Pediatric Cancer Predisposition and Surveillance
In this section, you will learn:
- Surveillance for early detection in pediatric cancers means structured monitoring of physical traits and/ or clinical signs in children who are at higher risk of developing cancer.
- Physical traits and personal or family history as well as genetic testing are routinely used to identify individuals with a cancer predisposition syndromes and to identify early signs of cancers in at-risk children.
- Genetic counseling helps families of at-risk children navigate through genetic testing–related decision-making and surveillance.
- Multi-disciplinary panels of experts in pediatric cancers and cancer genetics periodically issue guidelines for surveillance and screening in at-risk children.
- New frontiers in early detection of cancer predisposition syndromes and/or early signs of cancer in at-risk children include minimally invasive tests like liquid biopsies, artificial intelligence–based tools, and enhanced imaging strategies.
- Psychosocial and financial issues associated with surveillance and genetic testing pose a significant burden for children and their parents.
Pediatric or childhood cancers, although significantly less common than adult cancers, are the leading cause of disease-related deaths in children (ages 0 to 14) and adolescents (ages 15 to 19) (see Pediatric Cancer Trends in the United States). Over the past several decade, understanding of pediatric cancer biology has undergone a tectonic shift with advances in genomics and epigenomics, as well as in novel laboratory models that closely resemble pediatric cancers including brain tumors such as organoids and neurospheres. Although most childhood cancers are attributed to somatic alterations, available evidence shows that at least 10 percent to 18 percent arise from pathogenic germline alterations in cancer predisposition genes, although experts think this number will increase with refinements in, and access to, gene sequencing technologies, as well as increased awareness of cancer predisposition by clinical practitioners (36)Brodeur GM, et al. (2025) Clin Cancer Res, 31: 2581.(37)Roganovic J (2024) World J Clin Pediatr, 13: 95010.. Germline alterations are often inherited from parents but may also occur de novo in germ cells (egg and sperm) (see Unraveling the Genomics and Biology of Pediatric Cancers). The knowledge of molecular underpinnings of childhood cancers has enabled precise detection of a number of germline alterations that may increase the risk of cancer in children and adolescents (132)Mardis ER (2025) Annu Rev Genomics Hum Genet, 26: 279.. The sections below describe the role of surveillance for early detection of these cancers, the current state of the field, and what the future holds.
Identifying Children With Cancer Predisposition Syndromes
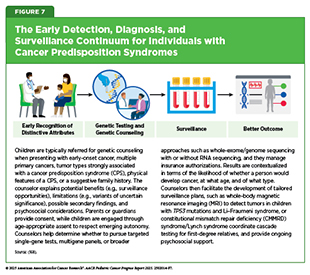
Early detection of cancer in children means closely monitoring a child for physical and/or clinical signs of a cancer predisposition syndrome (CPS) that can increase their risk of developing cancer (see Figure 4). If diagnosed with a CPS, the child is evaluated periodically by routine monitoring, or surveillance, for early signs of cancer using specific approaches. Conversely, a child may have a suspected CPS if diagnosed with more than one primary tumor throughout the body; a primary tumor in both organs of the paired set (e.g., in both kidneys) or multiple independent sites; more than one type of cancer; a cancer diagnosis at an earlier age than typically occurs in the population, such as colon cancer during adolescence; or specific types of tumors during childhood, such as choroid plexus carcinoma. Surveillance strategies are also used to find early signs that the cancer has come back and/or for early detection of second primary cancers (see Supporting Survivors of Pediatric Cancers). This approach contrasts with cancer screening for early detection in adults, which, for most common cancer types, is carried out at the population level in individuals with no signs or symptoms of the disease.
The Role of Distinctive Signs or Symptoms
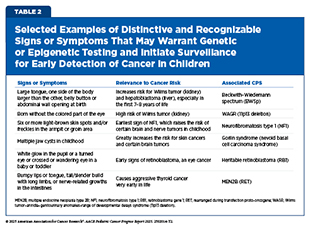
Traditionally, the clinical approach to genetic testing and surveillance begins after a child has been identified to have a strong or suggestive family history of a CPS, shows specific signs or symptoms associated with the syndrome, or has been diagnosed with specific cancers (see Table 2). The recently updated surveillance recommendations issued by the American Association for Cancer Research (AACR) Pediatric Cancer Working Group (PCWG), a multidisciplinary
group of experts in pediatric cancers and cancer genetics provide detailed, evidence-based guidance regarding the distinctive and recognizable signs or symptoms that may warrant surveillance (see Screening and Surveillance Recommendations).
Using signs or symptoms to inform whether a child should undergo surveillance and genetic testing can be highly efficient. This initial approach in settings where broad genetic screening is not yet feasible would allow health care providers to focus resources on children who have the highest likelihood of a CPS (see New Frontiers in Surveillance for Children With Cancer Predisposing Syndromes) (165)Goudie C, et al. (2021) JAMA Oncol, 7: 1806.. Because recognizable and distinctive signs and symptoms, such as skin lesions and morphologic features, are often incorporated into established criteria for the diagnosis of certain CPSs (e.g., the diagnostic criteria for neurofibromatosis type 1 by the International Consensus Group on Neurofibromatosis Diagnostic Criteria) (166)Legius E, et al. (2021) Genet Med, 23: 1506., the approach benefits from decades of clinical validation. It also minimizes unnecessary testing in children at lower risk, reducing the potential for uncertain findings that could lead to over-surveillance or psychological burden. When implemented systematically, the approach of using signs or symptoms can identify many children with a high-risk of CPS and facilitate the initiation of syndrome-specific, evidence-based surveillance recommendations in a timely manner (see Screening and Surveillance Recommendations).
