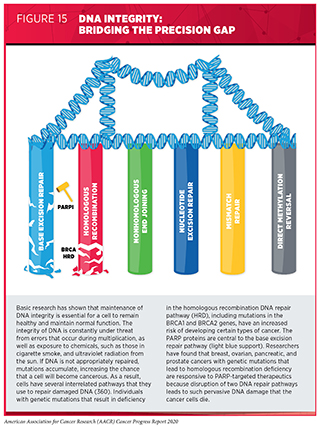
The development and FDA approval of PARP-targeted therapeutics was based on decades of basic and clinical research (358). PARP proteins have a key role in one of the many pathways that cells use to repair damaged DNA, the base excision repair pathway (359)Lord CJ, Ashworth A. PARP Inhibitors: The First Synthetic Lethal Targeted Therapy. Science [Internet]. Europe PMC Funders; 2017 [cited 2020 Jul 2];355:1152.. Blocking this pathway with PARP-targeted therapeutics reduces the ability of a cell to repair damaged DNA.
Deficiencies in other DNA repair pathways are found in some cancers. In some patients, these deficiencies are inherited. For example, inherited mutations in homologous recombination modifying genes such as BRCA1 and BRCA2 are associated with hereditary breast and ovarian cancer syndrome (see Table 2). However, mutations in these and other DNA repair pathway genes can also arise during a patient’s lifetime and are found in sporadic tumors.
Although PARP and BRCA proteins work in different DNA repair pathways, disruption to both pathways can ultimately trigger cell death (see Figure 15). Therefore, cancer cells harboring cancer-associated BRCA1 or BRCA2 (BRCA1/2) mutations that disable the BRCA proteins to repair damaged DNA are particularly susceptible to PARP-targeted therapeutics (359)Lord CJ, Ashworth A. PARP Inhibitors: The First Synthetic Lethal Targeted Therapy. Science [Internet]. Europe PMC Funders; 2017 [cited 2020 Jul 2];355:1152.. As a result of these discoveries, PARP-targeted therapeutics were first developed and approved for treating women with advanced ovarian cancer who have inherited a cancer-associated BRCA1/2 mutation.
As researchers learned more about the biology of cancer, it was determined that mutations in genes other than BRCA1/2 can disrupt the homologous recombination DNA repair pathway (359)Lord CJ, Ashworth A. PARP Inhibitors: The First Synthetic Lethal Targeted Therapy. Science [Internet]. Europe PMC Funders; 2017 [cited 2020 Jul 2];355:1152.. Homologous recombination deficiency (HRD) leads to the accumulation of DNA damage, a situation known as genomic instability, which, in turn, can lead to cancer. Therefore, researchers tested whether HRD might provide a new biomarker for cancers susceptible to PARP-targeted therapeutics. In October 2019, the FDA approved the PARP-targeted therapeutic niraparib (Zejula) for treating women with ovarian cancer, fallopian tube cancer, or primary peritoneal cancer that has progressed despite treatment with at least three different cytotoxic chemotherapy regimens and that tests positive for HRD using the myChoice CDx companion diagnostic. The myChoice CDx companion diagnostic determines HRD status by testing for the presence of cancer-associated BRCA1/2 mutations and three other markers of genomic instability. The approval was based on results from a phase II clinical trial that showed that 24 percent of patients who had HRD-positive advanced ovarian cancer had partial tumor shrinkage.
The success of the PARP-targeted therapeutics as treatments for breast and ovarian cancer fueled by an inherited BRCA1/2 mutation led researchers to investigate whether these molecularly targeted therapeutics might also benefit patients with other types of cancer-fueled mutations in BRCA1/2 and other genes that cause HRD.
Pancreatic cancer is one of the deadliest types of cancer; just 9 percent of patients are alive five years after diagnosis (5)Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin [Internet]. 2020;. Researchers estimate that 4 to 7 percent of pancreatic cancers diagnosed in the United States are attributable to an inherited BRCA1/2 mutation (361)T G, P H, M R, E VC, T M, MJ H, et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med [Internet]. N Engl J Med; 2019 [cited 2020 Jul 2];381.. In December 2019, the FDA approved the PARP-targeted therapeutic olaparib (Lynparza) for treating patients with metastatic pancreatic cancer that has not progressed during first-line treatment with a platinum-based chemotherapy regimen and who have an inherited BRCA1/2 mutation, as determined using the BRACAnalysis CDx companion diagnostic. The approval was based on results from a phase III clinical trial that showed that treatment with olaparib almost doubled the median time to disease progression (361)T G, P H, M R, E VC, T M, MJ H, et al. Maintenance Olaparib for Germline BRCA-Mutated Metastatic Pancreatic Cancer. N Engl J Med [Internet]. N Engl J Med; 2019 [cited 2020 Jul 2];381.. Progression-free survival was 7.4 months for those who received olaparib compared with 3.8 months for those who received placebo.
Prostate cancer is the most commonly diagnosed cancer among men living in the United States (5)Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin [Internet]. 2020;. In 2020 alone, more than 191,000 men are expected to be newly diagnosed with the disease. Research has shown that up to 30 percent of prostate cancers have mutations in genes that influence the homologous recombination DNA repair pathway, most commonly BRCA1/2 or ATM (362)J de B, J M, K F, F S, N S, S S, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med [Internet]. N Engl J Med; 2020 [cited 2020 Jul 2];382.. In May 2020, the FDA approved two PARP-targeted therapeutics for treating certain groups of men with metastatic prostate cancer fueled by mutations in genes that influence the homologous recombination DNA repair pathway, olaparib and rucaparib (Rubraca).
Initially, men who are diagnosed with metastatic prostate cancer are often treated with therapeutics that target androgens, the hormones that fuel prostate cancer growth. When the prostate cancer stops responding to these treatments, it is referred to as castration-resistant prostate cancer. Olaparib was approved for treating men who have metastatic castration-resistant prostate cancer that has progressed despite treatment with enzalutamide (Xtandi) or abiraterone (Zytiga) and that has a mutation in any gene that influences the homologous recombination DNA repair pathway, including BRCA1/2. The FoundationOne CDx companion diagnostic can be used to identify any mutation causing homologous recombination DNA repair pathway deficiency, including BRCA1/2 mutations, and the BRACAnalysis CDx companion diagnostic can be used to detect BRCA1/2 mutations. The approval was based on results from a phase III clinical trial that showed that treatment with olaparib improved overall survival compared with treatment with either enzalutamide or abiraterone (362)J de B, J M, K F, F S, N S, S S, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med [Internet]. N Engl J Med; 2020 [cited 2020 Jul 2];382..
Rucaparib was approved for a slightly different group of men who have metastatic castration-resistant prostate cancer, those whose cancer has progressed despite treatment with an androgen receptor–directed therapy and a taxane-based cytotoxic chemotherapeutic such as docetaxel and has a cancer-associated BRCA1/2 mutation. The approval was based on results from a phase II clinical trial that showed that 44 percent of patients who received rucaparib had partial or complete tumor shrinkage.
Increasing Options for Treating Breast Cancer
Breast cancer is the most commonly diagnosed cancer among women living in the United States (5)Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin [Internet]. 2020;. In 2020 alone, 276,480 women and 2,620 men are expected to be newly diagnosed with the disease. Even though remarkable progress is being made against the disease, as illustrated by the fact that the overall breast cancer death rate decreased by 40 percent from 1989 to 2017 (363)DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA Cancer J Clin [Internet]. 2019 [cited 2019 Oct 4];caac.21583., breast cancer remains the second-leading cause of cancer-related death for women in the United States (5)Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin [Internet]. 2020;.
