Unifying Cancer Science and Medicine: A Continuum of Innovation for Impact
In this section, you will learn:
- Researchers are leveraging insights into the cellular and molecular mechanisms of cancer initiation and progression to design innovative, patient-centric clinical trials that lead to safer and more effective treatments.
- Advances in novel approaches to surgery, radiotherapy, chemotherapy, molecularly targeted therapy, and immunotherapy—the five pillars of cancer treatment—are saving and improving lives.
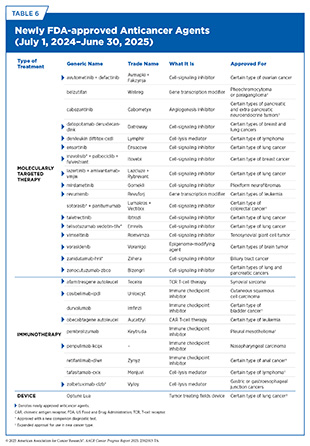
- From July 1, 2024, to June 30, 2025, the US Food and Drug Administration (FDA) approved 20 new therapeutics for treating various cancer types, approved one new device for treating lung cancer, and expanded the use of eight previously approved anticancer therapeutics to treat additional cancer types.
- Among FDA approvals are a new molecularly targeted therapy against a novel target for patients with lung cancer and pancreatic cancer, a new targeted therapy for a specific type of brain tumor, and a new form of immunotherapy—T-cell receptor (TCR) T-cell therapy—for patients with a rare sarcoma.
- While these exciting new advances have the potential to transform patient care, much work is needed to ensure all patient populations have access to these treatments.
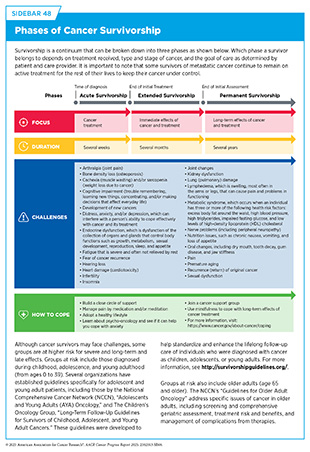
In the United States (US), the overall cancer death rate is declining steadily, and more individuals are living longer and fuller lives after a cancer diagnosis (see Cancer in 2025 and Supporting Cancer Patients and Survivors). This progress is attributable, in part, to the rapid advances in cancer treatment propelled by breakthroughs across the continuum of medical research.
Medical Research
Medical research is an iterative process that is set in motion when a discovery with the potential to affect the practice of medicine or public health is made in any area of research or clinical practice. One way that researchers build on a discovery is by asking questions that can be tested through experiments in a wide range of models that mimic healthy and diseased conditions (see Sidebar 6). Results from these experiments can lead to the identification of a potential preventive intervention or therapeutic target, or to the identification of a potential biomarker that can predict how a cancer might behave or how well a treatment might work. They also can feed back into the medical research cycle by providing new discoveries that lead to more questions or hypotheses (see Figure 5).
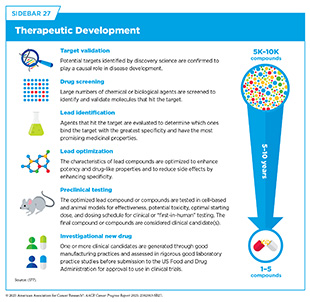
If a potential therapeutic target is identified, it takes many more years of preclinical research before a candidate therapeutic is developed and ready for testing in clinical trials (see Sidebar 27). During this time, several candidates are rigorously tested to identify any potential toxicity and to determine the appropriate doses and dosing schedules for testing in the first clinical trial.
Clinical Research
Clinical research, also known as clinical studies or clinical trials, evaluates the safety and efficacy of candidate agents before a therapeutic can be approved by the US Food and Drug Administration (FDA) and used as part of routine patient care. Institutional review boards critically review and approve all clinical studies before they can begin. Clinical trials are monitored throughout their duration. Patient safety and understanding of the clinical trial are prioritized through the informed consent process, which involves a discussion between the clinical research team and the patient about the trial’s purpose and what is expected of the patient, potential benefits and risks, alternative treatments, and the patient’s right to withdraw at any time.
There are several types of cancer clinical trials, including prevention trials, screening trials, treatment trials, and supportive or palliative care trials, each designed to answer different research questions. Clinical studies in which participants are randomly assigned to receive an investigational treatment or standard treatment are called randomized clinical trials and are considered the most rigorous.
Participating in a clinical trial has several benefits. These include access to potentially more effective treatments with careful monitoring before they are widely available, an active involvement in making health care decisions, and a direct contribution to lifesaving cancer research (455)Abu Rous F, et al. (2024) JAMA Oncol, 10: 416..
Along with private sector–sponsored clinical trials, National Cancer Institute (NCI)–supported studies play a vital role in the development of novel anticancer treatments. Researchers estimate that over the past four decades, clinical trials funded by NCI contributed to 14 million additional years of life for cancer patients in the United States (68). Additionally, NCI-funded clinical research has been critical in advancing our knowledge in key areas such as treatment de-escalation (see Minimizing the Use of Invasive Cancer Surgery), improving quality of life, and survivorship (see Supporting Cancer Patients and Survivors) (456)Abdou Y, et al. (2024) J Clin Oncol, 42: 3887.. Unfortunately, between 2008 and 2022, enrollment in federally funded cancer clinical trials remained flat while enrollment in private sector–sponsored trials doubled (457)Unger JM, et al. (2024) J Clin Oncol, 42: 3917.. While industry investment has accelerated the development of breakthrough cancer treatments, these data highlight the growing reliance on the private sector and concerning underinvestment in publicly sponsored research (see Investing in Research to Achieve a Healthier Future).
Clinical trials testing potential new cancer treatments have traditionally been conducted in successive phases, each involving more patients and typically focusing on a specific cancer type (e.g., breast cancer or prostate cancer). Phase I studies are designed to determine the optimal dose of an investigational anticancer therapeutic, how patients process it, and potential toxicities. Historically, phase I trials were not designed to evaluate anticancer efficacy of a therapeutic. However, because of rapid progress in clinical trial design and conduct, phase I trials are increasingly incorporating a preliminary evaluation of efficacy (458)Adashek JJ, et al. (2019) Nat Rev Clin Oncol, 16: 773.. Thanks to extraordinary advances in our understanding of cancer biology, patient responses to investigational therapies in phase I studies have also nearly doubled over the past two decades (459)Kingwell K (2022) Nat Rev Drug Discov, 21: 702..
Phase II studies are designed to determine the initial efficacy of investigational therapy, in addition to continually monitoring for potential toxicities. Phase III studies are large trials designed to determine therapeutic efficacy as compared to standard of care; when successful, the results of these trials have traditionally been used by FDA to approve new therapeutics or new indications for existing therapeutics. Phase IV studies are conducted after a therapy is approved by FDA and provide additional effectiveness or “real-world” data on the therapy. Sometimes phase 0 clinical studies are performed prior to traditional clinical trials wherein low doses of potential therapeutics are administered to a small number of patients to determine whether such treatments may have the desired effect.
The traditional, cancer-specific, multiphase clinical trial process requires large numbers of patients and takes many years to complete. However, this approach is not well suited to the current molecular era—in which the genetic alterations that drive cancer are being identified with greater frequency, and therapies designed to target those changes are actively being evaluated in clinical trials. Identifying and implementing more efficient clinical development strategies are areas of extensive investigation.
Innovations in Cancer Clinical Trials
Technological innovations in genomics, as well as in fields such as epigenomics, transcriptomics, proteomics, metabolomics, immunomics, and microbiome research, are giving researchers a more complete understanding of what drives tumor growth and the molecular changes that should be prioritized as targets for new therapeutics (see Cancer Development: Integrating Knowledge to Advance Precision Medicine). This growing knowledge has revealed the heterogeneous cellular and molecular landscape of advanced and metastatic cancers. Characterizing tumors at the cellular and molecular levels is also helping clinical researchers identify subsets of patients who are more likely to respond to investigational new therapies.
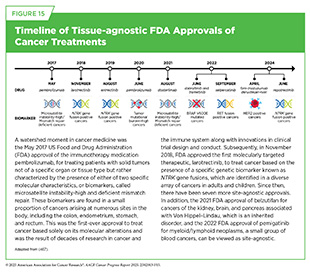
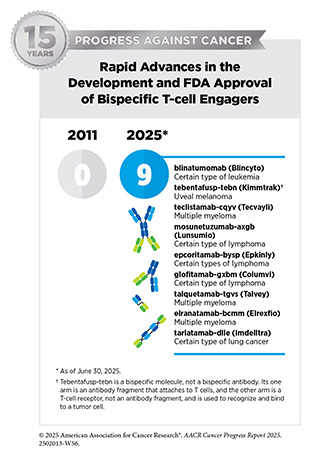
Consequently, over the past decade, clinical cancer research has undergone significant evolution from traditional drug-centric, tissue-specific trials to tissue-specific, genomically driven trials to most recently biomarker-driven clinical trials based on specific genetic or molecular features of a cancer, regardless of where in the body the cancer started (see Sidebar 28). Biomarker-driven clinical studies, which evaluate therapies in patients regardless of the site of their cancer’s origin, are more often being used to support tissue-agnostic regulatory approvals for all tumors that have the targeted biomarker. As of June 30, 2025, nine therapeutics have received tissue-agnostic approvals from FDA for treating patients with solid tumors that have specific genetic alterations (see Figure 15).
According to a recent analysis of nearly 300,000 tumor samples, more than 20 percent of tumors harbored at least one molecular alteration that is associated with tissue-agnostic treatment approval. Unfortunately, electronic health records of clinical outcomes indicated a poor uptake of treatments for tissue-agnostic indications; for example, less than 40 percent of patients with NTRK fusions received a corresponding targeted treatment (461)Sledge GW, Jr., et al. (2025) Nat Commun, 16: 2646..
Researchers continue to work on refining and reshaping aspects of clinical research to make trials more patient-focused (462)Nikanjam M, et al. (2025) CA Cancer J Clin, 75: 243.. There is an urgent call for a paradigm shift from traditional trial designs, in which patients are identified to fit an investigational therapy, to more patient-centric approaches, in which novel therapeutics are identified for each patient based on the unique features of their cancers (468)Marret G, et al. (2025) Cancer Cell, 43: 597.. By harnessing novel technologies, such as advanced DNA and RNA sequencing, researchers aim to develop patient-centric clinical trial designs that deliver therapies targeting genetic alterations, regardless of cancer site, offering novel treatment options for patients with shared molecular features, including those with rare cancers (469)Subbiah V, et al. (2025) Am Soc Clin Oncol Educ Book, 45: e100051..
A key feature of patient-centric trials is the ongoing monitoring of tumor characteristics throughout clinical care, rather than relying on a single assessment prior to the start of the study, as has been traditionally done. Using novel methodologies, such as liquid biopsy (see Envisioning the Future of Cancer Science and Medicine) and digital imaging, researchers are now able to identify dynamic changes in the cancer cells, the tumor microenvironment, and the patient’s immune system that reflect their cancer’s evolution (see Understanding the Path to Cancer Development).
Collecting such multidimensional data over time enables researchers to adapt therapeutic interventions, treatment dosages, or other aspects of the clinical trial protocol to more effectively manage tumor evolution and emerging resistance mechanisms. For example, researchers may decide, based on emerging molecular alterations, that combination therapy targeting multiple cancer drivers may be more effective.

In looking to the future, the design and conduct of clinical cancer research needs to keep pace with the new wave of scientific and technological advances. For example, precision medicine clinical trials must consider novel dosing strategies, optimal timing of therapeutic applications, and novel data collection methods.
Traditionally, clinical trials have focused on identifying the maximum tolerated dose of a treatment. This strategy was suited for older cytotoxic chemotherapeutics but is potentially harmful when applied to molecularly targeted therapeutics and immunotherapeutics, since higher doses may increase toxicity without improving efficacy and can negatively affect patients’ quality of life. In response, FDA has launched an evidenced-based initiative, Project Optimus, to reform dose optimization (see Applying Regulatory Science to Ensure Safe and Effective Cancer Therapies) (470)US Food and Drug Administration. Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases. Accessed: June 30, 2025..
While many precision therapeutics are evaluated in late-stage cancer, once standard treatments have failed, there is increasing evidence that supports applying these therapies earlier, even as the first line of treatment after diagnosis to achieve maximal benefit for patients.
Another shortcoming that has restricted the scope of current clinical research is underutilization of the rapidly accumulating vast amounts of nonclinical and clinical data generated through research and clinical practice. Leveraging existing knowledge from publicly available, open-access repositories such as chemical and drug databases, published literature, patient registries with clinical or genomic data, real-world data, electronic health records, past clinical trial data, patient reported outcomes, and insights from patient advocacy communities is pivotal to driving meaningful progress and transforming the future of cancer clinical trials (468)Marret G, et al. (2025) Cancer Cell, 43: 597..