The primary limitation of initiating surveillance based on signs or symptoms is its dependency on visible or otherwise recognizable indicators, which may may be absent entirely in some children carrying harmful genetic alterations or may appear only after disease has advanced. Studies have consistently demonstrated that the majority of children with a CPS lack recognizable or distinct features or family history that would suggest a need for surveillance or genetic testing or both (89)Zhang J, et al. (2015) N Engl J Med, 373: 2336.(167)Kim JW (2025) J Korean Neurosurg Soc, 68: 350.(168)Kuhlen M, et al. (2025) World J Pediatr, 21: 131.. The current approach also relies heavily on the expertise of the health care professional, often a pediatrician, because subtle features may be overlooked or misattributed to benign conditions. Collectively, this approach can cause delays in early detection of cancer in at-risk children, thus leading to missed opportunities for potentially less invasive interventions.
The Role of Genetic Testing
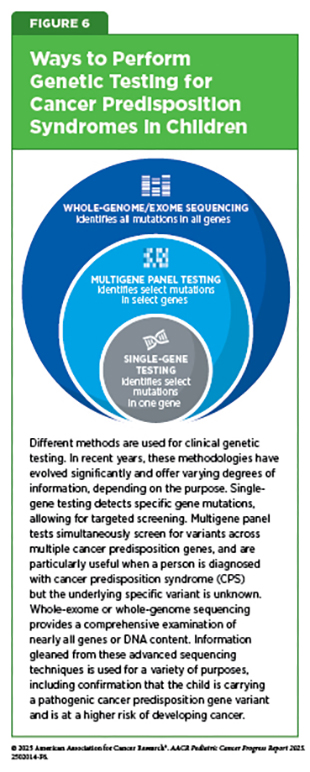
Genetic testing, also called genetic analysis, refers to a laboratory method that looks for changes in the germline chromosomes and genes that could alter the expression or function of specific genes or corresponding proteins in a person’s cells or tissues. Genetic tests are typically conducted on readily accessible biological samples, such as blood or saliva, with results generally becoming available within a time frame of 2 to 3 weeks (see Figure 6). In clinical practice, genetic testing may be performed for several reasons, including assessing risk for medical conditions such as cancer. Germline testing for cancer is a type of genetic testing that looks for inherited or de novo genetic changes that may increase the risk of developing cancer. For example, gene testing is performed if someone’s children, siblings, or other close family members have cancer or if there is an indication that a person may have a CPS (169)Alonso-Luna O, et al. (2023) Ann Hum Genet, 87: 81.. By contrast, somatic testing for cancer is done to search for genetic changes that occur during a person’s lifetime. Somatic testing is conducted using cancer tissue or other biospecimen from patients and can be used to diagnose the cancer, plan treatment, or determine how well the treatment is working (in samples taken after treatment has started).
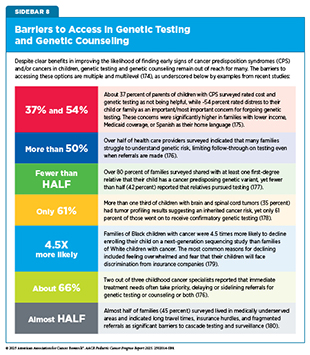
Studies have shown that distinctive physical traits, a strong family history of certain cancers, germline genetic testing, or a combination thereof, is the most effective way to identify children with CPS (170)Bakhuizen JJ, et al. (2024) Lancet Child Adolesc Health, 8: 751.. Genetic testing also helps determine whether specific conditions, such as retinoblastoma or Wilms tumor, are heritable or sporadic by identifying specific genetic alterations associated with these cancers. However, it is important to note that genetic testing and counseling is not readily available for many because it requires state-of-the-art infrastructure and trained health care professionals (see Sidebar 8). Another issue is the lack of education and understanding of the tests, their findings, and how they are used to inform clinical management among health care providers, patients, and family members (171)Capasso A, et al. (2025) J Genet Couns, 34: e1887.. Research has also found that pediatric patients and their siblings face higher rates of insurance denials for genetic testing (172)Zion TN, et al. (2023) Genet Med, 25: 100020..
The use of genetic testing of children for a CPS also raises ethical concerns about a child’s autonomy over health-related decisions. The lifelong psychosocial impact on children, including anxiety, guilt, and changes in family dynamics, presents yet another concern (173)Droin-Mollard M, et al. (2024) Eur J Hum Genet, 32: 1446.. These considerations underscore the need for all stakeholders working together to increase patient education to mitigate these risks and to ensure ethical, equitable access to childhood genetic testing.
Despite implementation challenges and ethical concerns associated with genetic testing, the identification of inherited mutations is a critical step for accurate cancer risk assessment, comprehensive genetic counseling, and the initiation of specific surveillance protocols. Recognizing the importance of genetic testing in developing a comprehensive surveillance and treatment plan, many pediatric oncology centers in the United States, some as part of the National Cancer Institute–designated Comprehensive Cancer Centers (NCI CCC), and some embedded in hospitals affiliated with an NCI CCC, have established dedicated cancer predisposition clinics staffed by genetic counselors, medical geneticists, and oncologists, who systematically evaluate and advise affected children and their families. These clinics often handle the unique issues of testing minors, coordinating follow-up screening, and providing counseling. Although identifying the balance of benefit and harm in genetically testing a child is an active area of research, such proactive measures are aimed not only to significantly improve health outcomes for children, but also to extend benefits to at-risk siblings and first-degree relatives.
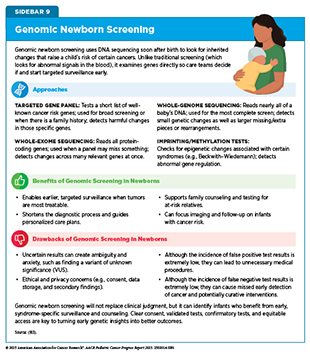
The idea of offering genetic screening at birth is gaining attention as a means to identify children at risk for serious conditions, including certain cancers, before symptoms appear. Traditional newborn screening already checks for a small set of metabolic and genetic diseases using blood tests. Advances in genetic sequencing now raise the possibility of expanding that window dramatically by flagging certain CPS that lead to cancer early in life, when careful monitoring or preventive steps might make the greatest difference. This approach can be especially impactful in newborns who are, effectively, born with cancer.
