Executive Summary
Remarkable progress against pediatric cancers is driven by discoveries across the basic, translational, clinical, and population sciences. Fueled by technological innovations, knowledge gleaned from these discoveries is improving diagnosis and surveillance, enabling personalized treatments, and reducing long-term treatment-related harm. As the world’s first and largest professional organization dedicated to preventing and curing all cancers, the American Association for Cancer Research (AACR) is committed to increasing public understanding of pediatric cancers, advocating for research funding, and supporting policies that accelerate the development and accessibility of effective treatments for our young patients.
The inaugural AACR Pediatric Cancer Progress Report 2025 highlights how research is transforming outcomes for children and adolescents, from molecularly targeted therapies and immunotherapies to genomic profiling that informs surveillance and treatment decisions. This first-of-its-kind report also underscores the gaps in our knowledge of pediatric cancers that are rare compared to most adult cancers, and are often understudied, and emphasizes the urgent need for increased federal investments, international collaborations, and innovative approaches to address these challenges.
Pediatric Cancer Trends in the United States
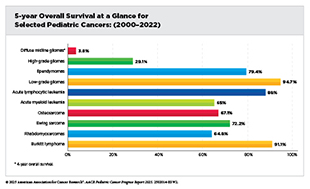
Decades of research and collaborations have transformed the outlook for cancers affecting children (ages 0 to 14) and adolescents (ages 15 to 19), collectively referred to as the pediatric cancers in this report. In the United States, the overall 5-year survival rate for pediatric cancers has risen from 63 percent in the mid-1970s to 87 percent in 2015–2021, although progress has slowed since 2000. Pediatric cancer mortality declined by 57 percent between 1970 and 2000 and by a further 19 percent from 2001 to 2023, reflecting continued progress driven by advances in risk-stratified therapy, precision medicine, and supportive care. Much of this progress stems from collaborative, multidisciplinary, international research initiatives supported by public and philanthropic funding sources.
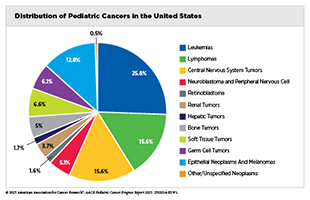
Pediatric cancers are rare. In 2025, nearly 15,000 children and adolescents will be diagnosed with cancer in the United States. Commonly diagnosed cancers among children include leukemias, brain and central nervous system (CNS) tumors, and lymphomas, and those among adolescents include lymphomas, thyroid cancer, and germ cell tumors. Although survival exceeds 90 percent for some cancers, such as Hodgkin lymphoma, thyroid carcinoma, and retinoblastoma, others, including high-grade gliomas and certain sarcomas, remain among the deadliest, with survival rates below 20 percent. The uneven pace of progress underscores the need for new research models and greater investments in drug discovery and development to improve outcomes for patients affected by aggressive and rarer subtypes of pediatric cancers.
The rarity of pediatric cancers has catalyzed broad national and international collaborations and partnerships. National initiatives, such as Project:EveryChild of the Children’s Oncology Group (COG) and Molecular Characterization Initiative (MCI) of the National Cancer Institute (NCI), are collecting comprehensive biospecimen and genomic data for common and rarer pediatric cancers. International partnerships like Cancer Grand Challenges and the COllaborative Network for NEuro-oncology Clinical Trials (CONNECT) are combining data and clinical expertise to develop novel therapeutics and expand access to innovative clinical trials. These collective efforts aim to ensure that all children and adolescents with cancer benefit from emerging therapies and precision medicine approaches.
Significant disparities in incidence and outcomes of pediatric cancers persist across racial, ethnic, geographic, and socioeconomic groups. For example, Hispanic children have the highest cancer incidence rates in the United States, while non-Hispanic Black children experience the lowest survival, with nearly a 30 percent higher likelihood of dying from select pediatric cancers than non-Hispanic Whites. Children and adolescents living in rural or economically disadvantaged areas also face higher mortality, often due to limited access to specialized centers, clinical trials, and supportive services.