Harnessing artificial intelligence (AI) and machine learning trained on diverse datasets can further improve clinical research. AI tools are being used in clinical research to identify patients eligible for trials; predict which patients are most likely to benefit from experimental treatments; simulate how new therapeutics work; create “digital twins” or virtual control groups using past patient data—allowing more trial participants to receive the new investigational therapy; and assess how well trial results apply to real-world patient populations (see Envisioning the Future of Cancer Science and Medicine) (471)Orcutt X, et al. (2025) Nat Med, 31: 457.(472)Gueguen L, et al. (2025) NPJ Precis Oncol, 9: 28.(473)Bickell NA, et al. (2024) JAMIA Open, 7: ooae131.(474)Katsoulakis E, et al. (2024) NPJ Digit Med, 7: 77.. However, there are limitations to the current use of AI, including lack of data diversity and proper regulatory oversight, that must be overcome before these tools can become part of regular clinical practice.
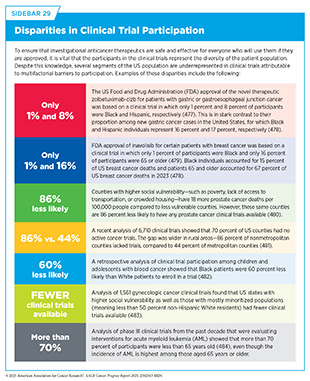
Low participation rate and a lack of sociodemographic diversity among those who do participate are two of the most pressing challenges in cancer clinical trials (see Sidebar 29). Based on recent estimates, only 7 percent of cancer patients nationwide participate in treatment trials (475)Unger JM, et al. (2024) J Clin Oncol, 42: 2139.. Low participation in clinical trials means that many trials fail to enroll enough patients to draw meaningful conclusions about the effectiveness of the investigational therapeutic. Lack of diversity in clinical studies means that the trial participant population does not match the actual national demographics of the cancer burden under study (476)National Academies of Sciences, Engineering, and Medicine. Improving Representation in Clinical Trials and Research: Building Research Equity for Women and Underrepresented Groups. Accessed: June 25, 2025.. Diversity of participants is critical because the efficacy and safety of an intervention may differ among populations, for example, among different ancestral groups or between men and women. Underrepresentation in clinical trials compromises the generalizability of the trial findings to the real-world patient population.
Understanding and eliminating barriers to clinical trial participation is vital if we are to accelerate the pace of progress against cancer for all patients. Numerous studies have investigated the existing barriers that limit participation of racial and ethnic minority groups and other medically underserved populations in cancer clinical trials. These studies have identified a range of factors, such as lack of awareness of clinical trials, financial challenges, limited health literacy, inadequate or complete lack of insurance, medical distrust, implicit biases among health care providers, lack of trial availability, and narrow eligibility criteria, among others (485)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2022. Accessed: June 30, 2025.. These barriers operate at the individual, systemic, and societal levels (486)Kahn JM, et al. (2022) Cancer, 128: 216..
As discussed in detail in AACR Cancer Disparities Progress Report 2024 (12)American Association for Cancer Research®. AACR Cancer Disparities Progress Report 2024. Accessed: June 14, 2025., increased knowledge of the barriers to clinical trial accrual is helping researchers, regulators, and policymakers design and implement evidence-based adaptations that can improve access to clinical research. Interventions aimed at addressing social drivers of health (see Figure 3), modifying trial design to ease patient participation, expanding eligibility criteria, improving the efficiency of data collection, including patient reported outcomes, and engaging in community outreach and patient navigation are being evaluated. Researchers are also leveraging AI to increase clinical trial participation and diversity by improving patient matching, identifying underserved populations, and streamlining recruitment processes. Additionally, a critical area of focus for all stakeholders in medical research is to ensure that the clinical research workforce is representative of the patient population it serves.
US lawmakers and FDA have been working on legislation and guidelines intended to increase the diversity of clinical trial participants (12)American Association for Cancer Research®. AACR Cancer Disparities Progress Report 2024. Accessed: June 14, 2025.. These include a diversity action plan that would require researchers and funders of clinical trials to submit concrete goals and needed steps for enrolling specific demographic groups in pivotal studies that are used by FDA to make regulatory decisions on new drugs (489)US Food and Drug Administration. Diversity Action Plans to Improve Enrollment of Participants from Underrepresented Populations in Clinical Studies. Accessed:. However, recent actions from the Administration are jeopardizing progress in this critical area of cancer medicine. Reductions in FDA staffing and funding, along with the elimination of clinical trial diversity initiatives, threaten to delay innovations and treatments that are essential not only for advancing care but also for ensuring all patients have equitable access to lifesaving therapies.
COVID-19, despite its adverse effects on all aspects of cancer research and patient care, enabled researchers to decentralize certain aspects of clinical trials so that lifesaving therapeutics could be brought quickly to as many patients as possible (490)American Association for Cancer Research. AACR Report on the Impact of COVID-19 on Cancer Research and Patient Care. Accessed: June 30, 2025.. Adaptations implemented during the pandemic, including consenting patients remotely, permitting telehealth for routine clinical assessments, delivering experimental drugs to patients, and allowing the use of local laboratory, imaging facilities, or community health centers accessible to patients, offered a blueprint to further reform clinical trials for the benefit of patients (see Increasing Access to and Decentralizing Trials). It is, therefore, not surprising that the number of decentralized trials rose sharply beginning in 2020, following only modest growth from 1998 to 2019 (491)Aiyegbusi OL, et al. (2024) Nat Med, 30: 3075..
Emerging data indicate that patient-centric approaches can improve outcomes. As an example, a supportive care clinical trial that involved partnerships between leading academic centers and community health centers reduced early deaths in patients with acute promyelocytic leukemia (APL), from an expected 30 percent to just 3 percent, across all care settings (492)Jillella AP, et al. (2025) JAMA Oncol, 11: 400.. Patients at community centers were treated locally using a consensus treatment plan developed through collaboration between the community oncologist and one of seven APL experts available from six lead academic centers.
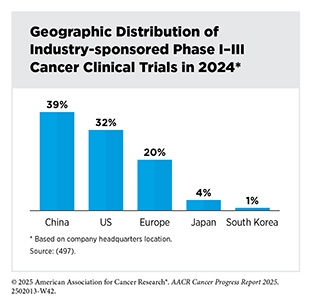
Ongoing research must continue to evaluate the impact of decentralized approaches on advancing clinical research and improving patient outcomes (493)Vanderpool RC, et al. (2024) J Natl Cancer Inst Monogr, 2024: 51.. A decentralized infrastructure may also improve the conduct of clinical trials globally. Unfortunately, despite investments of over $80 billion annually, less than 5 percent of cancer patients globally participate in clinical trials (494)Bloomberg New Economy International Cancer C, et al. (2024) Cancer Discov, 14: 2317.. Low participation is particularly pronounced in low- and middle-income countries (LMICs), where access to trials is limited (495)Izarn F, et al. (2025) ESMO Open, 10: 104086.. In fact, nearly 90 percent of all clinical trials are conducted in high-income regions and countries, including the United States, China, Japan, and Europe (494)Bloomberg New Economy International Cancer C, et al. (2024) Cancer Discov, 14: 2317.. While Africa comprises nearly 15 percent of the world’s population and carries 25 percent of the global burden of the disease, less than 2 percent of all cancer clinical trials are conducted there (494)Bloomberg New Economy International Cancer C, et al. (2024) Cancer Discov, 14: 2317.. In addition, based on a recent analysis of 50,073 oncology trials listed on the ClinicalTrials.gov website between January 2005 and March 2023, only 666 trials (1.3 percent) included study sites in Middle Eastern and North African countries (496)Taha T, et al. (2025) JAMA Oncol, 11: 668..
Expanding global access to cancer clinical trials must become a strategic priority for all stakeholders committed to accelerating breakthroughs in cancer care. Notably, multiregional clinical trials that enroll patients from multiple countries are becoming more common, especially for therapeutics developed by major multinational biopharmaceutical companies. This approach to clinical trials may offer many benefits, such as accelerating therapeutic development, including more diverse populations, and helping ensure new treatments work safely and effectively for people around the world (498)Mehta GU, et al. (2024) N Engl J Med..
Together with diversity action plans to improve representation of different racial, ethnic, age, and sex groups in trials, these global efforts can promote diversity in clinical research (see Increasing Access to and Decentralizing Trials). By carefully planning trial locations, for example, enrolling from countries in South and Central America, the Middle East, and Africa, where many underserved US patients have ancestral origins, researchers can build stronger, more inclusive studies that better represent the populations likely to use the treatments.
In this regard, in September 2024, the World Health Organization released new guidance to improve the design, conduct, and oversight of clinical trials, aiming to strengthen country-led research ecosystems, enhance trial quality and coordination, and ensure faster, more equitable access to safe and effective health interventions worldwide (499)World Health Organization. Guidance for Best Practices for Clinical Trials. Accessed: June 30, 2025..
Progress Across the Clinical Cancer Care Continuum
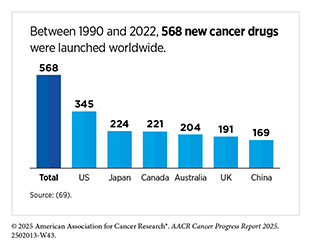
Research discoveries made as a result of innovative cancer science are continually being translated into new medical products for cancer prevention, early detection, diagnosis, and treatment. The United States has significantly benefited from its role as the world’s largest funder of medical research. Recent data show that it leads worldwide in the introduction of new cancer therapies and is most often the first to bring these treatments to the clinic (69)Li M, et al. (2024) BMJ Glob Health, 9..
Approval of new medical products, including new anticancer treatments, is not the end of a linear research process. Rather, it is an integral part of the medical research cycle (see Figure 5) because observations made during the routine use of new medical products can help to accelerate the pace at which similar products are developed and to stimulate the development of new, more effective products.
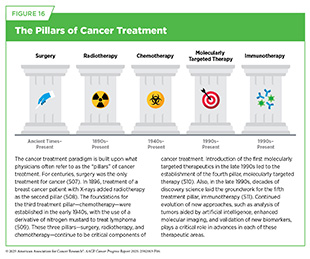
Traditionally, newly approved therapeutics are utilized alongside treatments already in use, including existing surgeries, radiotherapies, and chemotherapies, all of which continue to be the mainstays of clinical cancer care (see Figure 16). In recent years, the rapid rise of molecularly targeted therapies and immunotherapies—the two newest pillars of cancer treatment—has ushered in a new era of personalized cancer medicine. Additionally, researchers are continually evaluating new ways to refine the use of surgery, radiotherapy, and chemotherapeutics to improve survival and quality of life for patients.
Prostate cancer offers a clear example of advances in treatment refinement. Most tumors grow slowly, and the significant morbidity and costs associated with diagnosing and treating these tumors may outweigh the benefit for many patients. Treatments such as surgery can have lasting side effects, and many men with slow-growing prostate cancer may never experience problems during their lifetime. Research has shown that careful observation of disease in patients with nonaggressive prostate cancer (also referred to as watchful waiting or active monitoring) is a safe alternative to receiving immediate surgery or radiotherapy (500)Hamdy FC, et al. (2023) N Engl J Med, 388: 1547.(501)Newcomb LF, et al. (2024) JAMA, 331: 2084.. These findings are hopeful for patients who opt for active monitoring to avoid treatment-related adverse effects, such as sexual and incontinence problems. Recent data also indicate that among patients with nonaggressive prostate cancer the use of active monitoring has been increasing, particularly within certain sociodemographic groups, while surgical interventions are declining (502)Ajjawi I, et al. (2024) JAMA, 332: 2033.(503)Diven MA, et al. (2024) JAMA Netw Open, 7: e2429760.(504)Monda SM, et al. (2025) JAMA Oncol..
Active monitoring has also proven to be a safe approach for some patients with thyroid cancer and those with an early, non-invasive form of breast cancer, called ductal carcinoma in situ (505)Levyn H, et al. (2024) JAMA Otolaryngol Head Neck Surg.(506)Partridge AH, et al. (2025) JAMA Oncol, 11: 300..
The following sections focus on the recent advances across the five pillars of cancer treatment, in particular, the 20 new anticancer therapeutics approved by FDA in the 12 months spanning this report, July 1, 2024, to June 30, 2025 (see Table 6 and Supplementary Table 3). During the same timeframe, FDA expanded the use of eight previously approved anticancer therapeutics and one medical device for treating additional types of cancer. Furthermore, FDA expanded the use of several previously approved therapeutics to include treatment at different timepoints during the course of clinical care or treatment of a different subtype of the same cancer. Comprehensive information on all anticancer therapeutic approvals can be found on FDA’s website (512)US Food & Drug Administration. Oncology (Cancer) /Hematologic Malignancies Approval Notifications. Accessed: June 14, 2025.. Because many of these treatments, particularly molecularly targeted therapeutics and immunotherapeutics, are relatively new to the clinic, their long-term and late effects are still unknown (see Supporting Cancer Patients and Survivors). The fast pace of approval and increasing clinical use of these cutting-edge therapeutics warrant close monitoring of patients receiving such agents.