Across the globe, researchers are testing whether newborn genetic screening could spot cancer risks before the disease develops. Modeling studies suggest that sequencing panels for a handful of cancer predisposition genes could reduce childhood cancer deaths by nearly half and may become cost-effective as sequencing prices fall (181)Yeh JM, et al. (2021) Genet Med, 23: 1366.. Real-world studies demonstrate feasibility of the approach across families (182)Smith HS, et al. (2024) Am J Hum Genet, 111: 2094.(183)Linga BG, et al. (2024) Cancers (Basel), 16., while population-based studies show clinical benefits and cost-effectiveness of TP53 gene testing for Li–Fraumeni syndrome (LFS) in newborns (184)Kunst N, et al. (2022) J Natl Cancer Inst, 114: 722.. Additional studies, such as the Generation Study in the United Kingdom, which aims to sequence the genomes of 100,000 newborn babies to identify rare conditions, including CPSs, are ongoing (185)Genomics England. Newborn Genomes Programme. Accessed: August 31, 2025.. Key questions that remain, and require additional research, include which genes to evaluate, how to balance benefits against potential harms such as false positives, cost-effectiveness, and how families and health care systems will manage the ethical and practical challenges of sequencing every newborn (see Sidebar 9).
The Role of Genetic Counseling
Although the benefits of early surveillance are well proven in some CPSs, the evidence is limited in others, and screening may lead to unnecessary tests, false alarms, or anxiety for families. In addition, uncertainty may arise in interpreting some genetic findings, especially when the link between a genetic alteration and cancer risk is not fully understood. This means careful genetic counseling is essential to explain the results, outline the benefits and risks of surveillance, and avoid overtreatment (see Figure 7).
Genetic counseling combines multiple specialties, such as clinical genetics, cancer care, and psychosocial care, to guide families through the evaluation of inherited cancer risk in children (186)Zelley K, et al. (2024) Clin Cancer Res, 30: 3983.. It is a communication process in which a genetic counselor—a health professional who has specialized training in clinical genetics and counseling—helps the parents or guardians understand their child’s risk of developing cancer, as well as options for genetic testing, including its risks and benefits. After genetic testing is done, genetic counselors help parents or guardians understand genetic test results, including how the results can affect other family members, and provide counseling and support for next steps that may include surveillance planning and cascade testing. Recent pediatric oncology frameworks and guidelines emphasize that timely identification of a CPS can alter the course of care and improve outcomes by enabling early, targeted surveillance and risk-reduction strategies (see Screening and Surveillance Recommendations) (186)Zelley K, et al. (2024) Clin Cancer Res, 30: 3983.. In addition, there is emerging evidence that patients with constitutional mismatch repair deficiency (CMMRD)-related cancers can benefit from treatment with immune checkpoint blockade immunotherapies (187)Westdorp H, et al. (2017) Cancer Lett, 403: 159.(188)Henderson JJ, et al. (2022) JCO Precis Oncol, 6: e2100286.(189)Palova H, et al. (2024) NPJ Precis Oncol, 8: 110..
For children with inherited cancer risk and their families, genetic counseling is critical both for making informed decisions about the next steps and for considering the ethical implications of those decisions. Genetic counselors are trained to help families navigate complex results of genetic tests and support informed decision-making under stressful and often emotionally charged circumstances. Protocol-based surveillance—a planned schedule of medical checkups, imaging, and lab tests—for certain CPSs, notably LFS, has been shown to improve overall survival, underscoring the value of early counseling and structured monitoring (190)Villani A, et al. (2016) Lancet Oncol, 17: 1295., as is reflected by the experience of Chenia Lloyd-Gascho, an adolescent with LFS. As one example, in a long-term study with an 11-year follow-up, researchers evaluated the impact of a surveillance protocol—consisting of frequent physical examinations, biochemical and imaging studies, including whole-body MRI, brain MRI, breast MRI and mammography (adult women), abdominal and pelvic ultrasound, and colonoscopy (for adults)—for individuals with LFS (190)Villani A, et al. (2016) Lancet Oncol, 17: 1295.. Findings revealed that 84 percent of individuals who underwent surveillance and developed cancer were alive at a 4-year follow-up, compared to 49 percent of those not on surveillance (190)Villani A, et al. (2016) Lancet Oncol, 17: 1295..
Several recent studies have shown similarly improved survival outcomes for individuals who carry germline mutations with or without an LFS diagnosis and undergo surveillance protocols. In one study, 92 percent of the children undergoing surveillance with screening MRI were diagnosed with low-grade brain tumors before symptoms appeared. In contrast, 85 percent of children who were not undergoing routine surveillance and were diagnosed after symptoms appeared had high-grade tumors. All children with low-grade tumors whose tumors were surgically removed after they were diagnosed during screening MRI were alive at 30 months, compared to only half of those who were not undergoing routine surveillance and were diagnosed with high-grade tumors after the symptoms appeared (191)Patel N, et al. (2023) J Neurosurg Pediatr, 31: 258.. Another study of 31 children with LFS revealed that whole-body and brain MRI with ultrasound was accurate and feasible for early detection of cancer (192)Tewattanarat N, et al. (2022) Pediatr Radiol, 52: 1283.. Updated guidelines confirm that standardized surveillance enables early detection of cancer in LFS patients without symptoms, and guides treatment decisions that improve outcomes (see Screening and Surveillance Recommendations) (193)Greer MC, et al. (2024) Clin Cancer Res, 30: 5021.(194)Hansford JR, et al. (2024) Clin Cancer Res, 30: 2342..