The economic toll of pediatric cancers is substantial. The average cost of cancer care per child, including hospitalization and lost wages for parents, can approach $833,000 over the course of treatment and survivorship. Projections show that the cumulative cost of pediatric cancer care between 2020 and 2050 will exceed $594 billion globally, however, strategic investments can yield up to $2.6 trillion in lifetime productivity gains—a four-fold return on investment.
NCI allocated greater than $5 billion to pediatric cancer research between 2015 and 2024. Unfortunately, private-sector investments, which are pivotal to developing drugs and conducting clinical trials required for regulatory approvals, have lagged for pediatric cancers, making sustained federal and philanthropic support critical to continued progress. Philanthropic organizations focused on pediatric cancers have provided significant funding for basic research and clinical trials, bridging critical gaps left by the industry. Despite the public and philanthropic investments, the annual support for pediatric cancer research falls short. Sustaining the momentum of progress against pediatric cancers requires strengthening partnerships among federal agencies, industry, and philanthropic organizations so that every pediatric patient with cancer has the chance to survive and thrive.
Unraveling the Genomics and Biology of Pediatric Cancers
Pediatric cancers are biologically distinct from adult cancers. Many of these cancers arise early in development and are driven by normal growth pathways in immature cells that can normally become multiple tissue types, but are hijacked by tumor cells to fuel uncontrolled growth. Large-scale DNA sequencing studies have shown that pediatric cancers are typically driven by specific genetic, epigenetic, or structural changes that influence how cells grow, mature, and communicate, leading to the disruption of normal developmental programs.
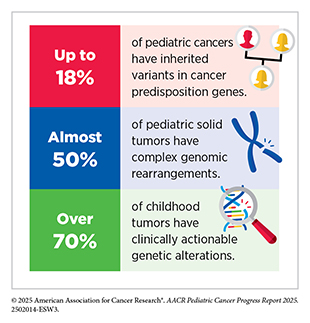
Advanced technologies, such as whole-genome and whole-exome sequencing, large-scale analyses of chemical changes in genes, and RNA sequencing, are offering insights into the molecular and cellular underpinnings of pediatric cancers. These approaches have revealed that both small mutations and large structural variants, such as gene fusions, chromosomal rearrangements, and amplifications, play critical roles in pediatric cancer development. While some gene fusions, for example those of NTRK and ABL genes, have become targets for precision therapies, many others are less well characterized or remain undruggable. Integrating tumor and germline sequencing has further revealed that over 70 percent of childhood tumors harbor clinically actionable alterations that can be used to make medical decisions, and up to 18 percent carry inherited mutations that predispose them to cancer. This knowledge has provided essential insights for improving diagnosis, guiding treatment, and identifying high-risk patients who may benefit from genetic counseling and surveillance.
Epigenetic alterations are another common driver of pediatric cancers, affecting how genes are switched on or off without changing the DNA sequence. Disruption of the proteins that regulate epigenetic changes can cause cells to lose identity and normal functions. A comprehensive understanding of the epigenetic landscape of normal and cancer cells is increasingly aiding tumor classification, diagnosis, and disease monitoring. As one example, profiling methylation, a common epigenetic alteration, has transformed tumor classification of brain tumors like medulloblastoma and glioma. Researchers are also exploring new therapeutics targeting epigenetic regulators to improve outcomes for pediatric patients with cancer. The tumor microenvironment (TME)—the ecosystem of cancer cells and supportive non-cancer cells, blood vessels, signaling molecules, and structural components surrounding a tumor—plays a pivotal role in how pediatric cancers progress and respond to therapy. The pediatric TME differs markedly from that of adults and is shaped by the developmental stage, with unique interactions between the immune system and cancer cells. Advanced technologies that enable understanding of cancer cells at the individual level and within the context of their surroundings have shown how chemotherapy and radiotherapy modify the TME, sometimes creating resistance to subsequent immunotherapy. These insights are guiding strategies to reprogram TMEs and effectively treat cancers in pediatric patients.