For patients with breast cancer, one factor determining what treatment options should be considered is the presence or absence of three tumor biomarkers, two hormone receptors and HER2. About 15 percent of breast cancers diagnosed the United States are characterized as HER2-positive (363)DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast cancer statistics, 2019. CA Cancer J Clin [Internet]. 2019 [cited 2019 Oct 4];caac.21583.. HER2-positive breast cancer tends to be aggressive, and the outcome for patients was typically poor until research led to the development and FDA approval of HER2-targeted therapeutics. Trastuzumab (Herceptin) was the first of these groundbreaking therapeutics to be approved by the FDA, in 1998.
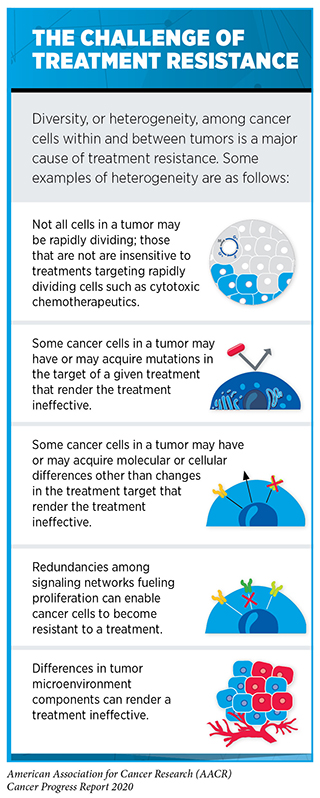
There are now numerous HER2-targeted therapeutics approved for treating advanced or metastatic HER2-positive breast cancer. Most patients are first treated with a combination of trastuzumab, a second HER2-targeted therapeutic called pertuzumab (Perjeta), and a cytotoxic chemotherapeutic. These therapeutics were always given intravenously until July 2020, when a combination of trastuzumab, pertuzumab, and hyaluronidase–zzxf called Phesgo that can be given subcutaneously (under the skin) was approved by the FDA as an alternative option. Despite the success of the trastuzumab–pertuzumab combination, most HER2-positive breast cancers eventually progress because they become treatment resistant (see sidebar on The Challenge of Treatment Resistance). Recent FDA decisions have provided two new molecularly targeted therapeutics, fam-trastuzumab deruxtecan-nxki (Enhertu) and tucatinib (Tukysa), to help address this challenge.
Fam-trastuzumab deruxtecan-nxki is a type of molecularly targeted therapeutic called an antibody-drug conjugate. It comprises a cytotoxic agent, deruxtecan, attached to the HER2-targeted antibody trastuzumab by a linker. When the antibody attaches to HER2 on the surface of breast cancer cells, the antibody-drug conjugate is internalized by the cells. This leads to deruxtecan being released from the linker and antibody. Once free, the deruxtecan is toxic to the breast cancer cells, which ultimately die.
Fam-trastuzumab deruxtecan-nxki was approved by the FDA in December 2019 for treating adults with metastatic HER2-positive breast cancer that has progressed despite treatment with two or more other HER2-targeted treatment regimens. The approval was based on results from a phase II clinical trial that showed that just over 60 percent of the patients who received the recommended dose of fam-trastuzumab deruxtecan-nxki had partial or complete tumor shrinkage (364)Modi S, Saura C, Yamashita T, Park YH, Kim S-B, Tamura K, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med [Internet]. Massachusetts Medical Society; 2020 [cited 2020 Jul 2];382:610–21..
Like all cancer treatments, fam-trastuzumab deruxtecan-nxki can have adverse effects, some of which can be very severe. One of the most concerning and, in some cases, life-threatening, is interstitial lung disease. Therefore, the FDA approved fam-trastuzumab deruxtecan-nxki with a boxed warning for interstitial lung disease and recommends that patients being treated with the molecularly targeted therapeutics be monitored for signs and symptoms of interstitial lung disease, including cough, dyspnea (difficult or labored breathing), fever and other new or worsening respiratory symptoms. If interstitial lung disease is suspected, further testing and intervention should be considered.
Tucatinib blocks HER2 from sending signals that promote the multiplication and survival of breast cancer cells. It was approved by the FDA in April 2020 for use in combination with trastuzumab and the cytotoxic chemotherapeutic capecitabine in the treatment of adults who have advanced or metastatic HER2-positive breast cancer that has progressed despite treatment with one or more other HER2-targeted therapeutics. The approval was based on results from a phase II clinical trial that showed that adding tucatinib to trastuzumab and capecitabine significantly improved median overall survival (365)RK M, S L, A O, E P, E H, SA H, et al. Tucatinib, Trastuzumab, and Capecitabine for HER2-Positive Metastatic Breast Cancer. N Engl J Med [Internet]. N Engl J Med; 2020 [cited 2020 Jul 2];382.. Among patients who had metastases in the brain, which are particularly hard to treat, the median time to disease progression was significantly longer for those who received tucatinib.
About 10 percent of all breast cancers diagnosed in the United States test negative for HER2 and the two hormone receptors (364)Modi S, Saura C, Yamashita T, Park YH, Kim S-B, Tamura K, et al. Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer. N Engl J Med [Internet]. Massachusetts Medical Society; 2020 [cited 2020 Jul 2];382:610–21.. This type of breast cancer, which is often referred to as triple-negative breast cancer, is highly aggressive. Patients often have poor outcomes, in part because cytotoxic chemotherapeutics were the only systemic treatment options for patients with metastatic triple-negative breast cancer until recently. Even with such treatments, the median overall survival is estimated to be less than 18 months (366)Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med [Internet]. Massachusetts Medical Society; 2018 [cited 2019 Jul 15];379:2108–21..
In April 2020, the FDA approved an antibody-drug conjugate called sacituzumab govitecan-hziy (Trodelvy) for treating adults who have metastatic triple-negative breast cancer that has progressed despite the patient having tried at least two other treatment regimens. Sacituzumab govitecan-hziy comprises an antibody that targets the protein Trop-2, which researchers have detected in a high proportion of triple-negative breast cancers, attached by a linker to a cytotoxic agent called SN-38. When the antibody attaches to Trop-2 on the surface of triple-negative breast cancer cells, the antibody-drug conjugate is internalized by the cells. This leads to SN-38 being released from the linker and antibody. Once free, the SN-38 is toxic to the cancer cells, which ultimately die. Sacituzumab govitecan-hziy was approved by the FDA after it was shown in a phase I/II clinical trial to cause partial or complete tumor shrinkage for 33 percent of patients who received the antibody-drug conjugate. Although more time is needed to determine there is also a survival benefit, sacituzumab govitecan-hzi has already transformed the lives of many patients with triple-negative breast cancer, such as Ferda Martin.
Adding Precision to Treatment for Blood Cancers
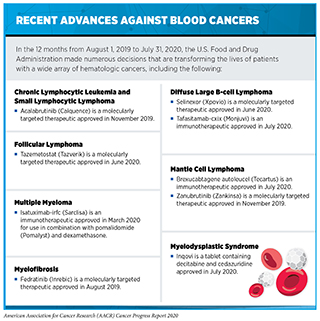
Cancers that arise in blood-forming tissues, such as the bone marrow, or in cells of the immune system are called blood cancers, or hematologic cancers. In the 12 months covered by this report, the FDA has made numerous decisions that are transforming the lives of patients with a wide array of hematologic cancers (see sidebar on Recent Advances against Blood Cancers). Among these decisions are the approval of two new molecularly targeted therapeutics for use in the treatment of certain types of these diseases and the expansion of the uses of three previously approved molecularly targeted therapeutics to include the treatment of additional types of blood cancer.