Evidence shows that counseling prior to genetic testing improves knowledge retention and decision confidence while helping families prepare for uncertain or unexpected results (175)Rapoport CS, et al. (2024) Cancer Rep (Hoboken), 7: e2119.. If a pathogenic variant is identified (see Sidebar 4), counselors coordinate testing of at-risk relatives, enabling risk reduction or early detection in additional family members. Pediatric cases carry unique ethical challenges. Genetic counselors are trained to balance immediate medical benefits against preserving the child’s right to make decisions later in life. Most approaches re-consent patients as they turn 18 years of age, to preserve ongoing surveillance in an adult setting.
Evidence shows that using telehealth for genetic counseling can provide knowledge and satisfaction comparable to that achieved with in-person visits, with the added benefit of reduced travel and wait times (195,196). Demand for pediatric cancer genetic counseling currently exceeds the available number of trained counselors, especially outside large academic centers (197)Bakhuizen JJ, et al. (2024) EJC Paediatric Oncology, 4: 100176.. Even with advanced sequencing, variants of uncertain significance remain common, requiring nuanced interpretation and long-term follow-up. Although the federal Genetic Information Nondiscrimination Act (GINA) prohibits discrimination based on genetic information in health insurance and employment, adolescents with cancer and their parents often report heightened anxiety and concern about future insurability after receiving genetic test results (198)Van Hoyweghen S, et al. (2025) Cancers (Basel), 17.. Several additional barriers to access to genetic testing and counseling may further affect the uptake of these services (see Sidebar 8).
Expanding the reach of genetic counseling and testing is becoming an urgent priority, especially because technological advances are enabling diagnoses of more children and adults carrying inherited risks for cancer and other serious conditions (197)Bakhuizen JJ, et al. (2024) EJC Paediatric Oncology, 4: 100176.. Several recent studies and reviews highlight both the promise of telehealth genetic counseling and the challenges that remain for its broader adoption (195)Danylchuk NR, et al. (2021) J Genet Couns, 30: 1361.(199)Finney J, et al. (2025) J Genet Couns, 34: e1982.. Evidence from more than 13,000 patients needing genetic counseling across dozens of studies indicates that telehealth, whether by phone or video, delivers counseling outcomes comparable to those achieved with in-person care (195)Danylchuk NR, et al. (2021) J Genet Couns, 30: 1361.. Patients and parents report high levels of satisfaction, reduced travel costs, and improved access, and many now prefer hybrid models that combine virtual and in-person visits (195)Danylchuk NR, et al. (2021) J Genet Couns, 30: 1361.(199)Finney J, et al. (2025) J Genet Couns, 34: e1982..
Professional guidelines, such as those from the National Society of Genetic Counselors, cautiously recommend telehealth as a safe and effective alternative, noting its potential to increase health equity by reaching rural or underserved families
(200)Green S, et al. (2023) J Genet Couns, 32: 4.. The AACR PCWG recently updated its guidelines for genetic counselor practice and surveillance of childhood CPS, emphasizing the need for universal access to genetic testing, early referral (within 1 to 2 months of diagnosis), and ongoing surveillance throughout survivorship care. The guidelines, among other recommendations, include incorporating the education of families through web-based tools, videos, and chatbots; banking DNA for children for whom genetic counseling and/or testing cannot be completed; and psychosocial care. Nevertheless, barriers persist (186)Zelley K, et al. (2024) Clin Cancer Res, 30: 3983., such as provider licensure rules that vary from state to state, and access to reliable Internet or devices to support web-based care (180)Vuocolo B, et al. (2024) J Genet Couns, 33: 1337..
In pediatric cancer care, additional challenges surface. Children with suspected CPS often face delays in diagnosis, with some studies showing that nearly 40 percent are recognized only after their first cancer develops (201)Kaffai S, et al. (2024) Front Pediatr, 12: 1410061.. Dedicated pediatric CPS programs that include access to oncologists, genetic counselors, psychologists, and social workers all in one place can improve detection and offer structured surveillance (202)Pan V, et al. (2025) J Genet Couns, 34: e70051.. However, limited workforce capacity, high demand, and gaps in psychosocial support remain pressing problems (203)Jenkins SM, et al. (2025) Pediatr Res, 97: 1261..
Taken together, these findings argue for sustained investment in telehealth infrastructure and educational initiatives for providers, patients, and families. Only then can the benefits of genomic medicine be delivered equitably, ensuring that lifesaving diagnoses, surveillance protocols, and therapies reach children and families when they are needed most.
Screening and Surveillance Recommendations
Genetic testing is uncovering CPS at an increasingly rapid pace (84)Grobner SN, et al. (2018) Nature, 555: 321.(204)Orbach D, et al. (2023) EJC Paediatric Oncology, 2: 100023.(205)MacFarland SP, et al. (2019) JCO Precis Oncol, 3.. For these children and families, structured surveillance, such as whole-body MRI in LFS or renal imaging in Wilms tumor predisposition, have already shown to improve care and outcomes (193)Greer MC, et al. (2024) Clin Cancer Res, 30: 5021.(206)Brzezinski JJ, et al. (2025) Clin Cancer Res, 31: 18.(207)Blake A, et al. (2024) JAMA Oncol, 10: 1060.. Advances in the identification of novel biomarkers for CPSs, as well as artificial intelligence (AI)–based solutions for streamlining surveillance strategies, offer the potential to expand predictive tools, creating time windows for earlier detection or targeted treatment (see Artificial Intelligence–based Solutions) (208)Apps J, et al. (2024) EJC Paediatric Oncology, 4: 100191..