Technological innovations are fueling progress against pediatric cancers. Single-cell and multi-omic profiling is mapping the diversity of cells within tumors, while CRISPR gene editing is enabling functional testing of genetic drivers of the disease. Artificial intelligence (AI) is accelerating the integration of genomic and imaging data to identify molecular subtypes of tumors and predict outcomes precisely.
Collaborations and data-sharing have become a cornerstone of progress in pediatric cancer research. Large-scale initiatives such as MCI, the Childhood Cancer Data Initiative (CCDI), and the Human Tumor Atlas Network (HTAN), are connecting genomic, clinical, and imaging data to accelerate discovery and guide precision medicine. These efforts are already improving diagnosis and therapy selection for thousands of pediatric patients. Global initiatives, such as the Cancer Grand Challenges, are bringing large-scale data analyses and clinical expertise together to unravel the mechanisms that drive pediatric cancers and develop innovative targeted treatments.
Pediatric Cancer Predisposition and Surveillance
Roughly 10 percent to 18 percent pediatric cancers arises from inherited genetic alterations that confer a predisposition to cancer. Advances in genomics have transformed how a child or adolescent with a cancer predisposition syndrome (CPS) is diagnosed, enabling clinicians to identify risk of cancer development long before symptoms appear. Surveillance—the structured, ongoing monitoring through physical exams, imaging, or molecular tests—has become a cornerstone of pediatric cancer precision medicine, leading to the early detection of cancers in children with CPS, as well as monitoring children with CPS who have already been diagnosed with cancer for relapse or the development of second primary cancers.
Traditionally, clinicians have suspected a CPS when a child exhibits recognizable physical attributes, a strong family history, or a suggestive cancer pattern. Classic signs, such as light to dark brown flat birthmarks in neurofibromatosis type 1 or white pupils in heritable retinoblastoma, continue to guide early testing and surveillance, especially in health care settings where universal genetic screening is not available. However, many children with CPS lack outward features or family history, resulting in delayed diagnosis and missed opportunities for early intervention.
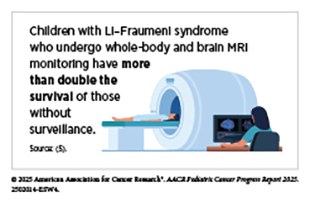
Modern approaches, such as single-gene tests, multigene panels, and whole-genome and whole-exome sequencing, can pinpoint inherited mutations responsible for CPS, for example those in the TP53 gene in Li–Fraumeni syndrome. Although test results from these approaches are usually available within days or weeks, limited infrastructure, high costs, and shortages of trained professionals restrict access, especially for families in rural areas or low-resource settings. Psychosocial and ethical concerns, from anxiety and misunderstanding of results to questions about consent, further complicate uptake of genetic testing. Despite these challenges, integrating genetic testing into pediatric oncology has proven transformative. Identifying inherited genetic variants associated with cancers allows clinicians to implement syndrome-specific monitoring strategies, such as periodic whole-body magnetic resonance imaging (MRI) for children carrying TP53 gene variants. Combined clinical and genetic assessments remain the most effective pathway for early and accurate risk detection in pediatric patients.
Genetic counseling is central to bridging scientific advances with coordinated care. Counselors guide families through testing decisions, explain results, and plan follow-up surveillance. They also help parents weigh the benefits and limitations of genetic testing, address ethical implications, and help manage a stressful situation for the patients and their families. Structured counseling paired with surveillance can substantially improve survival.
Dedicated cancer predisposition clinics, often a part of major cancer centers, provide multidisciplinary support to affected children and their parents. However, shortages of trained counselors and fragmented reimbursement continue to limit widespread availability of genetic counseling. Workforce expansion and licensure reform, among other interventions, can help mitigate these challenges.
Standardized surveillance guidelines, historically available only for a few CPSs, are now available for many CPSs. In 2023, the American Association for Cancer Research Pediatric Cancer Working Group updated its landmark 2017 consensus surveillance guidelines, emphasizing radiation-sparing imaging—MRI and ultrasound—and recommending that surveillance for many CPSs begin at or soon after birth. The next frontier is the genomic newborn screening to identify infants at risk before disease develops. Modeling studies suggest that sequencing for a small set of cancer risk genes could reduce childhood cancer mortality by nearly half. While promising, these efforts raise complex issues surrounding consent, privacy, and security of children’s health-related data. Experts are also concerned about findings of unknown significance that can cause unnecessary anxiety for the parents and/or medical procedures for the child.