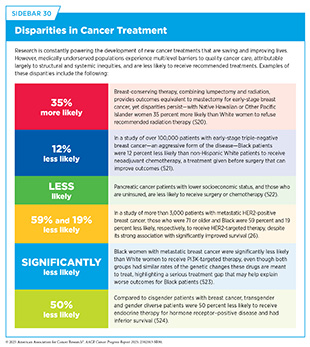
New medical products used across the continuum of clinical cancer care transform lives by extending survival and improving quality of life. However, not all patients in the United States receive the standard of care recommended for the type of cancer with which they have been diagnosed and for the stage of cancer at the time of diagnosis. Disparities in cancer treatment are driven largely by socioeconomic and structural factors, such as lack of health insurance or access to health care facilities, as well as high costs of cancer care (see Sidebar 30). There are also stark differences in the cost and accessibility of cancer treatments around the world, with major disparities between high-income and low-income countries (513)Tfayli AH, et al. (2025) Cancer, 131: e35590.. On the basis of a recent analysis, patients in most high-income countries can access cancer medications without significant out-of-pocket expenditure, while in lower-middle and low-income countries, 40 percent of essential chemotherapeutics are only available at full cost (514)Cherny NI, et al. (2025) Ann Oncol, 36: 247..
Research has shown that in the United States, disparities in survival for several cancer types can be eliminated when all patients have equivalent access to standard treatments (12)American Association for Cancer Research®. AACR Cancer Disparities Progress Report 2024. Accessed: June 14, 2025.. As one example, some studies have found no racial or ethnic disparities in cancer outcomes among patients who are treated at a single-payer system, such as the US Department of Veterans Affairs’ Veterans Health Administration, the nation’s largest integrated health care system (515)Kim RB, et al. (2024) J Racial Ethn Health Disparities, 00: 10.1007/s40615..
Medicaid expansion through the Patient Protection and Affordable Care Act (ACA) has been shown to increase insured status, early diagnosis, access to high-volume hospitals and timely cancer treatment, and reduce cancer disparities, leading to improved outcomes for patients (516)Tamirisa N, et al. (2023) Ann Surg: 00.(517)Hooda Z, et al. (2025) Ann Thorac Surg.(518)Lyons JM, et al. (2025) Cancers (Basel), 17.. Therefore, it is imperative that all stakeholders in public health work together to ensure equitable access to quality cancer treatments.
Educating health care providers about the approval processes for relevant therapeutics is critical if they are to adequately advise patients about the risks and benefits associated with these treatments. Unfortunately, according to a recent national survey of physicians including oncologists, only 41 percent of respondents reported moderate or better understanding of FDA’s drug approval process (519)Dhruva SS, et al. (2024) Health Aff (Millwood), 43: 27..
Advances in Cancer Treatment With Surgery
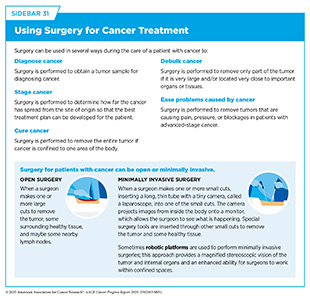
For centuries, surgery was the only pillar of cancer treatment (see Figure 16). Today, it remains the foundation of curative treatment for many patients. Surgery is used in several ways during the care of a patient with cancer (see Sidebar 31).
Sometimes, additional therapy is given before, after, or around the time of surgery based on the specific features of a patient’s tumor (see Sidebar 32). Researchers have found that this approach not only improves the surgeon’s ability to remove the tumor (e.g., by shrinking the tumor when given before the surgery) but also increases the patient’s overall survival and/or quality of life (525)Burotto M, et al. (2019) Semin Oncol, 46: 83.. Researchers are continuously innovating new and improved strategies to maximize the benefits and minimize harm from surgery for cancer patients.
Minimizing the Use of Invasive Cancer Surgery
Several recent studies have shown that performing less invasive surgeries or avoiding surgeries altogether can benefit certain patients by minimizing tissue damage and postprocedural complications while maintaining, and in some cases improving, long-term outcomes (526)Topal H, et al. (2022) JAMA Netw Open, 5: e2248147.(527)Son SY, et al. (2022) JAMA Surg, 157: 879.(528)Di Benedetto F, et al. (2023) JAMA Surg, 158: 46.(529)Bartels SAL, et al. (2023) Journal of Clinical Oncology, 41: 2159.. A few examples of such findings are discussed below.
The main purpose of neoadjuvant therapy is to shrink the tumor and reduce lymph node involvement before surgery, making it possible to perform a less extensive surgery. Based on a report, the use of neoadjuvant therapy has significantly increased since 2010, particularly for breast, pancreatic, and bladder cancers (530)Habermann EB, et al. (2025) J Am Coll Surg, 240: 95..
Emerging data suggest that certain patients who have achieved a complete response, that is, those who have no detectable cancer, after neoadjuvant treatments (see Sidebar 32), can skip surgery without compromising outcomes. As one example, a recent study conducted in the Netherlands suggests that some patients with locally advanced esophageal cancer may safely avoid surgery if they respond well to neoadjuvant chemotherapy and radiotherapy (531)van der Wilk BJ, et al. (2025) Lancet Oncol, 26: 425.. In the clinical trial, patients who showed no signs of cancer after neoadjuvant treatment were either closely monitored or underwent standard surgery. The study found that survival outcomes were similar in both groups, indicating that active monitoring may be a safe alternative to immediate surgery for selected patients.
Another new clinical trial showed that some women with certain types of invasive breast cancer may be able to safely avoid surgery after responding well to neoadjuvant systemic therapy (532)Kuerer HM, et al. (2025) JAMA Oncol, 11: 529.. In this trial, women who showed no detectable cancer after neoadjuvant treatment were treated with radiation alone instead of surgery. After 5 years of follow-up, none of these patients had cancer recurrence in the treated breast, and all remained cancer-free.
One area of increasing focus is identifying patient groups who may be able to avoid surgery after showing strong responses to neoadjuvant immunotherapy. Early findings across several cancer types highlight the significant potential of this approach (see Releasing the Brakes on the Immune System) (533)Cercek A, et al. (2025) N Engl J Med, 392: 2297..
Although these results are promising, more research is needed before such approaches can become standard clinical practice. Additionally, there is an urgent need to streamline research efforts, as a recent review of the global landscape of surgical de-escalation trials in breast cancer revealed significant duplication in ongoing studies (534)McCrorie AD, et al. (2025) NPJ Breast Cancer, 11: 32..
Lymph node dissection is another area of cancer surgery in which de-escalation is becoming increasingly common. This procedure involves removing lymph nodes near the tumor to determine whether the cancer has spread beyond the primary site, given the lymphatic system’s role in metastasis (see Circulatory and Immune Systems). Lymph node dissection is routinely performed in patients with solid tumors such as breast, colorectal, gastric, and thyroid cancers.
There are two main types of lymph node dissection: regional, which targets nodes closest to the tumor, and extended (or radical), which involves a wider removal of lymphatic tissue, often across multiple nodal regions. While regional dissection helps with accurate staging and treatment planning, extended dissection carries a higher risk of complications.
For example, in breast cancer, axillary lymph node dissection (ALND)—an extended procedure historically performed when cancer was suspected to have spread to underarm lymph nodes—can lead to morbidity such as lymphedema, numbness, shoulder stiffness, and long-term functional limitations. Recent studies suggest that ALND offers no survival benefit over regional or sentinel lymph node biopsy (SLNB) and can be safely omitted in certain breast cancer patients, including those with small tumors and normal lymph nodes on imaging or those who respond well to neoadjuvant chemotherapy (535)Gentilini OD, et al. (2023) JAMA Oncol, 9: 1557.(536)Montagna G, et al. (2024) JAMA Oncol..
A clinical study in patients with bladder cancer has shown similar results (537)Lerner SP, et al. (2024) N Engl J Med, 391: 1206.. Some patients with muscle-invasive bladder cancer may have cancer cells in the lymph nodes near the bladder at the time of diagnosis. Despite limited research comparing local lymph node dissections to extended lymph node dissection, the more extensive surgery has become the standard of care. The study, which included nearly 600 patients with muscle-invasive bladder cancer, found that extended lymph node removal during bladder surgery did not improve survival compared to limited node surgery but was linked to a higher risk of serious side effects and death within 90 days (537)Lerner SP, et al. (2024) N Engl J Med, 391: 1206..
Researchers are also evaluating whether less invasive procedures including robotic surgeries could replace traditional surgery for certain patients. For example, tumor ablation is a minimally invasive treatment that destroys tumors in organs like the liver, kidney, bone, or lung using extreme heat, extreme cold, or other energy-based methods that damage cancer cells. In a recent study that compared thermal ablation to surgical removal for patients with small colorectal tumors that had spread to the liver found that patients who received thermal ablation had similar survival rates compared to those who had surgery (538)van der Lei S, et al. (2025) Lancet Oncol, 26: 187.. Importantly, thermal ablation caused far fewer side effects and serious complications. Nonsurgical management with radiation-based ablative therapy was also shown to be an alternative option for localized pancreatic cancers, especially among patients for whom surgery is risky or could greatly worsen quality of life (539)Reyngold M, et al. (2025) JAMA Oncol, 11: 609.. Additionally, robotic surgery may offer important advantages for certain patients, for example, those with middle or low rectal cancer, as it was associated with fewer cancer recurrences and better outcomes after 3 years compared to laparoscopic surgery (540)Feng QY, et al. (2025) Jama-Journal of the American Medical Association..
Advances in Radiation-based Approaches to Cancer Care
Radiotherapy is the use of high-energy rays (e.g., gamma rays and X-rays) or particles (e.g., electrons, protons, and carbon nuclei) to control or eradicate cancer. Discovery of X-rays in 1895 allowed visualization of internal organs at low doses, and the effective use of X-rays at high doses to treat a breast cancer patient a year later established radiotherapy as the second pillar of cancer treatment (see Figure 16). Radiotherapy plays a central role in the management of cancer and works primarily by damaging DNA, leading to cancer cell death.
Globally, 50 percent of all patients with a new diagnosis of cancer need radiotherapy as their initial treatment, and 15 percent require follow-up radiotherapy (542)Abdel-Wahab M, et al. (2024) Lancet Oncol, 25: e545.. Unfortunately, there are significant disparities in the access to radiotherapy. According to a recent report, sub-Saharan Africa has the fewest radiotherapy machines per patient—with around 20 countries lacking any—while North America has the highest availability (542)Abdel-Wahab M, et al. (2024) Lancet Oncol, 25: e545.. In high-income countries, there is one radiotherapy machine for every 130,600 people, compared to just one for every 15.6 million people in low-income countries (542)Abdel-Wahab M, et al. (2024) Lancet Oncol, 25: e545..
There are many types of and uses for radiotherapy (see Sidebar 33). However, it is important to note that radiotherapy may also have harmful side effects, partly because of the radiation-induced damage to healthy cells surrounding the tumor tissue (543)Wang K, et al. (2021) CA Cancer J Clin, 71: 437.. Because of the central role of radiotherapy in the treatment and management of cancer, researchers are continually innovating radiotherapeutic approaches to maximize the benefits for patients while minimizing potential harms.
Long-term effects of radiation therapy can negatively impact a patient’s quality of life. Researchers are evaluating approaches to make radiotherapy safer and more effective, including using biomarkers to identify patients who are unlikely to benefit from radiation or may be more vulnerable to its toxic effects, allowing radiotherapy to be reduced or even avoided without affecting patient outcomes (544)Kishan AU, et al. (2025) Clin Cancer Res, 31: 2530.(545)Meattini I, et al. (2025) JAMA Oncol, 11: 329..
As one example, a major clinical trial led by the Children’s Oncology Group found that some patients with Wilms tumor, the most common type of kidney cancer in children, can safely skip radiation therapy, helping to reduce its long-term adverse effects (546)Dix DB, et al. (2018) J Clin Oncol, 36: 1564.. Traditionally, the treatment for patients with stage IV Wilms tumor that has spread to the lungs has been chemotherapy and surgery, followed by radiation therapy to the lungs. Data from the trial suggest that nearly half of children with advanced Wilms tumor can avoid lung radiation if they respond well to initial chemotherapy (546)Dix DB, et al. (2018) J Clin Oncol, 36: 1564.. Children whose lung nodules disappeared after 6 weeks of standard chemotherapy continued treatment without radiation and had a 4-year survival rate of over 96 percent, which was similar to the rate in those who received radiation. Omission of radiation may help reduce serious long-term side effects, such as heart and lung damage or future cancers (see Sidebar 48). The new findings mark an important step toward customizing treatment to preserve survival while maintaining quality of life.