Surveillance for children with CPS aims to find tumors early, ideally before symptoms appear so that treatment can be less intensive and outcomes are better. Recent evidence demonstrates clear benefits of surveillance for children with CPS. In a 2024 study at a major pediatric cancer center, 274 children and adolescents on protocol-based surveillance were followed for a median of 3 years (207)Blake A, et al. (2024) JAMA Oncol, 10: 1060.. Thirty-five tumors without any prior symptoms were identified in 27 patients, or about 10 percent of the cohort, and nearly one-third of these were discovered on the very first scan after the diagnosis of the CPS. Importantly, 83 percent of solid tumors, including brain tumors, found through surveillance were confined to one site at diagnosis, compared with roughly 57 percent of comparable tumors that were detected before CPS diagnosis, a difference that strongly favors a structured early detection or surveillance approach (207)Blake A, et al. (2024) JAMA Oncol, 10: 1060..
Historically, standardized surveillance protocols existed for only a few CPSs, and there are only a handful of cancer-focused organizations that issue and/or incorporate surveillance guidance for children with CPS. For example, some groups have had long-standing guidance on genetic testing for children with CPS, such as the National Comprehensive Cancer Network in the United States (209)National Comprehensive Cancer Network. NCCN Guidlines. Accessed: August 31, 2025. and the National Institute for Health and Care Excellence in the United Kingdom (210)National Institute for Health and Care Excellence. NICE guidance. Accessed: August 31, 2025.. The National Society of Genetic Counselors issues guidance on genetic counseling standards (211)National Society of Geentic Counselors. NSGC Practice Guidelines. Accessed: August 31, 2025., ensuring families understand results and implications for relatives. For surveillance, the Children’s Oncology Group integrates monitoring into treatment protocols, while the European Society for Paediatric Oncology sets continent-wide standards (212)International Society of Paediatric Oncology (SIOP). European Standards of Care for Children with Cancer. Accessed: August 31, 2025.. Several syndrome-specific groups, such as those focused on LFS or Beckwith–Wiedemann spectrum, issue their own recommendations (213)Frebourg T, et al. (2020) Eur J Hum Genet, 28: 1379.(214)Brioude F, et al. (2018) Nat Rev Endocrinol, 14: 229..
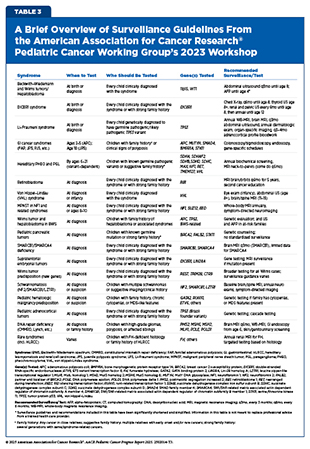
To standardize surveillance protocols for children with CPS, AACR convened a workshop in 2016 to develop consensus recommendations for early cancer detection in affected children (215)Brodeur GM, et al. (2017) Clin Cancer Res, 23: e1.. A follow-up workshop in 2023 expanded and updated these guidelines to reflect new data and include newly identified syndromes, while also exploring emerging surveillance technologies and potential prevention strategies for high-risk pediatric populations (36)Brodeur GM, et al. (2025) Clin Cancer Res, 31: 2581.(91)Kamihara J, et al. (2024) Clin Cancer Res, 30: 3137.(186)Zelley K, et al. (2024) Clin Cancer Res, 30: 3983.(193)Greer MC, et al. (2024) Clin Cancer Res, 30: 5021.(194)Hansford JR, et al. (2024) Clin Cancer Res, 30: 2342.(206)Brzezinski JJ, et al. (2025) Clin Cancer Res, 31: 18.(216)Rednam SP, et al. (2025) Clin Cancer Res, 31: 3368.(217)Wasserman JD, et al. (2025) Clin Cancer Res, 31: 3628.(218)Voss SD, et al. (2025) Clin Cancer Res, 31: 3638.(219)Rednam SP, et al. (2025) Clin Cancer Res, 31: 2271.(220)Achatz MI, et al. (2025) Clin Cancer Res, 31: 1831.(221)Kamihara J, et al. (2025) Clin Cancer Res, 31: 1573.(222)Perrino MR, et al. (2025) Clin Cancer Res, 31: 1400.(223)Michaeli O, et al. (2025) Clin Cancer Res, 31: 457.(224)Schultz KAP, et al. (2025) Clin Cancer Res, 31: 234.(225)Kalish JM, et al. (2024) Clin Cancer Res, 30: 5260.(226)Nakano Y, et al. (2024) Clin Cancer Res, 30: 5009.(227)Perrino MR, et al. (2024) Clin Cancer Res, 30: 4834.(228)MacFarland SP, et al. (2024) Clin Cancer Res, 30: 4566.(229)Maese LD, et al. (2024) Clin Cancer Res, 30: 4286.(230)Das A, et al. (2024) Clin Cancer Res, 30: 3378.(231)Kratz CP, et al. (2024) Clin Cancer Res, 30: 1733.. The updated guidelines for certain CPSs now recommend that surveillance begin at birth or during early childhood, depending on the syndrome, with blood tests and with ultrasound or MRI imaging prioritized over computed tomography (CT) to reduce radiation exposure. These protocols aim to catch tumors early when cure rates exceed 90 percent, such as for Wilms tumor or hereditary retinoblastoma (see Table 3).