New technologies are redefining early detection of cancers in children. Liquid biopsy—a minimally invasive technique that detects tumor DNA or cells in blood or cerebrospinal fluid—has been shown to detect cancer months before standard imaging in children with Li–Fraumeni syndrome. Multi-cancer early detection assays, an area of current intense research, could eventually enable broad, noninvasive screening across tumor types. Machine learning models that are trained using medical images or molecular data are showing promise in detecting patterns that can escape human observation. Smartphone-based applications, for example those capable of recognizing white pupils, a predictor of retinoblastoma, in family photographs offer a low-cost approach that is particularly useful in resource-limited settings. Similarly, other AI-driven tools, such as the McGill Interactive Pediatric OncoGenetic Guidelines application, are helping standardize evaluation for CPSs. Technological advances in imaging are improving safety and precision. As one example, standardized abdominal ultrasounds every 3 months during early childhood in children on the Beckwith–Wiedemann spectrum detect over 95 percent of Wilms tumors before metastasis, allowing organ-sparing surgery. Innovations, such as contrast-enhanced ultrasound, further enhance image resolution while minimizing radiation exposure.
Innovations in genomics, imaging, and artificial intelligence, together with equitable access and ethical oversight, can move pediatric oncology closer to a future in which cancer risk is detected early, monitored safely, and managed effectively, giving every child the best possible opportunity for surviving a cancer diagnosis and living a high-quality life.
Progress in Pediatric Cancer Treatment
Treatments for pediatric cancers have undergone a remarkable transformation over the past several decades, with 5-year survival from all cancers combined now exceeding 85 percent in the United States. These gains stem from advances in surgery, chemotherapy, and radiotherapy, coupled with a deeper understanding of cancer biology and the immune system that has enabled more personalized, less toxic therapies. Increasingly, clinicians are tailoring treatment intensity based on the molecular profile of a child’s cancer, reducing therapy for children with a favorable disease profile and intensifying therapy for those at a higher risk of recurrence.
Innovations in chemotherapy, a cornerstone of pediatric oncology, have shifted toward safer regimens to reduce toxicities associated with the treatment. Precision radiotherapy approaches are helping to minimize damage to developing organs. Clinical trials in patients with Wilms tumors, Hodgkin lymphoma, and hepatoblastoma have demonstrated that omitting radiotherapy altogether, reducing the radiation dose, or minimizing chemotherapy can still successfully treat some children with these cancers while sparing them the severe long-term side effects of these treatments, without compromising survival or health-related quality of life. Similarly, advances in surgery, particularly minimally invasive techniques, are improving recovery and reducing complications for certain patients.
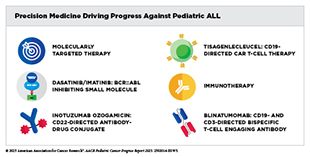
Precision medicine has been transformative for the treatment of certain pediatric cancers. Molecular profiling now routinely informs diagnosis, prognosis, and treatment selection for certain patients. For example, molecular testing in acute lymphoblastic leukemia (ALL), the most common childhood cancer, helps determine the level of risk based on genetic features, such as the presence of gene fusions ETV6::RUNX1 or BCR::ABL1, thus allowing clinicians to tailor therapy for maximal benefit. Similarly, the molecular classification of medulloblastoma, the most common malignant brain tumor in children, and neuroblastoma, the most common solid tumor outside the brain, guides tailored treatments. National initiatives, such as MCI and international efforts like, Zero Childhood Cancer, MAPPYACTS, and AcSé-ESMART are expanding access to genomic testing to ensure that children and adolescents with cancer can benefit from precision medicine.