For women with early-stage breast cancer, surgery followed by radiotherapy has been the standard treatment since it was believed that eradicating any remaining breast cancer cells with radiation after removal of the tumor would improve long-term outcomes. However, several studies have now shown that patients with early-stage breast cancer that are characterized as very low-risk based on certain molecular characteristics, can forgo radiation therapy after surgery without any excess risk of cancer recurrence, as long as they receive guideline-adherent treatment with hormone therapies (547)Jagsi R, et al. (2024) J Clin Oncol, 42: 390.(548)Whelan TJ, et al. (2023) N Engl J Med, 389: 612.(549)Mann GB, et al. (2024) Lancet, 403: 261.. In addition, a 30-year follow-up study found that radiotherapy after breast-conserving surgery did not improve overall survival for some patients with early-stage breast cancer, suggesting that radiation may be safely omitted in certain patients (550)Williams LJ, et al. (2024) Lancet Oncol, 25: 1213..
Researchers are also evaluating more sophisticated radiotherapy approaches that are safer and more effective. As one example, a recent study that compared intensity-modulated radiation therapy (IMRT) and three-dimensional conformal radiation therapy (3D-CRT) (see Sidebar 33) in non–small cell lung cancer (NSCLC) found IMRT was safer for patients (551)Chun SG, et al. (2024) JAMA Oncol, 10: 1111.. Over 5 years of follow-up, both treatment groups had similar survival rates and risk of developing new cancers. However, IMRT exposed the heart to less radiation than 3D-CRT and caused fewer serious cases of lung inflammation.
Stereotactic body radiotherapy (SBRT) is an advanced approach to radiotherapy that can target radiation to tumors more precisely than traditional radiotherapy. Higher doses and fewer sessions of radiation can be used compared with traditional radiotherapy, and healthy tissues surrounding a tumor are spared from damage caused by the radiation, which can reduce the long-term adverse effects. Given the potential benefits of SBRT, many clinical trials are testing ways to incorporate these treatments into clinical care. As one example, SBRT was shown to be a safe, convenient, and effective treatment option for certain patients with prostate cancer, and allowed men to complete their treatment in a much shorter time—five sessions compared to a minimum of 20 that is used traditionally—without compromising outcomes (552)van As N, et al. (2024) N Engl J Med, 391: 1413..
Historically, the main use of radiotherapy in the treatment of patients with metastatic cancer has been to reduce or control symptoms of disease. However, recent studies have shown that radiotherapy targeted to the initial cancer site from which tumors have metastasized can improve survival for patients who have metastatic tumors at a limited number of sites, referred to as oligometastatic tumors (553)van Moorselaar RJA, et al. (2022) Eur Urol Open Sci, 35: 70.. Moreover, studies have shown that stereotactic radiotherapy targeted to oligometastatic tumors can reduce the chances of disease progression and increase survival for patients who have solid tumors, such as prostate, lung, or gynecologic cancers (554)Palma DA, et al. (2020) J Clin Oncol, 38: 2830.(555)Donovan EK, et al. (2024) JAMA Oncol, 00: e241796.(556)Chinniah S, et al. (2022) Int J Radiat Oncol Biol Phys, 114: 684.(557)Mansouri A, et al. (2025) Nat Rev Clin Oncol, 22: 327..
Another recent advance in radiotherapy is the emergence of hypofractionated radiotherapy, whereby patients receive fewer but higher doses of radiotherapy compared to the traditional regimen (558)Cho WK, et al. (2024) JAMA Oncol, 10: 737.. Patients who receive hypofractionated radiotherapy complete their radiation over a shorter period and in fewer treatment sessions. In a recent review, researchers indicate that this approach is just as effective as traditional, longer radiation courses for treating breast cancer, while also offering benefits like fewer side effects, better cosmetic results, improved quality of life, and greater convenience for patients (559)Lee SF, et al. (2024) BMJ, 386: e079089.. Despite established benefits for patients, hypofractionated radiotherapy is underused and there are disparities in uptake in the United States (560)Booth S, et al. (2024) Pract Radiat Oncol, 14: e305..
Applying Precision to Radiation Therapy
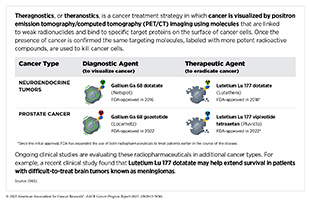
One of the most exciting and fastest growing areas in radiotherapy is the use of radiopharmaceuticals or molecularly targeted radiotherapeutics—radiation-emitting molecules that are linked to targeting molecules, which steer the radiation specifically to cancer cells. A particularly promising innovation is theranostics, which combines diagnostic imaging and molecularly targeted radiotherapy to deliver personalized treatment based on a patient’s unique tumor characteristics. A number of such diagnostic therapeutic pairs have been approved by FDA in recent years for the management of certain patients with neuroendocrine tumors and prostate cancer (177)American Association for Cancer Research. AACR Cancer Progress Report 2023. Accessed: Feb 29, 2025.(372)American Association for Cancer Research. AACR Cancer Progress Report 2021. Accessed: June 30, 2025.(561)American Association for Cancer Research. AACR Cancer Progress Report 2018. Accessed: June 30, 2025., and many more are at various stage of preclinical and clinical testing (see Radiopharmaceutical Therapy) (1)American Association for Cancer Research®. AACR Cancer Progress Report 2024. Accessed: June 11, 2025.(562)Giugliano F, et al. (2025) Cancer Treat Rev, 136: 102940.(563)Davis RA, et al. (2023) J Med Chem, 66: 9842.(564)Ganguly T, et al. (2023) J Nucl Med, 64: 639..
Molecularly targeted radiotherapeutics hold great promise for transforming cancer treatment, although challenges such as regulatory hurdles and complex manufacturing remain to be addressed. Additionally, access to these treatments has mostly been limited to high-income countries, as challenges like limited funding, shortages in sophisticated facilities for delivery, and insufficient trained medical personnel continue to hinder broader global use (542)Abdel-Wahab M, et al. (2024) Lancet Oncol, 25: e545..
Ongoing research in theranostics aims to improve tumor targeting while minimizing harm to healthy tissues. Scientists are exploring a range of novel radioisotopes that emit different types of radiation, along with various targeting molecules, such as engineered proteins and antibody derivatives, to enhance radiotherapeutic delivery and reduce toxicity. A recent analysis found that more than 60 novel radiotherapeutics, addressing over 25 unique targets across more than 20 solid and blood cancer types, are currently in various stages of clinical development (566)To J, et al. (2025) Nat Rev Drug Discov..
Advances in Treatment With Chemotherapy
Chemotherapy, which involves the use of chemicals to kill cancer cells, was first introduced as a pillar of cancer treatment in the early to mid-20th century (509)DeVita VT, Jr., et al. (2008) Cancer Res, 68: 8643.. Chemotherapy remains a backbone of cancer treatment, and its use is continually evolving to minimize potential harm to patients, while maximizing its benefits.
As with surgery and radiotherapy, chemotherapy is more commonly used to treat cancer in combination with one or more additional types of treatments. Newer and more effective chemotherapeutics continue to be evaluated in clinical research. In addition, researchers are continually evaluating the optimal dosage, novel formulations, treatment combinations, and sequence of chemotherapy delivery to improve patient outcomes.
As one example, a large international study that followed women with surgically resected, high-risk, early breast cancer for over 10 years to compare two chemotherapy schedules—one given every 3 weeks and one given every 2 weeks—found that the more frequent chemotherapy helped prevent breast cancer from coming back and improved overall outcome among this patient population (567)Matikas A, et al. (2024) J Clin Oncol, 42: 3077.. Another analysis found that people with pancreatic cancer who received their recommended chemotherapy regimen lived significantly longer than those who received lower than recommended dosage, suggesting that getting the recommended dosage of chemotherapy may improve survival (568)Patel SH, et al. (2025) Cancer, 131: e35759.. Yet another clinical trial conducted in France showed that among patients with advanced leiomyosarcoma, a rare tumor that develops in smooth muscle cells, those who received a combination of two chemotherapeutics, doxorubicin and trabectedin, followed by ongoing trabectedin lived longer than those who received doxorubicin alone (569)Pautier P, et al. (2024) N Engl J Med, 391: 789..
Treatment with chemotherapeutics can have adverse effects. These can occur during treatment and continue in the long term, or they can appear months or even years later (see Addressing the Challenges Faced by Survivors).
Researchers are investigating different approaches to making chemotherapeutics safer for patients. Areas of ongoing investigation include designing modifiable chemotherapeutics, such as those with “on” and “off ” switches, that are selectively delivered to tumors while sparing healthy tissue, as well as evaluating less aggressive chemotherapy regimens that can allow patients the chance of an improved quality of life without compromising survival.
As an example, data from a recent clinical trial from China found that children with high-risk retinoblastoma did just as well with three cycles of chemotherapy as with six, but experienced fewer side effects, better quality of life, and lower treatment costs, supporting a shorter, less intensive treatment approach (570)Ye H, et al. (2024) JAMA, 332: 1634.. Data from another clinical trial from the United Kingdom showed that among patients with early-stage anal cancer, reduced dosages of chemotherapy and radiotherapy showed similar short-term cancer control and fewer side effects compared to the standard-dose treatment, suggesting it may be a safer option while maintaining effectiveness (571)Gilbert A, et al. (2025) Lancet Oncol, 26: 707..
A critical area of ongoing research is identifying biomarkers to correctly predict which patients will or will not benefit from chemotherapy, as well as to predict which patients may suffer from chemotherapy-induced toxicities. Chemotherapeutics such as 5-fluorouracil (5-FU) and capecitabine may cause serious adverse effects. Dihydropyrimidine dehydrogenase (DPD) is a key enzyme that breaks down 5-FU by converting it into an inactive form for elimination from the body. Research has shown that some individuals may carry genetic changes known as DPD deficiency that reduce their ability to safely process this treatment, putting them at higher risk for severe side effects. To improve patient safety, FDA has recently updated drug labels to include information about this genetic risk and now recommends that clinicians consider testing patients for DPD deficiency before starting treatment (see Rapidly Delivering Safe and Effective Therapies to Patients) (572)US Food and Drug Administration. Safety announcement: FDA highlights importance of DPD deficiency discussions with patients prior to capecitabine or 5FU treatment. Accessed: June 25, 2025..
Another particularly exciting area of clinical cancer research is the evaluation of circulating tumor DNA as a biomarker to identify cancer patients who are at a low risk of recurrence and can safely forgo postsurgical chemotherapy (see Liquid Biopsy in Cancer Care) (573)Gottschalk Z, et al. (2024) Curr Oncol Rep, 26: 959.(574)Kasi PM, et al. (2024) Journal of Clinical Oncology, 42: 9..
Advances in Treatment With Molecularly Targeted Therapeutics
Remarkable advances in our understanding of cancer biology, including the discovery of numerous cellular and molecular alterations that drive tumor growth, have ushered in a new era of precision medicine. As a result, the standard of care is shifting away from a one-size-fits-all approach toward treatments tailored to the patient and the unique characteristics of their cancer (see Understanding the Path to Cancer Development).
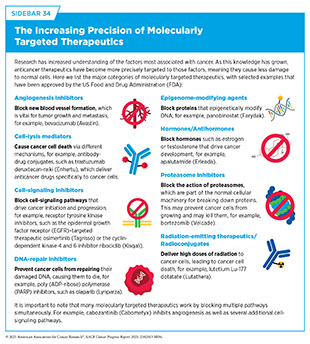
Therapeutics directed to molecules influencing cancer cell multiplication and survival target tumor cells more precisely than cytotoxic chemotherapeutics, which generally target all rapidly dividing cells, and thereby limit damage to healthy tissues. The greater precision of these molecularly targeted therapeutics tends to make them more effective and less toxic than cytotoxic chemotherapeutics (see Sidebar 34). As a result, they are not only saving lives but also allowing patients with cancer to have a higher quality of life.
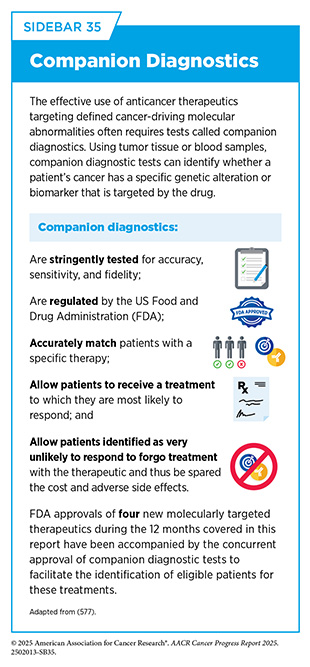
Diagnostic tests, such as sophisticated DNA sequencing or protein visualizing methods, are commonly used in the clinic to identify molecular changes in patient’s cancers (see Sidebar 35), helping to select those who are most likely to benefit from molecularly targeted therapeutics and spare others from unnecessary treatments and side effects. Companion diagnostics are tests that are required for the safe and efficacious use of certain FDA-approved treatments while complementary diagnostics are tests that are not mandatory but provide additional insights on clinical decision-making.