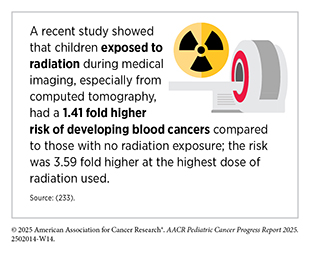
While structured surveillance protocols are beneficial for children with CPS, one of the key remaining concerns is radiation exposure of children as a result of surveillance protocols, many of which require imaging approaches involving radiation. The 2023 AACR PCWG update emphasizes the use of MRI (especially whole-body MRI) and ultrasound whenever possible, with the sparing use of CT for specific indications and syndromes. Recent studies have provided strong evidence to spare the use of CT when possible. In a study of nearly one million children, adolescents, and young adults who received computed tomography, those who received very high exposure to radiation had a 2.66-fold higher risk of blood cancers later in life, compared to those with very low exposure (232)Bosch de Basea M, et al. (2023) Nat Med, 29: 3111.. These principles should also be considered when imaging these patients for indications unrelated to cancer (218)Voss SD, et al. (2025) Clin Cancer Res, 31: 3638..
Another concern pertains to children’s risk of developing second primary cancers, either due to treatment of the primary cancer or because of a CPS. Although this risk is known, continued research can further help identify and refine surveillance strategies that will be most effective for this population (231)Kratz CP, et al. (2024) Clin Cancer Res, 30: 1733..
New Frontiers in Surveillance for Children With Cancer Predisposing Syndromes
Major strides have been made in genome sequencing and in understanding the role of genetic alterations in CPS in childhood cancer causation. However, the window in which a child’s risk for developing cancer can be detected and a care plan can be developed to mitigate the risk remains very brief, posing a serious challenge. Researchers are developing innovative new approaches and improving established methods that are noninvasive or minimally invasive and can detect children who may be at higher risk of developing cancer accurately and in a timely manner. In this section, we highlight some of the approaches that are either being implemented in the clinic now or are on the horizon to accelerate the pace of progress in early detection of childhood cancers.
Minimally Invasive Approaches
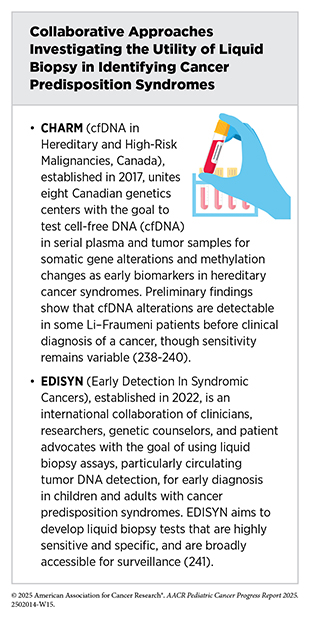
Liquid biopsies are emerging as a powerful, minimally invasive alternative to traditional, invasive tissue biopsies, with potential application across the continuum of care for children with cancer (see Liquid Biopsy) (234)Sundby RT, et al. (2022) Curr Opin Pediatr, 34: 39.(235)Janssen FW, et al. (2024) NPJ Precis Oncol, 8: 210.. Two recent studies point to a promising role for liquid biopsy in early detection among children with a CPS (236)Sundby RT, et al. (2024) Clin Cancer Res, 30: 4363.(237)Wong D, et al. (2024) Cancer Discov, 14: 104.. In a cohort of 89 people with LFS, including 26 children, researchers analyzed 193 blood samples using an approach that detects DNA alterations, variations in the size of the circulating tumor DNA (ctDNA) fragments, and epigenetic marks. In some cases, cancer-related signals appeared when the child had no clinical symptoms, and in several cases, these signals emerged months before standard surveillance detected any lesions. Among clinically cancer-free carriers, just over half of positive results (54 percent) reflected a true cancer signal, and a negative result was 95 percent accurate (237)Wong D, et al. (2024) Cancer Discov, 14: 104.. In individual samples, epigenetic marks were detectable about 20 months before traditional detection methods for osteosarcoma, a type of bone cancer, and combined DNA fragment and epigenetic signals preceded clinical diagnosis of leukemia or melanoma by about 6 to 18 months (237)Wong D, et al. (2024) Cancer Discov, 14: 104..
The second study evaluated plasma from 101 patients with neurofibromatosis type 1 and 21 controls for size variations in cell-free DNA (cfDNA) fragments—tiny DNA pieces released into the blood when cells die—and compared the findings with those from the tumor fraction test, which measures how much tumor DNA is present compared to normal DNA in a sample (236)Sundby RT, et al. (2024) Clin Cancer Res, 30: 4363.. The tumor fraction test detected many cancerous nerve tumors, but it could not detect the difference between benign and early-stage malignant tumors. In contrast, the accuracy of using cfDNA fragments was much better and detected 91.4 percent of malignant samples compared with 74.3 percent using tumor fraction alone. It also resolved several clinically ambiguous cases (236)Sundby RT, et al. (2024) Clin Cancer Res, 30: 4363.. Together, these results suggest that liquid biopsy can help detect cancers early in children with a CPS and inform surveillance decisions, while minimizing harm to children.
Multi-cancer early detection (MCED) tests, a variation of liquid biopsy, broadly aim to detect cancer-related signals from emergent cancers in the same assay. These tests typically detect molecular features of cfDNA, such as epigenetic marks it carries, known cancer predisposing mutations and somatic alterations in children, and/or size variations in DNA fragments. For the detection of cancer predisposing gene variants, MCED tests are carried out in specialized clinical settings designed for the detection of CPSs due to the complexity of the tests. The information gathered from these tests is integrated together to evaluate whether an individual should be screened for an emergent cancer and, if so, what type of cancer, including which area of the body should be imaged for further evaluation.
MCED tests carry enormous potential to revolutionize cancer screening in adults, although no MCED test has been approved for routine screening (242)Imai M, et al. (2025) Int J Clin Oncol, 30: 180.(243)Rubinstein WS, et al. (2024) CA Cancer J Clin, 74: 368.(244)Wade R, et al. (2025) Health Technol Assess, 29: 1.. Although studies evaluating the utility of MCED tests in children with a CPS or cancer are rare, those discussed above provide important groundwork for the utility of MCED tests in children who are at higher risk of developing cancer due to genetic predisposition. More research is needed to establish the utility of MCED tests in children with CPS. If these tests can detect very small amounts of tumor signal with very high accuracy, they could serve as a minimally invasive way to prompt urgent, targeted imaging in children who have other symptoms of a CPS or are already in well-defined high-risk groups, based on an earlier cancer diagnosis and clinical testing result indicating at CPS. With sufficient data to support the clinical utility of MCED surveillance, the time to diagnosis of an emergent cancer would be shortened as would the potential for improved outcome.