Molecularly targeted therapies are allowing successful treatments of some cancers that were once deemed intractable. Recent FDA approvals for childhood cancers, including revumenib for leukemia harboring KMT2A alterations; tovorafenib for low-grade glioma with BRAF alterations; and the first systemic therapy dordaviprone for H3K27M-mutated diffuse midline glioma, a fatal brain tumor, highlight how cancer genetics is driving clinical breakthroughs. Additional approvals, such as selumetinib and mirdametinib for neurofibromatosis type 1 and belzutifan for rare endocrine tumors, have increased treatment options for those with inherited CPSs. However, the pace of molecularly targeted drug development for childhood cancers lags far behind that for adult cancers, with very few new therapeutics specifically developed for and tested in pediatric patients.
Immunotherapy, which invokes a patient’s own immune system to eliminate cancer and represents one of the most exciting approaches to cancer treatment, has added a powerful new dimension to pediatric cancer treatment. CAR T-cell therapy has revolutionized care for children with relapsed or refractory ALL, offering long-term remission. Other immunotherapeutics, including dinutuximab for neuroblastoma, rituximab for non-Hodgkin lymphoma, and blinatumomab for ALL, have become standard of treatment, reducing the need for more toxic regimens. However, one class of immunotherapeutics known as immune checkpoint inhibitors, which have transformed the treatment of many adult cancers, have not been successful thus far in pediatric cancers.
Treatment of pediatric cancers faces major challenges that are attributable to multilevel barriers. Current approaches are insufficient to develop effective drugs against fusion proteins or epigenetic alterations that drive many pediatric cancers.
Low incidence rates of pediatric cancers and financial disincentives for the private sector limit patient recruitment for clinical trials, slowing discovery and regulatory approval. Racial and socioeconomic disparities persist, with Black and Hispanic children less likely to enroll in clinical trials and more likely to experience treatment-related complications. Evidence shows that expanding global clinical trial networks is critical to ensuring that progress against pediatric cancer benefits all children.
Although challenges remain, new technologies are poised to accelerate progress in pediatric cancer treatment. Innovative drugs, such as proteolysis-targeting chimeras, theranostics, and bispecific antibodies, are expanding treatment options by targeting historically intractable proteins that drive pediatric cancers. Liquid biopsy is showing promise for monitoring treatment response and detecting relapse in real time, especially in brain cancers and solid tumors for which the standard biopsy is highly invasive and carries significant risks. Artificial intelligence is accelerating diagnosis and trial design by analyzing imaging and molecular data to predict responses and simulate trial outcomes. Further, novel approaches in cellular engineering are rapidly extending the success of CAR T-cell therapies to additional subtypes of ALL, acute myeloid leukemia, and solid tumors, such as neuroblastoma and aggressive brain cancers.
Supporting Survivors of Pediatric Cancers
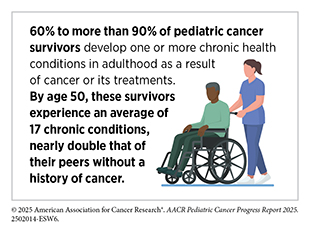
As of 2022, more than 521,000 pediatric cancer survivors were living in the United States, a number projected to exceed 580,000 by 2040. Yet, for many, survivorship is a lifelong journey shaped by the enduring physical, emotional, and financial consequences of cancer and its treatment. As more young people survive cancer, the focus of pediatric oncology has expanded to include promoting long-term health, improving quality of life, and delivering comprehensive survivorship care.
Pediatric cancer survivors are at higher risk for developing long-term health problems, known as late effects, that arise from cancer or from its treatments. These late effects may affect multiple organ systems and include heart disease, hormonal and growth disorders, infertility, hearing loss, neurocognitive impairment, and second primary cancers. Many survivors also experience accelerated aging—the premature onset of chronic, age-related diseases—driven by treatment-related DNA damage and inflammation.
Over the past several decades, reduced exposure to radiation and decreased use of anthracyclines (a class of chemotherapeutic drugs) has substantially decreased the risk of heart disease, hormonal and growth disorders, and second primary cancers among pediatric patients. The development of protective agents, such as dexrazoxane to prevent heart damage and sodium thiosulfate to reduce hearing loss, has further minimized chemotherapy-related toxicity. Moreover, advances in precision medicine are enabling tailored treatments based on molecular and genetic factors, thus improving outcomes and minimizing harm for certain patients.