Non-Hodgkin lymphoma is the most commonly diagnosed blood cancer in the United States. In 2020, 77,240 people in the United States are expected to be newly diagnosed with the disease and 19,940 to die from it (5)Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin [Internet]. 2020;.
The term non-Hodgkin lymphoma encompasses many different types of cancer, most of which arise in immune cells called B cells. Two molecularly targeted therapeutics to be recently approved by the FDA for treating different types of non-Hodgkin lymphoma arising in B cells—zanubrutinib (Zankinsa) and acalabrutinib (Calquence)—target a protein called BTK, which is one component of a signaling pathway that promotes the survival and expansion of non-Hodgkin lymphoma B cells.
Zanubrutinib is a new BTK-targeted therapeutic that was approved by the FDA in November 2019 for treating certain adults who have an aggressive type of non-Hodgkin lymphoma called mantle cell lymphoma. Zanubrutinib was approved for treating those patients whose mantle cell lymphoma has progressed despite at least one prior treatment. The approval was based on combined results from a phase I/II clinical trial and a phase II clinical trial. In both trials, 84 percent of the patients had partial or complete tumor shrinkage following treatment with zanubrutinib.
Acalabrutinib was approved for treating patients with mantle cell lymphoma in October 2017 (212). In November 2019, the FDA added an approval for using the BTK-targeted therapeutic for treating adults who have chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL), which are slow-growing types of non-Hodgkin lymphoma. CLL and SLL are essentially the same disease but have different names depending on where in the body the non-Hodgkin lymphoma cells accumulate. CLL cells are found mostly in the blood and bone marrow, whereas SLL cells are found mostly in the lymph nodes. The CLL and SLL acalabrutinib approval was based on results from two phase III clinical trials that showed that the time before disease progressed was significantly longer among patients who received acalabrutinib compared with patients who received alternative standard treatments (367)JP S, M E, W J, A S, JM P, IW F, et al. Acalabrutinib With or Without Obinutuzumab Versus Chlorambucil and Obinutuzmab for Treatment-Naive Chronic Lymphocytic Leukaemia (ELEVATE TN): A Randomised, Controlled, Phase 3 Trial. Lancet (London, England) [Internet]. Lancet; 2020 [cited 2020 Jul 2];395..
The most common type of non-Hodgkin lymphoma diagnosed in the United States is diffuse large B-cell lymphoma. There are several forms of this aggressive type of non-Hodgkin lymphoma but the most common is called diffuse large B-cell lymphoma, not otherwise specified. In June 2020, the FDA approved the molecularly targeted therapeutic selinexor (Xpovio) for treating patients with diffuse large B-cell lymphoma, not otherwise specified, whose disease has relapsed after or never responded to treatment with at least two other systemic treatments. Selinexor targets a protein called XPO1, which is found at elevated levels in diffuse large B-cell lymphoma cells. XPO1 helps move proteins out of a part of the cell called the nucleus. It is particularly linked to moving proteins that suppress tumor growth out of the nucleus. When selinexor targets XPO1, it forces these proteins to be retained in the nucleus where they can act to suppress tumor growth. The approval of selinexor was based on results from a phase II clinical trial that showed that 29 percent of patients had complete or partial tumor shrinkage after treatment with the new molecularly targeted therapeutic (368)N K, M M, F C, G F, A G, JSP V, et al. Selinexor in Patients With Relapsed or Refractory Diffuse Large B-cell Lymphoma (SADAL): A Single-Arm, Multinational, Multicentre, Open-Label, Phase 2 Trial. Lancet Haematol [Internet]. Lancet Haematol; 2020 [cited 2020 Jul 2];7..
Myelofibrosis is a rare type of blood cancer. It is estimated that there are about 20,000 people living with the disease in the United States (369)Palmer J, Mesa R. The role of fedratinib for the treatment of patients with primary orsecondary myelofibrosis. Ther Adv Hematol [Internet]. SAGE Publications; 2020 [cited 2020 Jul 2];11.. In many cases, myelofibrosis is driven by mutations in the JAK2 gene. In August 2019, the FDA approved a new JAK2-targeted therapeutic, fedratinib (Inrebic), for treating certain patients who have myelofibrosis.
Myelofibrosis is one of a group of six blood cancers called chronic myeloproliferative neoplasms: chronic myelogenous leukemia, polycythemia vera, primary myelofibrosis, essential thrombocythemia, chronic neutrophilic leukemia, and chronic eosinophilic leukemia. In some cases, polycythemia vera and essential thrombocythemia progress to become myelofibrosis. In this situation, the disease is referred to as secondary myelofibrosis.
Myelofibrosis usually develops slowly. Abnormal blood cells and fibers build up inside the bone marrow, which is where blood cells are made, leading to low levels of red blood cells (anemia). This causes tiredness, weakness, and shortness of breath. In addition, to make up for the low number of blood cells, an organ in the body called the spleen begins to make blood cells, which causes the spleen to enlarge dramatically, a condition known as splenomegaly.
The likely outcome for patients diagnosed with myelofibrosis is estimated based on several risk factors. Patients with more than one risk factor — including being age 65 or older; having anemia; experiencing fever, night sweats, or weight loss; having high white blood cell counts; and having at least 1 percent of cells in the blood being cancer cells — are classed as having intermediate-2 risk disease. Patients with four or more risk factors are classed as high risk.
Fedratinib was approved for treating adults with intermediate-2 or high-risk primary or secondary myelofibrosis. The approval was based on results from a phase III clinical trial that showed that treatment with fedratinib significantly reduced spleen volume and reduced myelofibrosis-related symptoms compared with placebo (369)Palmer J, Mesa R. The role of fedratinib for the treatment of patients with primary orsecondary myelofibrosis. Ther Adv Hematol [Internet]. SAGE Publications; 2020 [cited 2020 Jul 2];11..
Using Epigenetic Therapy to Treat Cancer
Research has shown that how and when genes are turned “on” or “off” is regulated by special factors called epigenetic modifications (see Cancer Development: Influences inside the Cell). These modifications tag DNA or attach to histones. The sum of these modifications across the entire genome is called the epigenome.
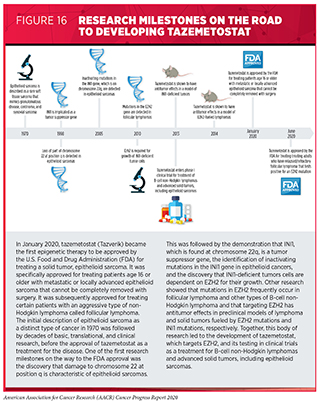
Genetic mutations that disrupt the epigenome can lead to cancer development. For example, mutations that lead to loss of the protein INI1, which is involved in epigenetic regulation of gene accessibility, drive more than 90 percent of a rare type of cancer called epithelioid sarcoma (370)Stacchiotti S, Schoffski P, Jones R, Agulnik M, Villalobos VM, Jahan TM, et al. Safety and efficacy of tazemetostat, a first-in-class EZH2 inhibitor, in patients (pts) with epithelioid sarcoma (ES) (NCT02601950). J Clin Oncol [Internet]. American Society of Clinical Oncology; 2019 [cited 2020 Jul 2];37:11003–11003.. Researchers found that the multiplication and survival of cancer cells lacking INI1 protein depends on a protein called EZH2, which adds epigenetic modifications called methyl groups to histones (371)Kim KH, Kim W, Howard TP, Vazquez F, Tsherniak A, Wu JN, et al. SWI/SNF-mutant cancers depend on catalytic and non-catalytic activity of EZH2. Nat Med [Internet]. Nature Publishing Group; 2015 [cited 2020 Jul 2];21:1491–6..