Unfortunately, because of multilevel barriers to health care, including inadequate health insurance and lack of access to quality cancer care, there are disparities in the utilization of diagnostic testing and molecularly targeted treatments (523)Podany EL, et al. (2025) JAMA Netw Open, 8: e2461899.(575)Heath E, et al. (2024) Cancer Res Commun, 4: 2598.. For example, despite overall increases in next-generation sequencing—a cutting-edge diagnostic testing method vital for making molecularly targeted treatment decisions—from 2015 to 2022, rising from 19 percent to 27 percent in metastatic prostate cancer and from 14 percent to 47 percent in advanced urothelial cancer, significant disparities persist. Black and Hispanic patients, individuals with low socioeconomic status, those with Medicaid or government insurance, and people living in certain geographic regions remain consistently less likely to receive this testing (576)Hage Chehade C, et al. (2024) JAMA Netw Open, 7: e2423186..
It is vital that ongoing research and future public health policies are aimed at ensuring equitable access to precision cancer medicine, including tumor genetic testing and the receipt of molecularly targeted therapeutics for all patients. Highlighted in the following sections are the 15 new molecularly targeted anticancer therapeutics that were approved by FDA in the 12 months covered in this report, from July 1, 2024, to June 30, 2025 (see Table 6).
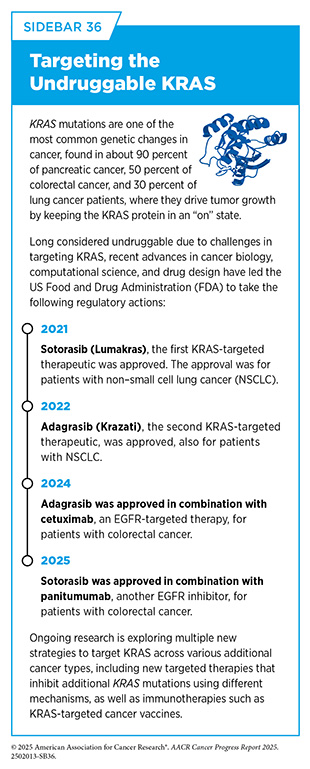
Although not discussed in detail, during the same 12-month period, FDA also expanded the use of four previously approved molecularly targeted therapeutics for treating new cancer types (see Table 6). As an example, in January 2025, FDA approved the KRAS-targeted therapeutic sotorasib (Lumakras) in combination with the EGFR-targeted therapy, panitumumab (Vectibix), for adults with metastatic colorectal cancer that has a mutation known as KRAS G12C (see Sidebar 36). Sotorasib was previously approved for certain patients with NSCLC (372)American Association for Cancer Research. AACR Cancer Progress Report 2021. Accessed: June 30, 2025..
Advancing Targeted Approaches in Breast Cancer Care
Thanks to major advances in early detection and treatment, breast cancer death rates have declined significantly in the United States. However, breast cancer still ranks as the second leading cause of cancer death in US women (6)American Cancer Society. Cancer Facts and Figures. Accessed: June 13, 2025.. Furthermore, rates of invasive breast cancer have been steadily rising since the mid-2000s, with women younger than 50 experiencing the fastest increase, attributable in part to changes in lifestyle and/ or hormonal risk factors (6)American Cancer Society. Cancer Facts and Figures. Accessed: June 13, 2025.. Therefore, continued innovation and investments in newer and more effective treatments are needed to address the evolving burden of the disease.
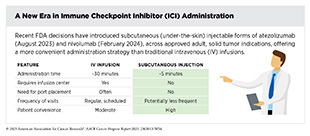
Two recent FDA decisions have the potential to further accelerate progress against breast cancer because they have provided new molecularly targeted treatment options for certain patients.
For patients with breast cancer, treatment decisions are often guided by the presence or absence of three key biomarkers—estrogen and progesterone hormone receptors (HR), and the HER2 protein—all of which can drive tumor growth. About 70 percent of breast cancers diagnosed in the United States are characterized as HR-positive/HER2-negative (4)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: June 31, 2025.. Treatment options for these patients include antihormone therapeutics, such as tamoxifen (prevents estrogen from attaching to its receptor), letrozole (lowers estrogen levels), or fulvestrant (destroys estrogen receptor), with or without another molecularly targeted therapy known as a cyclin-dependent kinase 4/6 inhibitor. Antihormone therapeutics, also known as endocrine therapy, work by reducing the hormonal signals that drive cancer growth and survival.
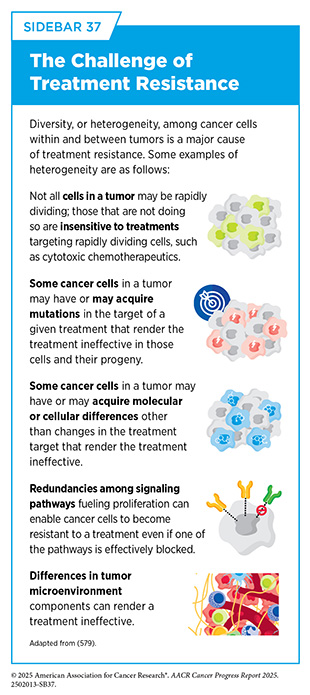
Unfortunately, most advanced HR-positive breast cancers that initially respond to endocrine therapy eventually progress because they have become treatment resistant (see Sidebar 37). Identifying novel targets for the development of new and effective therapies for this patient population is an area of active investigation.
In January 2025, FDA approved a new treatment called datopotamab deruxtecan-dlnk (Datroway) for adults with advanced HR-positive/HER2-negative breast cancer that cannot be removed with surgery or that has spread to other parts of the body. The approval was for patients whose disease has progressed despite previous treatments with antihormone therapy and chemotherapy (see Delivering Cytotoxic Agents Precisely to Cancer Cells).
Research has shown that a signaling pathway involving the protein PI3K-alpha and its partners is vital for normal cell multiplication and survival and is overactivated in approximately half of HR-positive/HER2-negative breast cancers (580)Turner NC, et al. (2023) N Engl J Med, 388: 2058.. PI3K-alpha is a key component of the pathway and activating mutations in the PIK3CA gene, which provides the code that cells use to make the PI3K-alpha protein, are observed in 35 percent to 40 percent of HR-positive breast cancers (581)Turner NC, et al. (2024) N Engl J Med, 391: 1584.. Overactivation of PI3K signaling in breast cancer has also been implicated in the development of resistance to endocrine therapy. A molecularly targeted therapeutic, alpelisib (Piqray), which blocks the function of PI3K-alpha protein, was approved for patients with breast cancer in 2019.
In October 2024, FDA approved a second PI3K-alpha inhibitor, inavolisib (Itovebi), in combination with a cyclin-dependent kinase 4/6 inhibitor, palbociclib, and the endocrine therapy, fulvestrant, for adults with HR-positive/ HER2-negative locally advanced or metastatic breast cancer that tests positive for PIK3CA mutation and has progressed during or after endocrine therapy. FDA also approved the FoundationOne Liquid CDx assay as a companion diagnostic test (see Sidebar 35) to identify patients with breast cancer who are eligible for treatment with inavolisib.
Unlike alpelisib, inavolisib not only blocks PI3K-alpha function but also causes degradation of the protein. Researchers believe that this novel approach may improve the effectiveness of the treatment compared to alpelisib (582)Mullard A (2024) Nat Rev Drug Discov, 23: 885.. FDA approval was based on results from a phase III clinical trial in which inavolisib combined with palbociclib and fulvestrant more than doubled the time patients lived without their cancer progressing compared to standard treatment (15.0 vs. 7.3 months) (581)Turner NC, et al. (2024) N Engl J Med, 391: 1584..
Matching Therapies to the Molecular Drivers of Lung Cancer
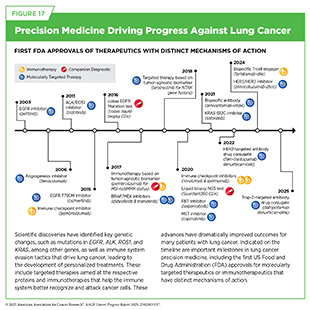
Lung cancer is the leading cause of cancer deaths in the United States. An estimated 226,650 new cases will be diagnosed, and 124,730 people will die from the disease in the United States this year alone (see Table 1). More than 80 percent of lung cancers diagnosed in the United States are classified as NSCLC. Research over the past decade has significantly increased our understanding of the genetic drivers of lung cancer, which has led to the development of precision medicine directed at many of these alterations (see Figure 17). Thanks to these major advances in treatment, as well as improved prevention and earlier detection, the decline in lung cancer death rates has accelerated in the past decade. Between 2013 and 2022, lung cancer mortality rates fell by an average of 4.8 percent per year in men and 3.7 percent per year in women (6)American Cancer Society. Cancer Facts and Figures. Accessed: June 13, 2025..
Dysregulation of the MET receptor protein plays a critical role in NSCLC by activating key processes including cell multiplication, survival, migration, and invasion. The MET receptor’s interaction with its binding partner, hepatocyte growth factor, is vital for epithelial-to-mesenchymal transition (EMT), a hallmark of cancer development. In May 2024, FDA approved a MET-targeted therapeutic telisotuzumab vedotin-tllv (Emrelis) for adults with locally advanced or metastatic NSCLC with high MET overexpression who have received prior systemic therapy (see Delivering Cytotoxic Agents Precisely to Cancer Cells).
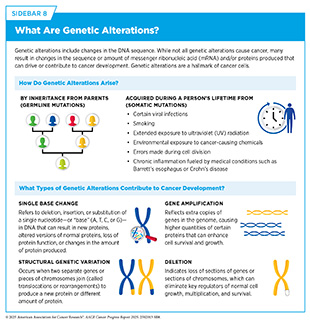
Alterations in the EGFR gene are found in 15 percent to 50 percent of advanced NSCLC cases (583)Cho BC, et al. (2024) N Engl J Med, 391: 1486.. The most common EGFR mutations are deletions called exon 19 deletions and single base changes known as L858R substitutions (see Sidebar 8). These mutations drive NSCLC growth and can be targeted with anti-EGFR therapeutics. However, most patients eventually become resistant to such treatments. In many cases, this resistance is linked to new mutations in EGFR, such as the single base change called T790M, or to activation of the MET pathway, though in many cases, the cause of resistance is still unknown.
Currently, the standard treatment for NSCLC patients with EGFR mutations is osimertinib (Tagrisso), an EGFR-targeted therapeutic, which specifically inhibits cancer-driving mutant forms of EGFR, including that produced by the EGFR T790M mutation, and has been shown to delay cancer growth better than earlier versions of EGFR-targeted drugs. Unfortunately, resistance to osimertinib arises in nearly all patients. Researchers are evaluating new therapeutics, alone or in combination, to address this challenge.
One such therapeutic is amivantamab-vmjw, which was approved by FDA in 2021 (372)American Association for Cancer Research. AACR Cancer Progress Report 2021. Accessed: June 30, 2025.. Amivantamab-vmjw binds to both EGFR and MET on the surface of lung cancer cells and blocks the cancer-driving signals triggered by these proteins while also engaging the immune system to attack cancer cells. It has shown promise when used alone or with chemotherapy, especially in patients who have stopped responding to osimertinib. In August 2024, FDA approved a new EGFR-targeted therapeutic, lazertinib (Lazcluze), in combination with amivantamab-vmjw for the initial treatment of locally advanced or metastatic NSCLC with EGFR exon 19 deletions or L858R substitutions, as detected by an FDA-approved test.
Lazertinib is a highly selective EGFR-targeted therapeutic that can block mutant forms of EGFR including EGFR T790M. In addition, unlike most EGFR-targeted treatments, lazertinib can reach the brain and may be effective against lung cancer that has metastasized to the brain. Researchers hypothesized that the combination of amivantamab-vmjw with lazertinib would prevent the development of treatment resistance (583)Cho BC, et al. (2024) N Engl J Med, 391: 1486..
FDA approval was based on the results of a phase III clinical trial that compared the effectiveness of amivantamab-vmjw plus lazertinib to osimertinib and showed that the combination therapy reduced the risk of disease progression or death by 30 percent compared to osimertinib. NSCLC patients receiving the new combination lived nearly 7 months longer without their cancer worsening (24 months vs. 17 months) (583)Cho BC, et al. (2024) N Engl J Med, 391: 1486.. Additional research is needed to identify effective strategies to manage and reduce the toxicities associated with the combination, and to evaluate whether the combination therapy can improve overall survival as well as its effectiveness in high-risk NSCLC patients, such as those with detectable circulating tumor DNA during treatment, coexisting TP53 mutations, and brain or liver metastases.
Research has shown that ALK gene mutations fuel 3 percent to 11 percent of NSCLC cases (584)Horn L, et al. (2021) JAMA Oncol, 7: 1617.. This knowledge has led to the development of several anticancer therapeutics targeting ALK. Crizotinib was the first of these to be approved by FDA, in August 2011, and became the standard initial treatment for patients with metastatic ALK-positive NSCLC. Unfortunately, not all patients with NSCLC driven by ALK have tumor shrinkage after crizotinib treatment. Moreover, the majority of patients whose cancer initially responds eventually experience relapse because the cancer becomes resistant to the agent. In many cases, crizotinib resistance emerges because NSCLC cells acquire additional ALK mutations.