Genetic predisposition plays an important role in determining which pediatric cancer survivors are most susceptible to late effects. Studies have identified inherited gene variants associated with DNA repair and cancer predisposition—for example, those of TP53, RB1, BRCA2, and FANCM genes—that can amplify the risk of second primary cancers or treatment-related heart disease. Integrating genetic information with treatment history helps identify survivors at a higher risk for late effects and develop targeted monitoring and prevention strategies.
The psychological and social toll of pediatric cancers can be profound. Survivors face an elevated risk of anxiety, depression, and posttraumatic stress, as well as learning and memory-related difficulties that can limit educational and employment opportunities. Young adult survivors (ages 20 to 39) of pediatric cancers are less likely to complete higher education, live independently, or marry, compared to peers without a cancer history. These effects underscore the importance of providing psychosocial support throughout the survivorship continuum.
Pediatric cancer survivors and their families also face lasting economic strain due to high medical costs, missed work, and reduced earning potential. Nearly two-thirds of survivors report some form of financial hardship, and many experience difficulty maintaining health insurance coverage or paying for follow-up care. Legislative measures and state insurance mandates for fertility preservation have helped to alleviate some of these burdens, but disparities persist, particularly among survivors from low-income households, rural communities, or racial and ethnic minority populations.
Parents of children and adolescents with cancer bear significant emotional burdens, including higher rates of anxiety, depression, and posttraumatic stress, as well as long-term financial insecurity including job loss or reduced work hours during their child’s treatment. These challenges highlight the need for care models that provide medical support, access to mental health services, financial counseling, and workplace protections for families of pediatric patients with cancer.
The complexity of pediatric cancer survivorship demands coordinated, multidisciplinary care across the lifespan. The COG Long-Term Follow-Up Guidelines provide a cornerstone for risk-based, lifelong surveillance. Updated in 2023, the guidelines include recommendations for genetic testing; monitoring after novel therapies, such as CAR T-cell treatment; and vaccination protocols. Tools, such as Passport for Care, help clinicians implement these guidelines through individualized, web-based survivorship care plans, ensuring that survivors receive consistent, evidence-based follow-up. Still, many pediatric cancer survivors forgo follow-up care or receive inconsistent care, particularly during the transition from adolescence to adulthood, which often complicates care continuity.
Collaborative models that integrate primary care providers, oncologists, and psychosocial specialists are emerging as best practices to improve coordination and address the full spectrum of survivorship needs. Patient-reported outcomes, digital health tools, and mobile applications are helping clinicians track symptoms, enhance communication, and promote engagement in care. These innovations, combined with improved coordination and training for primary care providers, are making survivorship care more accessible and effective.
Despite remarkable progress, many survivors continue to face lifelong health risks and social challenges. Holistic and equitable approaches that value both the years of life gained and the quality of those years, sustained investments in survivorship research and workforce development, and supportive health policies are essential to ensure that every pediatric cancer survivor can thrive in adulthood.
Understanding the Global Landscape of Pediatric Cancers
Pediatric cancer is a global health challenge, affecting nearly 400,000 children annually, with the vast majority of cases and deaths confined to low-income countries (LICs), lower middle-income countries (LMICs), and upper middle-income countries (UMICs). Despite tremendous advances in survival for certain pediatric cancers in high-income countries (HICs)—where 5-year survival for all cancers combined exceeds 85 percent—survival remains below 30 percent in LICs and LMICs, reflecting inequities in access to diagnostics, treatment, essential medicines, and a trained pediatric oncology workforce. The global burden is further compounded by the lack of population-based cancer registries in many low-resource settings, leading to incomplete data on incidence and outcomes and widespread underdiagnosis. Addressing these gaps requires strengthening health systems, building data infrastructure, and improving clinical capacity to ensure that every child, regardless of geography or income, can access timely and effective care.