Tazemetostat (Tazverick) targets EZH2, preventing it from adding methyl groups to histones. It was approved by the FDA in January 2020, after several decades of basic, translational, and clinical research, for treating patients age 16 or older with metastatic or locally advanced epithelioid sarcoma that cannot be completely removed with surgery (see Figure 16).
Epithelioid sarcoma is a type of soft-tissue sarcoma that usually arises in the soft tissue under the skin of a finger, hand, forearm, lower leg, or foot. In 2020, it is estimated that there will be 13,130 new cases of soft tissue sarcoma diagnosed in the United States in 2020 (5)Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin [Internet]. 2020;. Given that epithelioid sarcoma accounts for about 1 percent of soft tissue sarcomas (379)SB R, SB B. Epigenetic Therapy for Epithelioid Sarcoma. Cell [Internet]. Cell; 2020 [cited 2020 Jul 2];181., it is projected that about 1,300 people will be diagnosed with the disease in 2020.
Tazemetostat was approved for treating metastatic or locally advanced epithelioid sarcoma after it was shown in a phase II clinical trial to cause partial or complete tumor shrinkage for 15 percent of patients who received the molecularly targeted therapeutic (369)Palmer J, Mesa R. The role of fedratinib for the treatment of patients with primary orsecondary myelofibrosis. Ther Adv Hematol [Internet]. SAGE Publications; 2020 [cited 2020 Jul 2];11.. The approval made tazemetostat the first therapeutic approved by the FDA specifically for treating patients with epithelioid sarcoma, like Sandra Griego, and the first epigenetic therapy for treating patients with a solid tumor.
In June 2020, the FDA approved tazemetostat for treating certain patients with a different type of cancer, follicular lymphoma. Follicular lymphoma is an aggressive type of non-Hodgkin lymphoma that arises in B cells. Up to 25 percent of these cancers are fueled by mutations in EZH2 (380)A I, JC S, M T, JM M, C L, A V, et al. Tazemetostat, an EZH2 Inhibitor, in Relapsed or Refractory B-cell non-Hodgkin Lymphoma and Advanced Solid Tumours: A First-In-Human, Open-Label, Phase 1 Study. Lancet Oncol [Internet]. Lancet Oncol; 2018 [cited 2020 Jul 2];19.. Tazemetostat was approved for treating adults who have follicular lymphoma that has relapsed after or never responded to treatment with at least two other systemic treatments and that tests positive for an EZH2 mutation using the cobas EZH2 Mutation Test companion diagnostic. This approval was based on results from a phase II clinical trial that showed that 69 percent of patients with EZH2 mutation–positive follicular lymphoma had complete or partial tumor shrinkage after treatment with tazemetostat. Tazemetostat was also approved for treating adults who have follicular lymphoma without an EZH2 mutation so long as the lymphoma has relapsed after or never responded to other treatments and there are no satisfactory alternative treatment options after it was shown in the phase II clinical trial that 34 percent of patients in this medical situation had complete or partial tumor shrinkage.
Research has shown that the addition of methyl groups to DNA is particularly important for promoting the growth of many types of blood cancer, including a group of rare blood cancers called MDS (381)Kantarjian H, Issa J-PJ, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer [Internet]. Cancer; 2006 [cited 2020 Aug 3];106:1794–803.. This knowledge was harnessed in the development of the chemotherapeutic decitabine as a treatment for MDS in the mid 2000’s (381)Kantarjian H, Issa J-PJ, Rosenfeld CS, Bennett JM, Albitar M, DiPersio J, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer [Internet]. Cancer; 2006 [cited 2020 Aug 3];106:1794–803.. Decitabine disrupts the addition of methyl groups to DNA by proteins called DNA methyltransferases because it becomes incorporated into the DNA strands of MDS cells as they multiply and traps DNA methyltransferases in place, preventing them from adding further methyl groups to DNA.
Decitabine is given intravenously, which means that patients must travel to a health care facility to receive treatment. In July 2020, the FDA approved a tablet that contains decitabine together with another drug called cedazuridine, which prevents rapid breakdown of decitabine in the gut and liver, for treating certain patients with MDS. The new treatment, which is called Inqovi, has the potential to reduce the number of visits a patient must make to a health care facility, which is particularly important to patients during the COVID-19 pandemic.
MDS arise when the development of blood cells in the bone marrow goes awry. The type of MDS a person has, and therefore the symptoms he or she has, is determined by the type of blood cell affected and how it is affected. One of the most common systems for classifying MDS is the French-American-British system, which describes five main types of the disease: refractory anemia; refractory anemia with sideroblasts; refractory anemia with excess blasts; refractory anemia with excess blasts in transformation; and chronic myelomonocytic leukemia. The severity of a patient’s disease, which is an important factor in deciding the individual’s best treatment option, is determined using the International Prognostic Scoring System.
Inqovi was approved for treating adults who have refractory anemia, refractory anemia with sideroblasts, refractory anemia with excess blasts, or chronic myelomonocytic leukemia that is determined to be intermediate-1, intermediate-2, or high-risk using International Prognostic Scoring System group. The approval was based on cumulative data from two clinical trials that showed that about 20 percent of patients had a complete response following treatment with Inqovi (382)Savona MR, Odenike O, Amrein PC, Steensma DP, DeZern AE, Michaelis LC, et al. An oral fixed-dose combination of decitabine and cedazuridine in myelodysplastic syndromes: a multicentre, open-label, dose-escalation, phase 1 study. Lancet Haematol [Internet]. Lancet Haematol; 2019 [cited 2020 Aug 3];6:e194–203.(383).
Bringing the Promise of Precision Medicine to Patients with Rare Cancers
A cancer type is defined as rare by the NCI if fewer than 40,000 people are newly diagnosed with the disease in a given year (384)About Rare Cancers – National Cancer Institute [Internet]. [cited 2020 Jul 2].. Together, rare cancers account for about 27 percent of cancer cases and about 25 percent of cancer deaths each year in the United States.
Rare cancers can be challenging for researchers to study and for physicians to treat (see sidebar on The Challenges Posed by Rare Cancers). However, during the 12 months covered by this report, August 1, 2019, to July 31, 2020, the FDA approved new molecularly targeted therapeutics for treating several types of rare cancer, bringing the promise of precision medicine to patients who often have few treatment options.
Gastrointestinal stromal tumors (GISTs) are soft tissue sarcomas that arise anywhere along the gastrointestinal tract but most commonly in the stomach or small intestine. It is estimated that there are around 3,300 to 6,000 new cases of GIST each year in the United States (388)CL C, MC H. Molecular Pathobiology of Gastrointestinal Stromal Sarcomas. Annu Rev Pathol [Internet]. Annu Rev Pathol; 2008 [cited 2020 Jul 2];3.. The discovery that most GISTs are fueled by mutations in either the KIT gene or the PDGFRA gene led to the development and FDA approval of therapeutics that target KIT and PDGFRα. Imatinib (Gleevec) became the first of these molecularly targeted therapeutics approved by the FDA for treating GISTs in 2002.