These discoveries have led to the development of a new generation of ALK-targeted treatments, many of which are able to block the unique forms of ALK that result from these new mutations. In the past decade, FDA has approved four additional ALK-targeted therapeutics—ceritinib, alectinib, brigatinib, and lorlatinib—to treat ALK-positive NSCLC (585)Peng L, et al. (2022) Front Oncol, 12: 863461.. These newer treatments are more effective than crizotinib, especially at reaching and treating NSCLC that has spread to the brain. Initially used only in patients who stopped responding to crizotinib, these newer drugs have now become the preferred first treatment because they help patients live longer.
In December 2024, FDA approved the sixth ALK-targeted therapeutic, ensartinib (Ensacove) for patients with ALK-positive locally advanced or metastatic NSCLC who have not previously received an ALK-targeted therapy. The approval was based on the results of a phase III clinical trial showing that patients receiving ensartinib lived nearly twice as long without their cancer getting worse as those receiving crizotinib, 26 months versus 13 months. Ensartinib also worked better at treating brain metastases, a common concern in lung cancer. These data have established ensartinib as a potential new option for patients starting treatment for ALK-positive NSCLC.
Identifying which targeted therapy is most appropriate as the initial treatment for ALK-positive NSCLC and the order in which additional FDA-approved ALK-targeted therapeutics should be used to provide the maximum benefit for patients is an area of intensive research. A recent analysis comparing the effectiveness and safety of the six FDA-approved ALK inhibitors found that lorlatinib offers the greatest benefit in delaying disease progression, although it is associated with a higher risk of side effects (585)Peng L, et al. (2022) Front Oncol, 12: 863461.. While some clinicians recommend using lorlatinib first, others prefer starting with alectinib, ceritinib, brigatinib, or ensartinib, which tend to have fewer side effects, and reserving lorlatinib for later treatment if the disease progresses. The overall goal remains to maximize treatment effectiveness while minimizing toxicity and improving long-term outcomes for patients. Additional research is needed to establish a clear consensus on the best sequence of these therapies.
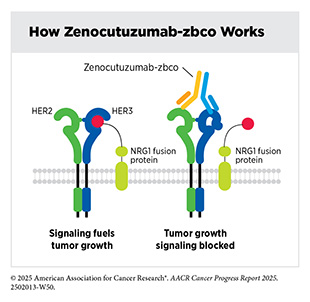
The protein neuregulin 1 (NRG1) plays an important role in the development and maintenance of the nervous system and heart. In less than 1 percent of all solid tumors, structural variations (see Sidebar 8) in the NRG1 gene can lead to the formation of NRG1 fusion proteins that contribute to cancer growth. NRG1 fusion proteins activate growth signals in cancer cells by binding to a cell surface protein called HER3, which then pairs with HER2, another cell surface protein involved in cell growth. The formation of the HER2/HER3 complex sets off a signaling cascade that promotes tumor development. As a result, targeting HER2 and HER3 has emerged as a promising treatment strategy for patients with NRG1 fusion–positive cancers, regardless of where the cancer originates in the body.
In December 2024, FDA approved zenocutuzumab-zbco (Bizengri) for patients with advanced, surgically unremovable, or metastatic NSCLC or pancreatic cancer harboring an NRG1 gene fusion whose disease has progressed despite systemic treatments. Zenocutuzumab-zbco blocks the NRG1 fusion protein from promoting the pairing of HER2 and HER3 proteins, thereby turning off the cancer-promoting signaling cascade initiated by the HER2/HER3 complex. Zenocutuzumab-zbco is the first HER3-targeted therapeutic approved by FDA, bringing a new hope to patients, such as Bob Fortin, who previously had limited options for their difficult-to-treat cancer.
FDA approval was based on the results of a clinical trial in which one-third of patients treated with zenocutuzumab-zbco experienced tumor shrinkage that lasted a median of 11 months (586)Schram AM, et al. (2025) N Engl J Med, 392: 566.. Most patients in the study had either NSCLC or pancreatic cancer, and while zenocutuzumab-zbco shrank tumors in people with different cancers, those with NSCLC and pancreatic cancer were most likely to respond. More than 30 percent of patients with NSCLC and 40 percent of patients with pancreatic cancer experienced tumor shrinkage (586)Schram AM, et al. (2025) N Engl J Med, 392: 566..
The study marks a significant step forward in treating cancers with NRG1 fusions by offering a new molecularly targeted option for patients who typically respond poorly to standard therapies. While the response rate of 30 percent is modest, the drug shows clear benefits, including low toxicity. Ongoing research aims to understand why some patients respond better than others and how resistance develops, with hopes of expanding the use of zenocutuzumab-zbco to more cancer types.
About 1 percent to 3 percent of NSCLCs are fueled by genetic alterations known as chromosomal translocations that involve the ROS1 gene and lead to the production of ROS1 fusion proteins (587)Perol M, et al. (2025) J Clin Oncol, 43: 1920.. These cancers often affect younger people, women, and those who have never smoked. In many patients, the cancer is diagnosed after it has already spread, including to the brain.
Several ROS1-targeted therapeutics have been approved by FDA, including crizotinib, entrectinib, and repotrectinib. However, these treatments have important limitations. Crizotinib is more effective than standard chemotherapy, but almost half of the patients treated develop brain metastases, which lead to poor outcomes. Many patients also become resistant to crizotinib attributable to newly acquired mutations in ROS1 gene, known as G2032R. Entrectinib was designed to be able to reach the brain but showed limited effect in NSCLC patients with brain metastases or those with acquired G2032R mutations. Repotrectinib, approved by FDA in 2023, is more effective against brain metastases and G2032R mutation but may cause serious side effects, especially neurologic problems, such as dizziness and lack of coordination.
Therefore, the approval of the newest ROS1-targeted therapy, taletrectinib (Ibtrozi), in June 2025 brings new hope for adults with locally advanced or metastatic ROS1-positive NSCLC. Taletrectinib is an oral drug that has been shown to work well in the brain, remains effective against G2032R mutation, and has fewer neurologic side effects.
FDA approval was based on results from two international phase II clinical trials showing that taletrectinib helped shrink tumors in about 85 percent to 90 percent of NSCLC patients who had never received ROS1-targeted therapy before. Among those who responded, about two-thirds to three-quarters had responses that lasted at least 1 year. In people who had already been treated with other ROS1-targeted drugs, taletrectinib still shrank tumors in about 52 percent to 62 percent of patients.
Taletrectinib also worked well in patients with brain metastasis and those with resistance mutations like G2032R. The side effects were mostly mild, with low rates of dizziness and other brain-related symptoms. Because of its strong and lasting effects, brain activity, and favorable safety profile, taletrectinib is expected to become a leading treatment option for people with ROS1-positive advanced lung cancer.
Despite the emergence of numerous molecularly targeted therapeutics as groundbreaking new treatments for NSCLC, and the evidence showing that targeted treatments guided by molecular testing of the tumor yield superior outcomes for patients with NSCLC (588)Scott JA, et al. (2024) JCO Oncology Practice, 20: 145., molecular testing rates and targeted therapy use remain low and there are wide variations across health care practices (589)Baron JM, et al. (2024) JCO Precis Oncol, 8: e2400039.
(590)Roberts TJ, et al. (2023) JAMA Netw Open, 6: e2310809.. Broad implementation of cutting-edge molecular testing to identify therapeutically targetable genetic alterations driving NSCLC offers meaningful benefits to patients, is cost-effective, and must be a priority in cancer medicine (591)Lemmon CA, et al. (2023) JCO Precis Oncol, 7: e2200294..
Delivering Cytotoxic Agents Precisely to Cancer Cells
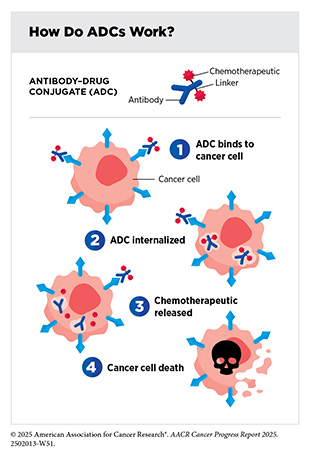
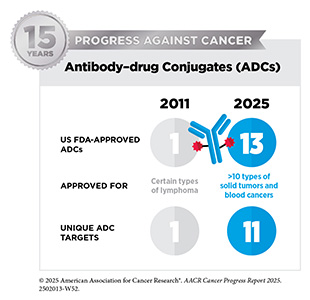
Two new anticancer treatments approved by FDA in the 12 months covered by this report belong to a class of molecularly targeted therapeutics known as antibody–drug conjugates (ADCs). ADCs consist of three main components: an antibody that specifically recognizes and binds to a target protein found mostly on cancer cell surface; a linker that connects the antibody to a chemotherapy; and a highly potent chemotherapeutic agent often referred to as a payload.
The linker in an ADC is designed to be stable in the bloodstream. Once the ADC attaches to its target on the surface of a cancer cell, it is internalized by the cell. This leads to the cleavage of the linker and the release of the cytotoxic chemotherapeutic from the antibody. Once free, the chemotherapeutic causes cancer cell death. The precision of antibody targeting reduces the side effects of the cytotoxic chemotherapeutic compared with traditional systemic delivery.
Trop-2 is a cell surface protein involved in key signaling pathways that regulate cell growth and survival. Trop-2 levels are frequently elevated in many cancers, including breast cancer and lung cancer, where it promotes tumor growth and spread. Elevated Trop-2 levels in breast cancer, particularly in HR-positive/HER2-negative and HR-negative/HER2-negative (also known as triple-negative) subtypes, have been linked to worse survival, making it a promising target for new therapies.
Datopotamab deruxtecan-dlnk is an ADC comprising the cytotoxic agent, deruxtecan, attached to a Trop-2–targeted antibody by a linker. In January 2025, datopotamab deruxtecan-dlnk was approved by FDA for the treatment of adult patients with unresectable or metastatic, HR-positive/HER2-negative breast cancer who received prior endocrine-based therapy and chemotherapy. Datopotamab deruxtecan-dlnk is the second Trop-2–targeted ADC to receive FDA approval for breast cancer patients; the first, sacituzumab govitecan-hziy (Trodelvy), was approved in April 2020 for adults with metastatic triple-negative breast cancer (592)Sengupta R, et al. (2020) Clin Cancer Res, 26: 5055..
FDA approval was based on results from a phase III clinical trial showing that patients who received datopotamab deruxtecan-dlnk lived longer without their cancer getting worse—nearly 7 months compared to 5 months for those who received standard chemotherapy (593)Bardia A, et al. (2025) J Clin Oncol, 43: 285.. Additionally, treatment with datopotamab deruxtecan-dlnk, compared to chemotherapy, was associated with fewer side effects. However, more research is needed to show whether the new therapeutic can extend overall survival compared to chemotherapy. Researchers are currently evaluating the effectiveness of datopotamab deruxtecan-dlnk, alone or in combination with other treatments, in additional subtypes of breast cancer, including the type Michelle Anderson-Benjamin was diagnosed with (594)Schmid P, et al. (2025) Ther Adv Med Oncol, 17: 17588359251327992.(595)Shatsky RA, et al. (2024) Nat Med, 30: 3737.(596)Sands J, et al. (2025) J Clin Oncol, 43: 1254..
In June 2025, FDA expanded the approval of datopotamab deruxtecan-dlnk to include the treatment of adults with advanced or metastatic NSCLC that has a mutation in the EGFR gene, the most common mutation in NSCLC, and has progressed after an EGFR-targeted therapy and platinum-based chemotherapy. The approval was based on results from two clinical trials showing that datopotamab deruxtecan-dlnk shrank tumors in 45 percent of patients (597)US Food and Drug Administration. FDA grants accelerated approval to datopotamab deruxtecan-dlnk for EGFR-mutated non-small cell lung cancer. Accessed: June 30, 2025..
The second ADC approved by FDA during the 12 months covered by this report is telisotuzumab vedotin-tllv. It was approved in May 2024, for adults with locally advanced or metastatic NSCLC who have received prior systemic therapy and whose cancers have certain biomarkers known as high MET overexpression. FDA also approved the VENTANA MET (SP44) RxDx Assay as a companion diagnostic test to identify NSCLC patients who are eligible for treatment.
Three types of alterations in the MET protein are observed in NSCLC: Mutations (known as exon 14 skipping mutations) in the MET gene, seen in about 2 percent to 4 percent of patients; extra copies of the MET gene (known as gene amplification), seen in about 5 percent of patients; and excessive amounts of MET protein (known as MET overexpression), seen in up to 40 percent of patients (598)Camidge DR, et al. (2024) J Clin Oncol, 42: 3000.. These alterations can help tumors grow and resist treatment, making MET a critical target for precision therapies in NSCLC.