Numerous studies have shown that liquid biopsies are also a superior choice for monitoring disease progression and the patient’s response to treatment following a cancer diagnosis. A powerful example of the benefits of liquid biopsy is its application in brain cancers, one of the most common cancers among children (see Pediatric Cancer Trends in the United States). Detecting brain cancers in children often relies on procedures that are invasive and potentially harmful. MRI scans, while essential, usually require sedation or anesthesia in young patients, which carries risks when repeated over time. In many cases, diagnosis also involves surgical biopsy of brain tissue, a procedure that may have significant potential complications, depending upon the location of the cancer. Even the collection of cerebrospinal fluid (CSF), though less invasive than surgery, is still uncomfortable and carries its own, albeit minimal, risks.
Liquid biopsy offers a promising new approach by analyzing tumor DNA or cells in blood or CSF samples. A simple blood draw, in particular, could provide critical diagnostic and monitoring information without exposing children to the potential harms of surgery or repeated anesthesia, marking a major step forward in safer care (235)Janssen FW, et al. (2024) NPJ Precis Oncol, 8: 210.(245)Doculara L, et al. (2022) Front Mol Biosci, 9: 885597.. Research has demonstrated that the detection of tumor DNA in CSF or in plasma can identify subtypes of brain tumors and help track disease over time, often earlier than MRI and cytology (157)Liu APY, et al. (2021) Cancer Cell, 39: 1519.(246)Izquierdo E, et al. (2021) Neurooncol Adv, 3: vdab013.. More recent studies have shown further promise of liquid biopsy in childhood cancer care. For example, in children with embryonal brain tumors, testing tumor DNA fragments in CSF found cancer signals in 92 percent of samples versus 17 percent with the tissue biopsy (247)Crotty EE, et al. (2024) Neurooncol Adv, 6: vdae126.. In neuroblastoma, a personalized blood test was negative for tumor DNA in every follow-up sample from children who remained well, but was positive for tumor DNA in all four cases of relapse, including one detected 78 days earlier than with the standard testing. This approach also outperformed five routinely surveyed markers in detecting relapse (248)Rahmqvist I, et al. (2024) Biomark Res, 12: 148.. In another recent study in which researchers evaluated samples collected at diagnosis from 233 children with hematologic, solid and brain tumors, ctDNA was detectable in all 177 children with hematologic malignancy; in 19 of 38 solid tumor patients and in 1 of 18 brain tumor patients. The assay also detected DNA sequence alterations, copy number variations, and structural variations responsible for oncogenic gene fusions (154)Lei S, et al. (2025) Leukemia, 39: 420..
Artificial Intelligence–based Solutions
Artificial Intelligence (AI) is a general term that applies to training a computational model to perform tasks commonly associated with human intelligence, such as how to reason, and learn. The use of AI carries enormous potential across the continuum of cancer care for adults, including in early detection of cancer, as is increasingly evident from US Food and Drug Administration (FDA) approvals and the integration in the clinic of AI-based software and devices. However, the field remains nascent for surveillance and screening in children with CPSs (see Sidebar 10) (249)Hashem H, et al. (2025) Front Med (Lausanne), 12: 1555893.(250)Elsayid NN, et al. (2025) Cureus, 17: e77524.(251)Ramesh S, et al. (2021) JCO Clin Cancer Inform, 5: 1208.(252)Hassan M, et al. (2025) Cancers (Basel), 17..
Recent studies, although investigating small patient cohorts and often in a single institute, are underscoring the immense potential of AI-enabled tools for surveillance in childhood cancers. Leukocoria, or white pupil, is an eye condition in which the pupil reflects light in a way that makes it appear white instead of the usual red (253)Kanukollu VM, et al. Leukocoria. StatPearls. Treasure Island (FL)2025.. Leukocoria is one of the most common signs leading to the diagnosis of retinoblastoma (254)Global Retinoblastoma Study G, et al. (2020) JAMA Oncol, 6: 685., which accounts for about 2 to 3 percent of all childhood cancers around the globe (255)Kaur K, et al. Retinoblastoma. StatPearls. Treasure Island (FL)2025.. Easy-to-perform approaches, such as CRADLE—a smartphone app that uses computer vision to scan photos and identify leukocoria as a way to screen for early detection of retinoblastoma—have been effective in real-world settings (256)Munson MC, et al. (2019) Sci Adv, 5: eaax6363..
Researchers are now leveraging AI to further improve detection of leukocoria in family photos. In this regard, a research team recently developed EyeScreen, a smartphone app designed to detect leukocoria through a combination of low-cost hardware and machine learning. The study involved smartphone-taken eye photos from 1,500 children who participated in the study (257)Bernard A, et al. (2022) Ophthalmol Sci, 2: 100158.. Eighty percent of the participant images were used to train a machine learning model. When tested for accuracy using images from 291 participants, the model showed 87 percent sensitivity (meaning detected true positive results) and 73 percent specificity (meaning avoided false positive results). It is important to note that the study was performed in Ethiopia and required only Android smartphones, which are less costly and readily available in the country (257)Bernard A, et al. (2022) Ophthalmol Sci, 2: 100158., indicating an easy-to-perform approach to flagging potential early signs of retinoblastoma, especially in resource-limited settings.