Despite the success of imatinib as a treatment for patients with GIST that is metastatic, recurrent, or unresectable (meaning that it cannot be surgically removed), some tumors do not respond to the molecularly targeted therapeutic and most of those that do initially respond eventually progress because they have become treatment resistant. Two recent FDA decisions are helping to address these challenges.
GISTs fueled by a specific mutation called D842V in a region of the PDGFRA gene known as exon 18 do not respond to imatinib or other FDA-approved molecularly targeted therapeutics (389)Cassier PA, Fumagalli E, Rutkowski P, Schoffski P, Van Glabbeke M, Debiec-Rychter M, et al. Outcome of Patients with Platelet-Derived Growth Factor Receptor Alpha-Mutated Gastrointestinal Stromal Tumors in the Tyrosine Kinase Inhibitor Era. Clin Cancer Res [Internet]. American Association for Cancer Research; 2012 [cited 2020 Jul 2];18:4458–64.. Avapritinib (Ayvakit) is a new molecularly targeted therapeutic that can block the effects of D842V mutations. In January 2020, the FDA approved avapritinib for treating adults with metastatic or unresectable GIST that tests positive for any PDGFRA exon 18 mutation, including D842V mutations. The approval was based on results from a phase I clinical trial that showed that 89 percent of patients who had GIST fueled by D842V mutations had complete or partial tumor shrinkage. In a larger group of patients with any mutation in exon 18 of PDGFRA, 84 percent had complete or partial tumor shrinkage.
In May 2020, the FDA approved another new molecularly targeted therapeutic to help address the challenge of treatment resistance, ripretinib (Qinlick). Ripretinib targets KIT and PDGFRα, like imatinib does. It was approved for treating adults with advanced GIST that has progressed despite treatment with three or more other molecularly targeted therapeutics, including imatinib, after it was shown in a phase III clinical trial to dramatically increase the time to disease progression compared with placebo (390)JY B, C S, MC H, J Z, S B, H G, et al. Ripretinib in Patients With Advanced Gastrointestinal Stromal Tumours (INVICTUS): A Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Oncol [Internet]. Lancet Oncol; 2020 [cited 2020 Jul 2];.
Tenosynovial giant cell tumors are a group of rare tumors that arise in and around the joints and tendons (391)Tenosynovial Giant Cell Tumor – NORD (National Organization for Rare Disorders) [Internet]. [cited 2020 Jul 2].. These tumors are benign, but they cause damage to the joints, which leads to pain, swelling, and limitation of movement of the joint. Surgery is the main treatment option. If patients do not have surgery or if the tumor continually recurs, patients suffer damage and degeneration of the affected joint and surrounding tissues or structures. In some cases, this can cause significant disability.
Research has shown that some cells in tenosynovial giant cell tumors have genetic alterations, called chromosomal translocations, that lead to overproduction of the protein CSF1, which attracts large numbers of immune cells to the site of the tumor. The immune cells that accumulate form the bulk of the tumor and cause damage to surrounding tissue. Pexidartinib (Turalio) is a new molecularly targeted therapeutic that targets the protein that CSF1 attaches to on the surface of immune cells, CSF1R, preventing CSF1 from attaching and, thereby, recruiting the immune cells to the tumor. It was approved by the FDA in August 2019 for treating adults who have a tenosynovial giant cell tumor that is causing them severe morbidity or functional limitations and is not amenable to surgery. The approval was based on results from a phase III clinical trial that showed 38 percent of patients who received pexidartinib had complete or partial tumor shrinkage compared with none of the patients who received placebo (392)Tap WD, Gelderblom H, Palmerini E, Desai J, Bauer S, Blay J-Y, et al. Pexidartinib versus placebo for advanced tenosynovial giant cell tumour (ENLIVEN): a randomised phase 3 trial. Lancet [Internet]. Elsevier; 2019 [cited 2020 Jul 2];394:478–87..
Pexidartinib was the first molecularly targeted therapeutic approved specifically for treating tenosynovial giant cell tumors. In April 2020, patients with another rare type of cancer, cholangiocarcinoma, or bile duct cancer, also gained a first molecularly targeted therapeutic treatment option, pemigatinib (Pemazyre).
Cholangiocarcinoma arises in cells that form the bile ducts, which are small tubes that connect the liver and gallbladder to the small intestine. There are two forms of the disease, named depending on whether the bile ducts in which the cancer begins are inside (intrahepatic cholangiocarcinoma) or outside (extrahepatic cholangiocarcinoma) the liver. Most patients have advanced disease at the time they are first diagnosed with cholangiocarcinoma. Despite treatment with cytotoxic chemotherapy, the prognosis for these patients is poor (393)Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin Cancer Res [Internet]. American Association for Cancer Research; 2018 [cited 2020 Jul 2];24:4154–61..
Researchers discovered that the growth of up to 20 percent of intrahepatic cholangiocarcinomas is fueled by alterations in the FGFR2 gene (393)Lowery MA, Ptashkin R, Jordan E, Berger MF, Zehir A, Capanu M, et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin Cancer Res [Internet]. American Association for Cancer Research; 2018 [cited 2020 Jul 2];24:4154–61.. Pemigatinib targets FGFR2. It was approved by the FDA for treating adults who have previously treated, unresectable locally advanced or metastatic cholangiocarcinoma that tests positive for an FGFR2 gene alteration using the FoundationOne CDx companion diagnostic. The approval was based on results from a phase II clinical trial that showed that treatment with pemigatinib led to complete or partial tumor shrinkage for 36 percent of patients (394)GK A-A, V S, A H, G V, D M, R A-R, et al. Pemigatinib for Previously Treated, Locally Advanced or Metastatic Cholangiocarcinoma: A Multicentre, Open-Label, Phase 2 Study. Lancet Oncol [Internet]. Lancet Oncol; 2020 [cited 2020 Jul 2];21..
Neurofibromatosis type 1 (NF1) is a genetic disorder that causes severe symptoms and complications including a significantly increased risk for developing various types of tumors. Although the tumors that develop in patients with NF1 are usually benign, some patients develop malignant tumors, usually in adolescence or adulthood. Plexiform neurofibromas are tumors that arise in cells that form the covering of peripheral nerves. These benign tumors occur in up to 50 percent of patients with NF1 and can cause pain, disability, and disfigurement. They can also go on to become cancerous.
Research has shown that the growth of plexiform tumors in patients with NF1 is fueled by a signaling pathway that includes proteins called MEK proteins (395)Dombi E, Baldwin A, Marcus LJ, Fisher MJ, Weiss B, Kim A, et al. Activity of Selumetinib in Neurofibromatosis Type 1–Related Plexiform Neurofibromas. N Engl J Med [Internet]. Massachusetts Medical Society; 2016 [cited 2020 Jul 2];375:2550–60.. In April 2020, the FDA approved the MEK-targeted therapeutic selumetinib (Koselugo) for treating pediatric patients age 2 and older who have NF1–related plexiform neurofibromas that cannot be safely removed surgically. The approval was based on results from a phase II clinical trial that showed that 66 percent of pediatric patients who received selumetinib had partial tumor shrinkage (395)Dombi E, Baldwin A, Marcus LJ, Fisher MJ, Weiss B, Kim A, et al. Activity of Selumetinib in Neurofibromatosis Type 1–Related Plexiform Neurofibromas. N Engl J Med [Internet]. Massachusetts Medical Society; 2016 [cited 2020 Jul 2];375:2550–60.. In addition, many of the children reported experiencing reduced pain, which is one of the most common neurofibroma-related symptoms, along with disfigurement and motor dysfunction.