MET overexpression may occur alone or coexist with other MET alterations and is linked to worse outcomes for patients. Based on the abundance of the protein, MET overexpression in NSCLC patients is classified as absent, low, intermediate, or high.
Telisotuzumab vedotin-tllv is the first FDA approved ADC to target MET-overexpressing cells and the second to be approved for patients with NSCLC. It comprises a cytotoxic agent, monomethyl auristatin E (MMAE), attached to an MET-targeted antibody by a linker. Telisotuzumab vedotin-tllv specifically binds to MET protein with high affinity, facilitating the targeted delivery of MMAE directly to tumor cells and leading to their destruction. FDA approval was based on data from a phase II clinical trial in which 35 percent of patients with high MET overexpression saw their tumors shrink in response to the treatment (598)Camidge DR, et al. (2024) J Clin Oncol, 42: 3000..
ADCs are the fastest-growing therapeutic approach for solid tumors worldwide. In 2024, 284 clinical trials were launched globally, nearly 100 more than in 2023, highlighting the rapid acceleration of research in this field (451)Parsons DW, et al. (2016) JAMA Oncol, 2: 616.. Ongoing investigation is focused on improving linker stability, identifying novel targets, and developing more potent payloads to enhance the precision, durability, and applicability of ADCs across a broader range of cancers.
Innovating Ovarian Cancer Treatment Through a Novel Combination
An estimated 20,890 new cases of ovarian cancer will be diagnosed in the United States in 2025, and 12,730 women will die from the disease (6)American Cancer Society. Cancer Facts and Figures. Accessed: June 13, 2025.. Low-grade serous ovarian cancer (LGSOC) is a rare and distinct form of ovarian cancer, accounting for less than 10 percent of cases. Compared to high-grade serous ovarian cancer, patients with LGSOC are usually diagnosed at a younger age but have better survival (599)Grisham R, et al. (2025) Int J Gynecol Cancer.. Additionally, unlike high-grade ovarian cancer, LGSOC typically has normal BRCA and TP53 genes but shows frequent changes in the RAS, RAF, and MAPK signaling pathways that affect how cells grow and divide.
Standard treatments used for other ovarian cancers, such as chemotherapy and molecularly targeted therapy, often have limited effectiveness in LGSOC and can cause significant side effects. This means patients with LGSOC often undergo many lines of treatment, and because they have longer survival, patients often face ongoing health challenges over time. There were no FDA-approved treatments specifically for LGSOC until this year. This changed in May 2025, when FDA approved a combination of two novel molecularly targeted therapeutics, avutometinib (Avmapki) and defactinib (Fakzynja), for patients with KRAS-mutated LGSOC that has returned after prior systemic treatment.
The RAF proteins and their signaling mediator, MEK, play a critical role in cell growth and drive many cancers including LGSOC. Molecularly targeted therapeutics that target RAF and MEK individually have been approved by FDA and are used in combination for treating several cancer types. Avutometinib is a new type of molecularly targeted therapeutic, referred to as a molecular clamp. It works by binding MEK and RAF together in a way that keeps them inactive (600)McNamara B, et al. (2024) Gynecol Oncol, 183: 133.. By gluing MEK and RAF together, avutometinib blocks both proteins at once and shuts down the RAF/MEK signaling pathway more efficiently.
Defactinib blocks another key protein called focal adhesion kinase (FAK), which supports cancer cell growth, survival, and spread (600)McNamara B, et al. (2024) Gynecol Oncol, 183: 133.. Upregulation of FAK signaling can also serve as a compensatory backup pathway that cancer cells use to resist treatment with RAF and MEK inhibitors. By shutting down FAK signaling, defactinib can slow cancer progression and make tumors more responsive to avutometinib.
FDA approval was based on results from a phase II clinical trial showing that the combination treatment shrank tumors in 44 percent of patients (601)Mullard A (2025) Nat Rev Drug Discov, 24: 408.. This new combination is now providing effective treatment for patients such as Mary Catherine Riley.
Personalizing Treatments for Patients With Rare Cancers
Rare cancers are defined by NCI as cancers that occur in fewer than 15 out of 100,000 people each year. Rare cancers can be challenging for researchers to study and for physicians to treat (see Sidebar 38). During the 12 months covered by this report, FDA approved a number of molecularly targeted therapeutics and immunotherapeutics for treating rare cancers, bringing the promise of precision medicine to patients who often have fewer treatment options.
Tenosynovial giant cell tumors are a group of rare tumors that arise in and around the joints and tendons. These tumors are benign, but they cause damage to the joints, which leads to pain, swelling, and limitation of joint movement. Surgery is the main treatment option. If patients do not have surgery or if the tumor continually recurs, patients suffer damage and degeneration of the affected joint and surrounding tissues or structures. In some cases, this can cause significant disability.
Research has shown that some cells in tenosynovial giant cell tumors have genetic alterations, called chromosomal translocations (see Sidebar 8), that lead to overproduction of the protein CSF1, which attracts large numbers of immune cells to the site of the tumor. The immune cells that accumulate form the bulk of the tumor and cause inflammation and damage to surrounding tissue. Vimseltinib (Romvimza) is a new molecularly targeted therapeutic that specifically inhibits the function of CSF1R, the protein on the surface of immune cells that CSF1 attaches to (603)Smith SM, et al. Clinical Cancer Advances 2021: ASCO’s Report on Progress Against Cancer. Journal of Clinical Oncology2021. p JCO.20.03420.
Vimseltinib works by locking CSF1R in an inactive state, preventing it from turning on and sending signals that fuel tumor growth and inflammation.
Vimseltinib was approved in February 2025 for adults with symptomatic tenosynovial giant cell tumors for which surgical resection may cause worsening functional limitations or severe morbidity. The approval was based on results from a phase III clinical trial showing that 40 percent of patients had significant tumor shrinkage, compared to none in the placebo group (604)Gelderblom H, et al. (2024) Lancet, 403: 2709.. Patients also experienced meaningful improvements in joint movement, physical function, and pain relief, with most responses lasting at least 6 months.
Vimseltinib is the second CSF1R-targeted therapeutic approved for treating tenosynovial giant cell tumors. The first, pexidartinib, was approved in 2019. However, a unique mechanism of action of vimseltinib is attributable to a greater selectivity for CSF1R and fewer adverse effects, providing a potentially safer option for patients.
Biliary tract cancers are a group of tumors that develop in the organs involved in producing, storing, and transporting bile for digestion, namely, the gallbladder, bile ducts, or the area where the bile and pancreatic ducts meet the small intestine. These cancers are rare—affecting approximately 12,610 individuals in 2025—but typically diagnosed at advanced stages when surgery is no longer an option. Systemic therapies, therefore, are the mainstay of treatment for most patients. Chemotherapy regimens have long been approved to treat advanced biliary tract cancers, while in recent years, immunotherapy and molecularly targeted therapies have been added to the treatment landscape. Despite these advances, many patients with biliary tract cancers eventually relapse, underscoring the need for more effective personalized treatment strategies.
Research has shown that HER2 alterations are common in biliary tract cancer, present in up to 31 percent of gallbladder cancer cases, 19 percent of bile duct cancers in the liver, and 5 percent of bile duct cancers outside the liver (605)Harding JJ, et al. (2023) Lancet Oncol, 24: 772.. These findings suggest that HER2 targeting may potentially benefit these patients. In November 2024, FDA approved zanidatamab-hrii (Ziihera), a new HER2-targeted therapy, for patients with advanced or inoperable HER2-positive biliary tract cancer who have already received chemotherapy. This is the first HER2-targeted therapy approved for this rare cancer and brings new hope to patients such as Dawn Varrati. FDA approval was based on results from a phase II clinical trial, in which over half of the patients responded to the drug, with responses lasting nearly 15 months.
Zanidatamab-hrii is an engineered protein called a bispecific antibody that binds to two distinct regions of the HER2 protein. This dual binding helps block cancer-promoting signals, promotes HER2 receptor degradation, and enhances the immune attack on cancer cells to more effectively treat HER2-positive tumors.
Bringing the Promise of Precision Medicine to Children, Adolescents, and Young Adults With Cancer
In the United States, an estimated 9,550 children (ages 0 to 14 years) and 85,480 adolescents and young adults (AYA; ages 15 to 39 years) will be diagnosed with cancer in 2025 (4)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: June 31, 2025.. Enormous progress has been made in the treatment of childhood and AYA cancers over the past several decades, as reflected in the greater than 85 percent 5-year relative survival rates for all cancers combined for both populations (see Figure 2). In the 12 months covered in this report, FDA approved a number of new therapeutics that will continue the momentum of progress against pediatric and AYA cancers.
Brain and other nervous system tumors are the second leading cause of cancer deaths among AYA. Low-grade gliomas are slow-growing brain tumors. They can often be cured with surgery alone, but depending on their location, some low-grade gliomas cannot be fully removed if they are adjacent to vital structures in the brain. In some cases, low-grade gliomas may grow back even after surgical removal. Low-grade gliomas with mutation in the IDH1 or IDH2 genes are the most common malignant primary brain tumors diagnosed in young adults, such as Alex Hepner, a young adult who was diagnosed with this cancer (606)Yan H, et al. (2009) N Engl J Med, 360: 765.. Patients with IDH mutant gliomas often receive a combination of radiation and chemotherapy after surgery, especially if they are at high risk of disease progression. While this regimen can keep the cancer in check for years, it is not curative and can lead to serious long-term side effects.
Research has shown that mutations in the IDH1 or IDH2 genes and subsequently abnormal IDH1 and IDH2 proteins lead to the production of an abnormal molecule called 2-hydroxyglutarate, which causes widespread epigenetic changes that disrupt normal cell function and drive brain tumor development (see Epigenetic Changes) (607)Dang L, et al. (2009) Nature, 462: 739.(608)Duncan CG, et al. (2012) Genome Res, 22: 2339.(609)Koivunen P, et al. (2012) Nature, 483: 484.(610)Turcan S, et al. (2012) Nature, 483: 479.. These findings led to the investigation of therapeutic approaches for treating IDH1 and IDH2 mutant brain tumors by blocking the production or effects of 2-hydroxyglutarate. Building on this work, scientists developed the molecularly targeted therapeutic vorasidenib (Voranigo), which blocks the altered IDH1 and IDH2 proteins and substantially reduces levels of 2-hydroxyglutarate and associated epigenetic changes related to IDH mutations (611).
In August 2024, vorasidenib was approved by FDA for patients 12 years and older with certain slow-growing gliomas, known as grade 2 astrocytoma or oligodendroglioma, that have IDH1 or IDH2 mutation, after patients have undergone surgery, whether a full removal, partial removal, or just a biopsy of the tumor. FDA approval was based on results from a clinical trial showing vorasidenib significantly delayed tumor progression and the need for additional treatments. Patients who received vorasidenib had a 61 percent lower risk of tumor progression compared to those who received a placebo (611)Mellinghoff IK, et al. (2023) Nat Med, 29: 615.. Ongoing research is evaluating potential mechanisms of resistance to vorasidenib as well as its effectiveness in combination with immunotherapy.
Neurofibromatosis type 1 (NF1) is an inherited genetic disorder that causes severe symptoms and complications including a significantly increased risk for developing various types of tumors (see Figure 6). Although the tumors that develop in individuals with NF1 are usually benign, some patients develop malignant tumors, usually in adolescence or adulthood. Plexiform neurofibromas are tumors that arise in cells that form the covering of peripheral nerves. These benign tumors occur in up to 50 percent of patients with NF1 and can cause pain, disability, and disfigurement. They can also go on to become cancerous.
Research has shown that the growth of plexiform tumors in patients with NF1 is fueled by a signaling pathway that includes the MEK family of proteins (612)Moertel CL, et al. (2025) J Clin Oncol, 43: 716., and in 2020, FDA approved the MEK-targeted therapeutic selumetinib (Koselugo) for treating pediatric patients ages 2 years and older who have NF1-related plexiform neurofibromas that cannot be safely removed surgically. In February 2025, FDA approved a second MEK-targeted therapeutic, mirdametinib (Gomekli), for both adult and pediatric patients 2 years of age and older with NF1 who have symptomatic plexiform neurofibromas not amenable to complete resection. The approval was based on results from a phase II clinical trial showing that 52 percent of pediatric patients and 41 percent of adult patients who received mirdametinib had tumor shrinkage (612)Moertel CL, et al. (2025) J Clin Oncol, 43: 716..
Mirdametinib offers the first treatment option for adult patients with NF1-related plexiform neurofibromas. Unlike selumetinib, which is only available as capsules, mirdametinib can be taken as a capsule or oral suspension, making it a promising new option for children who may have difficulty swallowing.
In addition to the new molecularly targeted therapeutics discussed above, in the 12 months covered in this report, FDA expanded the use of two previously approved anticancer therapeutics for treatment of childhood cancers. These include belzutifan (Welireg) as the first oral therapy for the treatment of children 12 years and older and adults with pheochromocytomas or paragangliomas—rare tumors that develop in the adrenal glands or nearby nerves—that have spread or are not surgically removable and cabozantinib (Cabometyx) for the treatment of children 12 years and older and adults with pancreatic or non-pancreatic neuroendocrine tumors—tumors in hormone-producing cells inside or outside the pancreas—that have spread or are not surgically removable and have not responded to earlier treatments.