The World Health Organization (WHO) Global Initiative for Childhood Cancer (GICC) with its CureAll framework represents the most ambitious effort to address global disparities in the burden of pediatric cancers. Launched in 2018 with the goal of achieving at least 60 percent survival for pediatric cancers globally by 2030, GICC provides a roadmap for integrating childhood cancer care into national cancer control plans through four pillars—centers of excellence, universal health coverage, standardized treatment regimens, and monitoring and evaluation—supported by advocacy, financing, and governance. More than 80 countries are already working with GICC to develop or strengthen national pediatric cancer care strategies.
Innovative partnerships are the driving force behind progress against pediatric cancers globally. The St. Jude–WHO Global Platform for Access to Childhood Cancer Medicines, launched in 2021, aims to deliver essential medicines to at least 120,000 children in LMICs over 7 years. This initiative, which is already operational across Asia, Africa, and Latin America, is expanding to include national programs, such as Ghana’s plan to provide free essential medicines for children with cancer by 2026. Similarly, the Adapted Resource and Implementation Application (ARIA) Guide, developed through collaboration among several global organizations focused on pediatric cancers, provides clinicians with resource-adapted, evidence-based protocols to care for pediatric patients with cancer in regions with limited infrastructure.
Precision medicine and clinical research are offering molecularly guided treatment options that improve survival and reduce toxicity. New multinational and adaptive trial platforms are expanding opportunities for children with relapsed or refractory cancers to access novel therapies matched to their tumor’s molecular profile. Programs, such as the Netherlands’ iTHER, Australia’s Zero Childhood Cancer Program, and Europe’s MAPPYACTS, have demonstrated the feasibility and clinical impact of integrating molecular profiling into pediatric cancer care. However, access to these technologies remains highly uneven. In some LMICs, resource-adapted approaches are helping to fill gaps in access to advanced technologies needed for molecular profiling.
Access to treatment remains the greatest challenge globally. The availability of WHO essential medicines for childhood cancers varies widely, and treatment abandonment rates in LICs and LMICs can exceed 30 percent due to high out-of-pocket costs, travel burdens, distrust in modern medicine, and lack of supportive services. Studies have shown dramatically improved treatment retention and survival through locally adapted protocols and social interventions, as seen in Guatemala and Malawi, where treatment abandonment rates have dropped below 1 percent and survival has doubled. Regional collaborations in Africa and Latin America promoting standardized protocols, shared expertise, and improved supportive care are aiming to close survival gaps for pediatric cancers, such as ALL, Burkitt lymphoma, and Wilms tumor.
The shortage of a skilled pediatric oncology workforce is another critical barrier. Across Africa, there is fewer than one clinician specialized in pediatric cancer for every one million children, and only four countries have the capacity to treat pediatric brain tumors. Expanding region-specific training programs, building multidisciplinary teams, and investing in infrastructure for radiotherapy and surgery are essential to achieve the GICC goal by 2030. Global partnerships, such as the Pediatric Oncology East and Mediterranean network and the Franco-African Pediatric Oncology Group, demonstrate that coordinated regional training and mentorship can increase workforce capacity and improve outcomes.
The global landscape of pediatric cancer reflects extraordinary progress in some regions and deep inequities in others. While HICs continue to benefit from advances in precision medicine, immunotherapy, and supportive care, most children worldwide lack access to standard of care treatment. Achieving the GICC goal requires sustained international collaboration and national policy commitments, as well as investments in health care infrastructure and workforce development.
Advancing Pediatric Cancer Research and Patient Care Through Evidence-based Policies
Federal government programs and policies are critical to catalyze progress against pediatric cancers. Robust and sustained investment in agencies, such as NIH and FDA, play key roles in driving progress, enabling scientific breakthroughs, supporting the next generation of researchers and physician-scientists, and improving patient care.
Although NIH and NCI are global leaders in providing funding for pediatric cancer research, challenges persist, including workforce shortages, inadequate infrastructure for research and clinical trials, and inequities in support across cancer types and for survivorship research. Increased federal funding, more flexible grant models, and improved transparency in funding allocation are urgently needed to continue making significant progress and ensure equitable care for all children with cancer. Crucially, any cuts to federal agencies and their staffs or programs would drastically impact pediatric cancer research, stalling scientific discovery, reducing innovation, and harming patients and their families.