Kaposi sarcoma arises in the skin; the mucous membranes lining the mouth, nose, and throat; lymph nodes; or other organs. Tumors often arise in more than one place in the body at the same time. They are associated with human herpesvirus-8, also known as Kaposi sarcoma herpesvirus.
There are several types of Kaposi sarcoma. The most common type in the United States is epidemic Kaposi sarcoma, also known as AIDS-related Kaposi sarcoma. Treating patients who have HIV with highly active antiretroviral therapy (HAART) often keeps Kaposi sarcoma at bay, but not always. In May 2020, the FDA expanded the use of a molecularly targeted therapeutic previously approved for treating multiple myeloma, pomalidomide (Pomalyst), to help address this challenge. Pomalidomide works against cancer in several ways, including by modulating aspects of the immune system and by reducing the production of molecules called VEGFs, which leads to disruption of new blood and lymphatic vessel networks. It was approved by the FDA for treating adults who have AIDS-related Kaposi sarcoma after failure of HAART and Kaposi sarcoma in adult patients who are HIV-negative after it was shown in a phase I/II clinical trial to cause partial or complete tumor shrinkage for 67 percent of HIV-positive patients and 80 percent of HIV-negative patients (396)Polizzotto MN, Uldrick TS, Wyvill KM, Aleman K, Peer CJ, Bevans M, et al. Pomalidomide for Symptomatic Kaposi’s Sarcoma in People With and Without HIV Infection: A Phase I/II Study. J Clin Oncol [Internet]. American Society of Clinical Oncology; 2016 [cited 2020 Jul 2];34:4125–31..
Advancing Bladder Cancer Treatment
Bladder cancer is the sixth most commonly diagnosed cancer in the United States, with more than 81,000 new cases expected to be diagnosed in 2020 (5)Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin [Internet]. 2020;.
More than 90 percent of bladder cancers diagnosed in the United States are classified as urothelial cancers because they arise in cells that comprise the transitional cell urothelium that lines the bladder. Research has shown that up to 60 percent of bladder cancers are characterized by elevated levels of a protein called nectin-4 (397)PM C-E, D S, P Y, Z A, K M, Y S, et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res [Internet]. Cancer Res; 2016 [cited 2020 Jul 2];76..
Enfortumab-vedotin-ejfv is an antibody-drug conjugate that comprises a cytotoxic agent, monomethyl auristatin E, attached to a nectin-4–targeted antibody by a linker. When the antibody attaches to nectin-4 on the surface of bladder cancer cells, the antibody-drug conjugate is internalized by the cells. This leads to monomethyl auristatin E being released from the linker and antibody. Once free, the monomethyl auristatin E is toxic to the bladder cancer cells, which ultimately die.
In December 2019, the FDA approved enfortumab vedotin-ejfv for treating adults who have locally advanced or metastatic urothelial cancer that has progressed despite treatment with an immune checkpoint inhibitor and a platinum-containing cytotoxic chemotherapy regimen. The approval was based on results from a phase II clinical trial that showed that 44 percent of patients had complete or partial tumor shrinkage after treatment with enfortumab vedotin-ejfv (397)PM C-E, D S, P Y, Z A, K M, Y S, et al. Enfortumab Vedotin Antibody-Drug Conjugate Targeting Nectin-4 Is a Highly Potent Therapeutic Agent in Multiple Preclinical Cancer Models. Cancer Res [Internet]. Cancer Res; 2016 [cited 2020 Jul 2];76..
Treatment with Immunotherapy
Cancer immunotherapy refers to the use of therapeutics that unleash the power of a patient’s immune system to fight cancer. Not all these therapeutics, which are known as immunotherapeutics, work in the same way (see sidebar on How Immunotherapeutics Work).
In the past decade, cancer immunotherapy emerged as the fifth pillar of cancer care (see Figure 14). It is one of the most exciting approaches to cancer treatment to have ever entered the clinic. This is in part because some of the patients with metastatic disease who have been treated with these revolutionary treatments have had remarkable and durable responses, as Dr. Al Stroberg has had after being treated with ipilimumab almost 10 years ago because he had advanced melanoma. Unfortunately, only a minority of patients have such incredible responses. In addition, the current FDA-approved immunotherapeutics do not work against all types of cancer. Identifying ways to increase the number of patients for whom treatment with an immunotherapeutic yields a remarkable and durable response is an area of intensive basic and clinical research investigation.
Fortunately, our scientific understanding of the immune system and how it interacts with cancer cells is rapidly increasing, and there are already clinical trials underway testing many novel immunotherapeutics and testing new ways to use those that we already have (399)J XY, JP H, C O, ST N, VM H-L, J T. Trends in Clinical Development for PD-1/PD-L1 Inhibitors. Nat Rev Drug Discov [Internet]. Nat Rev Drug Discov; 2020 [cited 2020 Jul 2];19.(400)Yu JX, Upadhaya S, Tatake R, Barkalow F, Hubbard-Lucey VM. Cancer cell therapies: the clinical trial landscape. Nat Rev Drug Discov [Internet]. 2020 [cited 2020 Jul 2];. The new immunotherapeutics and treatment strategies that are on the horizon hold extraordinary promise for the future. Here, however, we focus on new immunotherapeutics that were approved by the FDA in the 12 months covered by this report, August 1, 2019 to July 31, 2020, and previously approved immunotherapeutics that were approved for use against additional types of cancer during the same period (see Table 6).
Releasing the Brakes on the Immune System
Research has shown that immune cells called T cells are naturally capable of destroying cancer cells. It has also shown that some tumors evade destruction by T cells because they have high levels of proteins that attach to and trigger “brakes” on T cells, stopping the T cells from attacking the tumor. These brakes, which are proteins on the surface of T cells, are called immune-checkpoint proteins.
This knowledge led researchers to develop therapeutics that release certain T-cell brakes, freeing the T cells to destroy the cancer cells. These immunotherapeutics are called checkpoint inhibitors (see Figure 17).
As of July 31, 2020, there are seven checkpoint inhibitors approved by the FDA. Ipilimumab, which was the first of these immunotherapeutics to be approved by the FDA, in March 2011, targets the immune-checkpoint protein CTLA-4. Ipilimumab protects CTLA-4 from the proteins that attach to it and trigger it to put the brakes on killing by T cells. The other six FDA-approved checkpoint inhibitors release a different T-cell braking system. They target either the immune-checkpoint protein PD-1 or PD-L1, which is one of the proteins that applies the PD-1 brake on T cells.
Checkpoint inhibitors have broad utility in the treatment of cancer; most of these groundbreaking immunotherapeutics are approved by the FDA for treating multiple types of cancer (see Figure 18). During the 12 months spanning this report, August 1, 2019 to July 31, 2020, the FDA approved expanding the uses of five of the checkpoint inhibitors—atezolizumab (Tecentriq), durvalumab (Imfinzi), ipilimumab, nivolumab, and pembrolizumab—to include the treatment of additional types of cancer. These approvals mean that as of July 31, 2020, one or more checkpoint inhibitor were approved for treating 16 types of cancer and for treating any type of solid tumor characterized by the presence of certain molecular characteristics, microsatellite instability–high, DNA mismatch–repair deficiency, and tumor mutational burden–high.
One of the expanded uses for PD-1/PD-L1–targeted checkpoint inhibitors approved by the FDA during the 12 months spanning this report was the June 2020 approval of pembrolizumab for treating certain adults and children with solid tumors characterized by the presence of a specific molecular characteristic, or biomarker, called tumor mutational burden–high. These biomarkers are found in a proportion of cancers arising at numerous sites in the body, including melanoma and lung cancer (410). This is the second FDA approval of pembrolizumab based on a common biomarker and not the location in the body where the cancer originated (see Figure 19).