Beyond smartphone photos, researchers tested a deep learning model trained on clinical-grade images of the eye that can distinguish between normal eye images and those showing signs of retinoblastoma (258)Aldughayfiq B, et al. (2023) Diagnostics (Basel), 13.. On a test dataset containing images from children with or without retinoblastoma, the model distinguished between the two groups with 97 percent accuracy and 99 percent precision, indicating that it could reliably detect cases while minimizing missed diagnoses (258)Aldughayfiq B, et al. (2023) Diagnostics (Basel), 13.. While results are promising, the model needs to be validated in real-world clinical settings with larger and more diverse datasets.
Two recent studies highlight how AI and digital tools can improve early tumor detection in children with TP53 variants. In one study, a machine-learning model, trained and validated using DNA methylation profiles from blood draws of 301 TP53 variant carriers, reached about 93 percent accuracy in a test group of 79 children with TP53 variants, correctly flagging most cancers before age six while sparing many low-risk children from extra scans (259)Subasri V, et al. (2025) Nat Commun, 16: 7976.. In the second study, the McGill Interactive Pediatric OncoGenetic Guidelines (MIPOGG) app standardized evaluation for CPSs, identifying 99.5 percent of 412 children with cancer, and when compared directly with genetic testing, showed high sensitivity (90.7 percent), a very strong ability to rule out disease (negative predictive value 98.6 percent), but only a modest ability to confirm cases (positive predictive value 17.6 percent) (165)Goudie C, et al. (2021) JAMA Oncol, 7: 1806..
AI based tools are an emerging frontier in early detection of cancer, but their use in surveillance and treatment decisions in pediatric oncology remains sparse. Additionally, large-scale studies will be critical to realize the general applicability and integration of AI-based solutions into clinical decision-making.
Innovative Imaging Enhancements
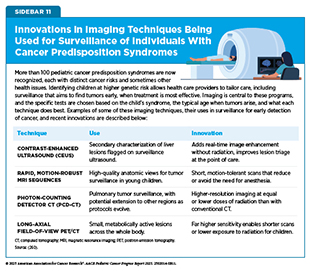
Imaging is the backbone for finding tumors early and tailoring tests according to specific risks associated with different CPSs, as well as with children’s ages of onset (260)Perrino M, et al. (2025) AJR Am J Roentgenol.. In recent years, many advances and innovations in imaging techniques have significantly improved the surveillance of children with CPS (see Sidebar 11).
Over the past decade, whole-body MRI has become a backbone of cancer surveillance for children with certain CPSs, such as LFS and constitutional mismatch repair deficiency (261)Grasparil AD, 2nd, et al. (2020) AJR Am J Roentgenol, 215: 1002.. Unlike CT or positron emission tomography (PET) scans, whole-body MRI avoids radiation, making it safer for repeated use in children, with studies confirming that it can detect asymptomatic but treatable tumors during follow-up scans (193)Greer MC, et al. (2024) Clin Cancer Res, 30: 5021.. Importantly, evidence indicates that interventions based on whole-body MRI scans improve outcomes for children with LFS who have central nervous system (CNS) tumors. One study showed that whole-body MRI detected low-grade CNS lesions in 92 percent of children with LFS on the surveillance protocol. Importantly, early surgical interventions led to a significant survival advantage in children with low-grade lesions, with an overall survival of 100 percent at 30 months (191)Patel N, et al. (2023) J Neurosurg Pediatr, 31: 258.. The consensus among experts is that whole-body MRI works best when integrated into syndrome-specific protocols rather than as a general screening tool (193)Greer MC, et al. (2024) Clin Cancer Res, 30: 5021.(261)Grasparil AD, 2nd, et al. (2020) AJR Am J Roentgenol, 215: 1002..
For syndromes such as the Beckwith–Wiedemann spectrum, in which errors in growth regulation lead to larger-than-expected growth in children and increase their risk of developing certain tumors, ultrasound-based surveillance has proven equally transformative. In Beckwith–Wiedemann spectrum, standardized abdominal ultrasound scans every 3 months during early childhood detect more than 95 percent of Wilms tumors, usually before cancer spreads, enabling surgery that spares the kidney (262)Murphy AJ, et al. (2023) Front Pediatr, 11: 1122390.(263)Mussa A, et al. (2019) J Cancer Res Clin Oncol, 145: 3115.. In retinoblastoma, imaging of the eye with handheld spectral domain optical coherence tomography (HH-SD-OCT) has revolutionized clinical care. Enhancements in HH-SD-OCT—higher-speed scanning, wider field of view to the periphery, and optimization for use for children (264)Papageorgiou E, et al. (2022) Expert Review of Ophthalmology, 17: 87.—can also enable earlier and more eye-sparing therapies by helping to detect microscopic retinal tumors invisible on fundus exam, a test in which an eye doctor uses a special instrument to examine the back part of the eye (the fundus), which includes the retina, optic nerve, and blood vessels (265)Allphin MT, et al. (2024) Fam Cancer, 24: 12.. Together, these imaging innovations are shifting care toward earlier, safer, and less invasive interventions.
Despite advances in imaging for surveillance of children with CPS, major gaps remain. Evidence linking survival outcomes with detection of tumors during surveillance remains scarce, since randomized trials are not feasible or considered ethical. Operational barriers, such as the need for anesthesia in very young children, limited pediatric MRI availability, and false positives leading to unnecessary biopsies, further complicate implementation (266)Mobley EM, et al. (2023) Cancer Med, 12: 18281.. Equally concerning is the uneven access: Many high-risk children still lack routine surveillance because of geographic differences in the availability of resources and expertise (266)Mobley EM, et al. (2023) Cancer Med, 12: 18281.. Addressing these gaps will be essential to ensure that these advances translate equitably into real-world improvements in survival and quality of life for children with cancer.
Next Section: Progress in Pediatric Cancer Treatment Previous Section: Unraveling the Genomics and Biology of Pediatric Cancers