Bipartisan congressional support and key legislation have significantly advanced pediatric cancer research, data-sharing, and drug development over the past decade. Landmark legislations, such as the Creating Hope Act, RACE for Children Act, and the STAR Act, have incentivized pharmaceutical innovation, expanded clinical trials, and enhanced federal data infrastructure. However, ensuring that pediatric drug studies are completed and research is successfully translated into approved therapies for children with cancer continues to pose significant challenges. Reauthorization and continued funding of these initiatives, along with stronger enforcement, are essential to sustain momentum and improve outcomes for pediatric cancer patients.
Advances in regulatory science and specific regulatory reforms policy have further translated research into new treatments for children with cancer. FDA plays a critical role in advancing pediatric cancer treatment by ensuring that drug development processes account for the unique biological and clinical needs of pediatric cancer patients, promoting early integration of pediatric considerations and innovative trial designs, and maintaining tools like the Pediatric Molecular Targets List to guide regulatory decisions.
However, and even despite these advances, pediatric cancer patients continue to face numerous challenges, including limited access to clinical trials (particularly for those in rural or underserved communities), financial burdens on families during and after treatment, and inadequate survivorship support. These challenges highlight the urgent need for continued legislative and policy action. A range of new and proposed legislation—such as the Innovation in Pediatric Drugs Act, Give Kids a Chance Act, EPIC Act, and Accelerating Kids’ Access to Care Act—aims to strengthen drug development pipelines, enforce timely pediatric studies, enhance care access, and expand molecular diagnostics. Additionally, policies that promote comprehensive health insurance coverage, mitigate barriers to health care access, address health disparities, and enhance legislative implementation will be essential to sustain momentum, accelerate the development of innovative treatments, and ultimately improve care for pediatric patients. It is especially important for new evidence-based policies to prioritize research and drug discovery and development tailored to the unique biology of pediatric cancers and the needs of pediatric patients to ensure that all children can benefit equitably from scientific and medical progress.
AACR Call to Action
Congress plays a crucial role by funding vital research programs and advancing policies that improve the lives of children with cancer and pediatric cancer survivors. Unfortunately, the current political climate, budget cuts, and funding instability threaten to curtail scientific advancement, weaken America’s biomedical enterprise, and stymie future progress. AACR calls on all stakeholders to engage with members of Congress and leaders at federal agencies to prioritize pediatric cancer research and patient care.
AACR recommends the following actions:
- Provide robust and sustained federal funding of no less than $51.303 billion for NIH and $7.934 billion for NCI in FY 2026 and increase support for the federal agencies and programs that are focused on pediatric cancer research and patient care.
- Expand access to clinical trials and promising therapies for children and adolescents with cancer through regulatory reform and policies to address barriers.
- Modernize and evaluate current pediatric cancer research programs and policies to better support the discovery and development of treatments as well as to improve patient care.
- Support efforts that leverage and harmonize all available data to aid pediatric cancer research including the objectives and proposals outlined in the Administration’s recent Executive Order from September 30, 2025, to prioritize the harnessing of American artificial intelligence innovation to unlock cures for pediatric cancer.
- Foster global and public–private partnerships to accelerate pediatric cancer research and the development of innovative treatments for pediatric cancer patients.
- Strengthen survivorship and long-term care for pediatric cancer survivors by ensuring comprehensive, accessible, and reimbursable long-term care services.
By following these recommendations, the United States will foster innovative research, accelerate scientific discovery, create groundbreaking cures, and remain the global leader in pediatric cancer research. Robust and sustained investment will improve our nation’s health and deliver on the promise of a future without cancer. The progress against pediatric cancers is at a critical juncture, and now is the time for a renewed commitment to scientific research that can help save and improve the lives of millions of children and adolescents with cancer.
Next Section: A Snapshot of Progress Against Pediatric Cancers in 2025 Previous Section: A Message from the AACR