The approval was based on data from a phase II clinical trial showing that pembrolizumab treatment led to tumor shrinkage in about 30 percent of patients with an unresectable or metastatic, tumor mutational burden–high solid tumor that had progressed despite prior treatment. The patients included in the analysis had been shown to have tumors that were tumor mutational burden–high using the FoundationOneCDx assay companion diagnostic, which the FDA approved for identifying patients eligible for pembrolizumab treatment. Thus, the approval provides new treatment options and new hope to patients with a wide range of types of cancer, like Leonard Ganz who has urothelial carcinoma and Barbara Bigelow who has triple-negative breast cancer.
The other biomarkers that can be used to identify patients with solid tumors that have progressed after prior treatment and who are now eligible for treatment with pembrolizumab are microsatellite instability–high and DNA mismatch–repair deficiency (30). About 15 percent of the colorectal cancer cases diagnosed in the United States are characterized by these biomarkers (411)Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol [Internet]. BioMed Central; 2017 [cited 2020 Aug 3];12:24.. The use of pembrolizumab was expanded in July 2020 to include the initial treatment of patients with colorectal cancer that is characterized by either microsatellite instability–high or DNA mismatch–repair deficiency. The approval was based on results from a phase II clinical trial that showed that the time before disease progression was almost double among patients who received pembrolizumab compared with patients who received standard treatments.
Endometrial cancer is another type of cancer that is frequently characterized by microsatellite instability–high and DNA mismatch–repair deficiency. In September 2019, however, the FDA expanded the use of pembrolizumab to include the treatment of women who have advanced endometrial cancer that is neither microsatellite instability–high nor mismatch repair–deficient and who have disease progression following prior systemic therapy but are not candidates for curative surgery or radiation. When being used in this way, pembrolizumab should be used in combination with the molecularly targeted therapeutic lenvatinib (Lenvima). The approval was based on data from a phase I/II clinical trial showing that pembrolizumab and lenvatinib treatment led to tumor shrinkage in about 40 percent of patients (412)V M, MH T, C A, A O, J M, AL C, et al. Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer. J Clin Oncol [Internet]. J Clin Oncol; 2020 [cited 2020 Jul 2];.
In June 2020, the FDA also added cutaneous squamous cell carcinoma as a type of cancer for which pembrolizumab is an approved treatment option. Most patients diagnosed with this type of skin cancer are cured by surgery and/or radiation. However, in some cases, the disease does advance, and pembrolizumab was approved to help address this challenge. The approval of pembrolizumab for treating patients with recurrent or metastatic cutaneous squamous cell carcinoma that is not curable by surgery or radiation was based on results from a phase II clinical trial that showed that 34 percent of patients who received the immunotherapeutic had complete or partial tumor shrinkage.
Durvalumab is another PD-1/PD-L1–targeted checkpoint inhibitor to have its use expanded to a new type of cancer by the FDA in the 12 months spanning this report. In March 2020, the FDA approved the checkpoint inhibitor for use in combination with two cytotoxic chemotherapeutics—etoposide and either carboplatin or cisplatin—for the initial treatment of certain patients with advanced SCLC. The approval was based on results from a phase III clinical trial that showed that adding durvalumab to standard cytotoxic chemotherapy improved overall survival (413)L P-A, M D, Y C, N R, K H, D T, et al. Durvalumab Plus Platinum-Etoposide Versus Platinum-Etoposide in First-Line Treatment of Extensive-Stage Small-Cell Lung Cancer (CASPIAN): A Randomised, Controlled, Open-Label, Phase 3 Trial. Lancet (London, England) [Internet]. Lancet; 2019 [cited 2020 Jul 2];394..
The use of nivolumab was also expanded during the 12 months spanning this report. In June 2020, it was approved for treating certain adults who have advanced, recurrent, or metastatic, squamous cell carcinoma of the esophagus that has progressed despite cytotoxic chemotherapy. Although esophageal cancer is a rare type of cancer, which is expected to be a diagnosis received by 18,440 people in the United States in 2020, it is also one of the deadliest; the 5-year relative survival rate for patients diagnosed with the disease is just 20 percent (2)Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2017, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2 [Internet]. [cited 2020 Jun 29].. The approval was based on results from a phase III clinical trial in which it was shown that nivolumab improved overall survival compared with standard cytotoxic chemotherapy (414)K K, BC C, M T, M O, CY L, K C, et al. Nivolumab Versus Chemotherapy in Patients With Advanced Oesophageal Squamous Cell Carcinoma Refractory or Intolerant to Previous Chemotherapy (ATTRACTION-3): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet Oncol [Internet]. Lancet Oncol; 2019 [cited 2020 Jul 2];20..
Nivolumab has been previously approved by the FDA as a treatment for NSCLC and hepatocellular carcinoma, which is the most common type of liver cancer. These approvals, which were granted in March 2015 and September 2017, respectively, yielded remarkable and durable responses for only some patients and the 5-year relative survival rates for those diagnosed with these types of cancer at an advanced stage remain below 20 percent (2)Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975-2017, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2 [Internet]. [cited 2020 Jun 29].. Thus, researchers are testing various ways to increase the number of patients who benefit from nivolumab, including evaluating how well it works in combination with other immunotherapeutics. In March 2020, the FDA approved using nivolumab in combination with ipilimumab to treat patients with hepatocellular carcinoma that has progressed after standard treatment when it was shown in a phase I/II clinical trial to cause tumor shrinkage in 33 percent of patients. In May 2020, the same combination of checkpoint inhibitors was approved by the FDA for treating patients who have tumors that express at least 1 percent PD-L1, as measured using the PD-L1 IHC 28-8 pharmDx companion diagnostic, and that are not fueled by mutations in either the EGFR gene or the ALK gene. This approval for NSCLC was based on results from a phase III clinical trial in which it was shown that the combination improved overall survival compared with platinum-based cytotoxic chemotherapy (415)MD H, L P-A, R BC, B Z, SW K, E CC, et al. Nivolumab Plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med [Internet]. N Engl J Med; 2019 [cited 2020 Jul 2];381..
The fifth checkpoint inhibitor to have its use expanded by the FDA during the 12 months spanning this report is atezolizumab. In May 2020, it was approved for use in combination with a molecularly targeted therapeutic called bevacizumab (Avastin) as a new initial treatment for patients with hepatocellular carcinoma that cannot be removed by surgery or metastatic hepatocellular carcinoma. Most patients who receive these diagnoses are initially treated with the molecularly targeted therapeutic sorafenib (Nexavar). Unfortunately, the majority of those whose tumors initially respond to this treatment eventually have disease progression, highlighting the need for new, more effective treatment options. The atezolizumab–bevacizumab combination was approved by the FDA after it was shown in a phase III clinical trial to improve overall survival compared with sorafenib (416)RS F, S Q, M I, PR G, M D, TY K, et al. Atezolizumab Plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N Engl J Med [Internet]. N Engl J Med; 2020 [cited 2020 Jul 2];382..
In July 2020, the FDA further expanded the use of atezolizumab to include melanoma, which is the deadliest type of skin cancer. This approval is for the use of atezolizumab in combination with two molecularly targeted therapeutics, cobimetinib (Cotellic) and vemurafenib (Zelboraf), which were approved for treating melanoma fueled by mutations in the BRAF gene called V600 mutations in November 2015 (57). The new combination of atezolizumab, cobimetinib, and vemurafenib was approved for treating patients with melanoma that is metastatic or cannot be removed by surgery and that tests positive for a BRAF V600 mutation. The approval was based on results from a phase III clinical trial in which it was shown that adding atezolizumab to cobimetinib and vemurafenib significantly increased the time before disease progression (417)Gutzmer R, Stroyakovskiy D, Gogas H, Robert C, Lewis K, Protsenko S, et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAFV600 mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England) [Internet]. Lancet; 2020 [cited 2020 Aug 3];395:1835–44..
Even though checkpoint inhibitors have yielded extraordinary benefit for patients with a diverse array of cancer types, not all patients have tumors that respond to these immunotherapeutics and many whose tumors do respond, initially, develop resistance after a while. Therefore, researchers are working hard to determine how to increase the number of patients who benefit from these lifesaving immunotherapeutics, as discussed in Expanding the Scope of Checkpoint Inhibitors.
Increasing the Cancer-killing Capacity of the Immune System
In some patients with cancer, it is not an issue of T-cell brakes being triggered that prevents the patient’s immune system attacking and destroying the cancer cells; rather, it is an issue of there being insufficient cancer-killing T cells.
One of the most recently developed ways to dramatically increase the number of functional cancer-killing T cells that a patient has is an approach to immunotherapy called adoptive T-cell therapy (418)June CH, O’Connor RS, Kawalekar OU, Ghassemi S, Milone MC. CAR T cell immunotherapy for human cancer. Science (80- ) [Internet]. 2018;359:1361–5.. Adoptive T-cell therapy is a complex medical procedure that is customized for each patient. During treatment, T cells are harvested from a patient, expanded in number and/or genetically modified in the laboratory, and then returned to the patient, where they attack and potentially eliminate the cancer cells (see sidebar on Types of Adoptive T-Cell Therapy).
In July 2020, the FDA approved a third adoptive T-cell therapy, brexucabtagene autoleucel (Tecartus). Similar to the first two of these revolutionary new types of immunotherapy approved by the FDA—axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah)—it is categorized as chimeric antigen receptor (CAR) T-cell therapy. Given that CAR T-cell therapy involves genetic modification of a patient’s cells, it is sometimes referred to as cell-based gene therapy. For all three of the CAR T-cell therapies approved by the FDA, a patient’s T cells are genetically modified to have a CAR that targets the molecule CD19.
CD19 is a protein found on the surface of immune cells called B cells. Several types of leukemia and lymphoma arise in B cells, including an aggressive type of non-Hodgkin lymphoma called mantle cell lymphoma.
The latest estimates show that the mantle cell lymphoma incidence rate has been increasing steadily since the turn of the century (420)Teras LR, DeSantis CE, Cerhan JR, Morton LM, Jemal A, Flowers CR. 2016 US lymphoid malignancy statistics by World Health Organization subtypes. CA Cancer J Clin [Internet]. American Cancer Society; 2016 [cited 2020 Aug 3];66:443–59.. There are now more than 3,300 new cases of the disease diagnosed in the United States each year. Brexucabtagene autoleucel was approved for treating adults who have mantle cell lymphoma that has not responded to or that has relapsed following at least one other treatment. The approval was based on results from a phase II clinical trial that showed that more than 60 percent of patients who received brexucabtagene autoleucel had complete responses, meaning no cancer was detectable during at least one follow-up examination (421)Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N Engl J Med [Internet]. N Engl J Med; 2020 [cited 2020 Aug 3];382:1331–42..
Like the other CAR T-cell therapies, brexucabtagene autoleucel can sometimes cause severe or life-threatening cytokine-release syndrome and other serious adverse effects, including potentially life-threatening swelling in the brain. Therefore, the FDA has put in place a risk evaluation and mitigation strategy that requires that health care facilities using brexucabtagene autoleucel be specially certified. Researchers also are working hard to identify new ways to reduce the severe adverse effects of CAR T-cell therapies without decreasing the therapeutic benefit of these immunotherapeutics.
Directing the Immune System to Cancer Cells
An immune cell must find a cancer cell before it can destroy it. Many immunotherapeutics that have been approved by the FDA for treating cancer work, at least in part, by helping immune cells find cancer cells (see Cell Lysis Mediators in Supplemental Table 2, Part 2). The most recent additions to this group of immunotherapeutics are isatuximab-irfc (Sarclisa), which was approved by the FDA in March 2020 for treating certain patients with multiple myeloma, and tafasitamab-cxix (Monjuvi), which was approved by the FDA in July 2020 for treating certain patients with diffuse large B-cell lymphoma.
Multiple myeloma is one of the most commonly diagnosed blood cancers in the United States, with 32,270 new cases expected to be diagnosed in 2020 (5)Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin [Internet]. 2020;. In recent years, the development and FDA approval of new therapeutics—including proteasome inhibitors like bortezomib (Velcade) and carfilzomib (Kyprolis), immunomodulatory agents like lenalidomide (Revlimid) and pomalidomide, and immunotherapeutics like the CD38-targeted daratumumab (Darzalex)—have improved outcomes for patients. Despite the advances, unfortunately, many patients whose disease initially responds to the new therapeutics eventually relapse owing to treatment resistance.
Isatuximab-irfc is a CD38-targeted immunotherapeutic, like daratumumab, which was approved by the FDA in November 2015. CD38 is a protein found at high levels on the surface of myeloma cells. When isatuximab-irfc attaches to CD38, it has several effects on myeloma cells, one of which is to flag them for immune cells, which upon attaching to another part of isatuximab-irfc are triggered to destroy the myeloma cells. Isatuximab-irfc was approved for use in combination with pomalidomide and dexamethasone for treating patients with multiple myeloma that has relapsed or not responded to at least two other treatments, including lenalidomide and any one of the proteasome inhibitors. The approval was based on data from a phase III clinical trial that showed that adding isatuximab-irfc to pomalidomide and dexamethasone almost doubled the time before disease relapse (422)M A, PG R, SV R, J S-M, M B, I S, et al. Isatuximab Plus Pomalidomide and Low-Dose Dexamethasone Versus Pomalidomide and Low-Dose Dexamethasone in Patients With Relapsed and Refractory Multiple Myeloma (ICARIA-MM): A Randomised, Multicentre, Open-Label, Phase 3 Study. Lancet (London, England) [Internet]. Lancet; 2019 [cited 2020 Jul 2];394..
Diffuse large B-cell lymphoma is the most common type of non-Hodgkin lymphoma diagnosed in the United States (see Adding Precision to Treatment for Blood Cancers). Tafasitamab-cxix targets CD19, which is found on the surface of B cells, including diffuse large B-cell lymphoma cells. When tafasitamab-cxix attaches to CD19, it has several effects on diffuse large B-cell lymphoma cells, including flagging the cells for immune cells. Tafasitamab-cxix was approved for use in combination with lenalidomide for treating patients who have diffuse large B-cell lymphoma, not otherwise specified, that has relapsed or not responded to other treatment and who are not able to have an autologous stem cell transplant. The approval was based on data from a phase II clinical trial that showed that more than 50 percent of patients who received tafasitamab-cxix and lenalidomide had complete or partial tumor shrinkage, with the majority of these patients having complete tumor shrinkage (423)G S, J D, E GB, O T, W J, AM L, et al. Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol [Internet]. Lancet Oncol; 2020 [cited 2020 Aug 15];21.. Additional follow-up and additional studies are needed to determine whether these immunotherapeutics also extend survival for patients.