Advances in Treatment With Immunotherapeutics
The immune system is a complex network of cells (called white blood cells; see Sidebar 39), tissues (e.g., bone marrow), organs (e.g., thymus), and the substances they make (e.g., cytokines) that help the body fight infections and other diseases, including cancer. The immune system actively monitors threats from external (such as viruses and bacteria) and internal sources (such as abnormal or damaged cells) and works to eliminate them from the body.
The immune system is highly effective in detecting and eliminating cancer cells, a process also known as cancer immune surveillance (613)Hiam-Galvez KJ, et al. (2021) Nat Rev Cancer, 21: 345.. However, as tumor cells acquire new properties during the course of cancer development (see Understanding the Path to Cancer Development), some cells find ways to “hide” from the immune system, such as by decreasing or eliminating the numbers and/or amounts of proteins on the surface of tumor cells that are used by the immune system to recognize cancer; triggering certain brakes on immune cells that prevent them from eradicating cancer cells; and releasing molecules that weaken the ability of immune cells to detect and destroy cancer cells (614)Mishra AK, et al. (2022) Diseases, 10: 60.. The field of cancer immunology is focused on better understanding how tumor cells evade the immune system and leveraging this knowledge to develop novel cancer treatments.
Unprecedented advances in cancer immunology over the past two decades have firmly established immunotherapy as the fifth pillar of cancer medicine (615)Kaufmann SHE (2019) Front Immunol, 10: 684.. Cancer immunotherapy refers to any treatment that works by using the immune system to fight cancer. There are various ways in which different immunotherapeutics unleash the immune system to fight cancer (see Sidebar 40).
Releasing the Brakes on the Immune System
Decades of research have revealed that some tumor cells have increased levels of certain proteins on their surface that attach to and activate “brakes” on T cells, thus stopping them from attacking cancer cells. These brakes are proteins on the surface of T cells and are called immune checkpoint proteins. Immune checkpoint inhibitors (ICIs) are a class of transformative new therapeutics that block the checkpoint proteins from attaching to their binding partners on cancer cells and can thereby release the brakes on T cells which trigger previously restrained T cells to attack and destroy cancer cells (616)Marin-Acevedo JA, et al. (2021) J Hematol Oncol, 14: 45..
The first ICI approved by FDA was ipilimumab (Yervoy) in 2011, which blocks the checkpoint protein CTLA-4 and was authorized for treating metastatic melanoma (see Figure 18). This was followed in 2014 by the approval of two additional ICIs—nivolumab (Opdivo) and pembrolizumab (Keytruda)—which target a different checkpoint protein, PD-1. These agents work by preventing PD-1 from binding to its partner protein, PD-L1, on cancer cells, thereby lifting the immune system’s brake. Both were also initially approved for metastatic melanoma. In 2016, a fourth ICI, atezolizumab (Tecentriq), which targets PD-L1 directly, was approved for certain types of bladder cancer.
The third immune checkpoint protein to be targeted by an FDA-approved therapy is LAG-3. In March 2022, FDA approved relatlimab-rmbw, the first and, so far, only ICI that targets LAG-3, for use in combination with nivolumab (as Opdualag) in patients with metastatic melanoma. While additional ICIs have been approved since then, relatlimab represents the most recent example of an ICI targeting a novel immune checkpoint pathway.
Since 2011, when the inaugural edition of the AACR Cancer Progress Report was published, the use of ICIs has rapidly expanded and these therapeutics are currently considered one of the most exciting approaches to cancer treatment (see Figure 18 and Figure 19). This is in part because some patients with metastatic cancers who have been treated with these therapeutics have had remarkable and durable responses. For example, long-term results from a clinical trial testing the ICI pembrolizumab in patients with advanced NSCLC showed that 23 percent of patients lived 5 or more years after the treatment, which stands in stark contrast to the historically low 5-year relative survival rate for these patients of just about 5 percent (617)Garon EB, et al. (2019) Journal of Clinical Oncology, 37: 2518..
Another clinical trial that monitored advanced melanoma patients for at least 7.5 years showed that half of the patients treated with the combination of nivolumab and ipilimumab lived at least 6 years (618)Wolchok JD, et al. (2025) N Engl J Med, 392: 11.. Before ipilimumab was approved in 2011, most patients lived less than a year. Building on these advances, researchers are now studying which ICIs offer the best long-term outcomes and whether single drugs used alone or combinations like nivolumab plus ipilimumab lead to better survival and fewer side effects over time (618)Wolchok JD, et al. (2025) N Engl J Med, 392: 11.(619)Long GV, et al. (2025) J Clin Oncol, 43: 938..
During the 12 months spanning this report (July 1, 2024 to June 30, 2025), FDA approved two new ICIs—cosibelimab-ipdl (Unloxcyt) and penpulimab-kcqx—and expanded the uses of three previously approved ICIs—durvalumab (Imfinzi), pembrolizumab (Keytruda), and retifanlimab-dlwr (Zynyz)—to include additional cancer types. With these latest approvals the total number of ICIs approved by FDA has reached 15 as of June 30, 2025. These groundbreaking treatments are now approved for treating 22 cancer types as well as any type of solid tumor characterized by the presence of certain molecular characteristics (see Figure 19).
In December 2024, FDA approved cosibelimab-ipdl, a new PD-L1–targeting ICI, for the treatment of adults with advanced cutaneous squamous cell carcinoma (CSCC) that has spread or that is locally advanced and cannot be treated with surgery or radiation intended to cure the disease.
CSCC is the second most common form of skin cancer in the United States. While existing PD-1–targeted ICIs have improved outcomes for patients with CSCC, treatment options are still limited for patients with advanced or metastatic disease who cannot undergo curative surgery or radiation.
Additionally, currently approved ICIs are not effective or well tolerated for all patients, highlighting the need for new options that may be fulfilled by cosibelimab-ipdl.
FDA approval was based on results from a phase I clinical trial demonstrating that cosibelimab-ipdl helped shrink tumors in nearly half of the patients (620)Clingan P, et al. (2023) J Immunother Cancer, 11.. These responses were also long-lasting, with many patients continuing to benefit for more than a year. These findings, together with the lower rates of reported adverse events compared to other ICIs, suggest that cosibelimab-ipdl could offer a safer and effective new treatment option for patients with advanced CSCC.
The second new ICI approved by FDA in the 12 months covered in this report is penpulimab-kcqx, a PD-1–targeted ICI. It was approved by FDA, along with chemotherapy, in April 2025 for treating adults with non-keratinizing nasopharyngeal carcinoma (NPC) whose cancer has metastasized or come back and who have not yet received treatment for advanced disease. In addition, penpulimab-kcqx was approved to be used alone for adults whose cancer has worsened after receiving platinum-based chemotherapy and at least one other treatment.
NPC is a rare form of head and neck cancer that starts in the upper part of the throat, behind the nose. The risk of developing NPC is influenced by a combination of factors, including inherited genes, certain viral infections, and environmental or dietary habits. There are different types of NPC. Non-keratinizing NPCs are characterized by the absence of keratin protein in cancerous cells. NPCs are rare in North America and Europe but have a higher incidence in China, North Africa, and India (621)Roy Chattopadhyay N, et al. (2017) Drug Discov Ther, 11: 170..
FDA approval of penpulimab-kcqx was based on results from two clinical studies. In the first trial, a phase III study, patients with metastatic or recurrent NPC who had not received prior treatment for their advanced disease received either penpulimab-kcqx with chemotherapy or chemotherapy alone. Those who received penpulimab-kcqx lived longer without their cancer worsening—about 9.6 months compared to 7 months for those who received only chemotherapy (622)Hu C, et al. (2025) Cancer Research, 85: CT011.. In a second study, a phase II clinical trial, patients whose cancer had progressed after at least two prior treatments received penpulimab-kcqx alone. About 28 percent of patients saw their tumors shrink, and for many, the benefit lasted a long time (623)Chen X, et al. (2024) Signal Transduct Target Ther, 9: 148.. Unlike other PD-1-targeted ICIs, penpulimab-kcqx was engineered with unique structural features aimed at reducing its adverse effects.
In addition to the two new ICIs discussed above, in the 12 months covered in this report, FDA expanded the use of three previously approved anticancer therapeutics for the treatment of new cancer types. These include retifanlimab-dlwr (Zynyz), approved for patients with advanced anal cancer, the first approval of an ICI for this disease; durvalumab (Imfinzi), approved for certain patients with bladder cancer; and pembrolizumab, approved for patients with advanced mesothelioma, each to be used in combination with standard chemotherapy.
ICIs have yielded extraordinary benefits for many patients. However, they can also have adverse effects, particularly the induction of immune-related adverse events. According to a new analysis, 40 percent of patients receiving ICIs may experience such events, some of which can be life threatening (625)Jayathilaka B, et al. (2025) Br J Cancer, 132: 51.. Immune-related adverse events occur because activated immune cells unleashed by ICIs not only attack cancer cells but can also target and injure healthy tissue.
To predict which patients are likely to experience serious adverse events and design interventions to combat these events without compromising the anticancer efficacy of ICIs, researchers must better understand why and how adverse events arise (626)Nielsen DL, et al. (2024) JAMA Oncol, 10: 1390.(627)Ruiz-Esteves KN, et al. (2024) JAMA Oncol, 10: 1409..
Managing side effects from ICIs in older adults can be especially difficult, particularly when they have comorbidities or are physically frail. Therefore, an urgent medical need is to identify strategies for addressing ICI-related toxicities among older adults (628)Eochagain CM, et al. (2025) Lancet Oncol, 26: e90.. Notably, research has shown that although the immune system undergoes complex changes with aging, some cancer patients age 65 and older may still respond well to ICIs, though responses may vary depending on cancer type and study design (629)Kao C, et al. (2025) Nat Commun, 16: 3531..
While ICIs have been transformative for many cancers, only 20 percent of patients respond to these treatments (624)Haslam A, et al. (2025) Int J Cancer, 156: 2352.. Identifying cellular and molecular biomarkers that can predict whether ICIs are likely to work in a patient is an area of extensive research investigation. Biomarkers can also help identify patients unlikely to benefit from immune checkpoint therapy, sparing them from unnecessary side effects, high treatment costs, and delays in receiving more effective alternatives.
Using advanced technologies, researchers are exploring several strategies—both before and during ICI treatment—to identify the best biomarkers for predicting response, including measuring cancer-specific proteins (called neoantigens) that help the immune system recognize and attack cancer cells, analyzing the activity of key immune-related genes, and assessing the abundance and spatial distribution of immune cells within tumor microenvironment over time (630)Alban TJ, et al. (2024) Nat Med, 30: 3209.(631)Campbell MJ, et al. (2024) Cell Rep Med, 5: 101799.(632)Hernando-Calvo A, et al. (2024) JCO Precis Oncol, 8: e2400100.(633)Usset J, et al. (2024) Nat Genet, 56: 2112.
(634)Yan Y, et al. (2025) Nat Genet, 57: 126.. One large study in patients with several advanced cancer types showed that five key factors—number of mutations in the tumor, immune cell infiltration of tumors, a molecule that suppresses the immune system, prior treatments, and tumor growth rate—can reliably predict response to immunotherapy and provide a framework for future biomarker research (633)Usset J, et al. (2024) Nat Genet, 56: 2112..
AI and machine learning are increasingly being used as powerful tools to advance research in this area (see New Advances in Artificial Intelligence). Recent studies have shown that AI-based algorithms developed using pathologic images, RNA or DNA sequence analyses, or even information from routine clinical tests derived from patients can successfully predict response to ICIs (635)Hamidi H, et al. (2024) Cancer Cell, 42: 2098.(636)Patkar S, et al. (2024) NPJ Precis Oncol, 8: 280.(637)Rakaee M, et al. (2025) JAMA Oncol, 11: 109.(638)Yoo SK, et al. (2025) Nat Med, 31: 869.. As one example, a new AI tool called SCORPIO, which was developed using information from routine blood tests and medical records accurately predicted which cancer patients were likely to benefit from immunotherapy and how long they might live, potentially improving treatment decisions and reducing unnecessary side effects (638)Yoo SK, et al. (2025) Nat Med, 31: 869..
Another important area of scientific inquiry is to identify behavioral and clinical factors, such as diet, physical activity, gut microbiome composition, duration and route of delivery of ICI treatment, and optimal combinations with other therapeutic modalities that can boost the efficacy of ICIs and increase the number of patients who respond favorably to these lifesaving treatments.
Increasing evidence is showing that the gut microbiome—bacteria, viruses, and all other microorganisms that live in the digestive system—can impact response to ICIs (639)Lin Y, et al. (2025) Med, 6: 100530.(640)Park JS, et al. (2023) Nature, 617: 377.(641)Rosario SR, et al. (2024) Nat Commun, 15: 10609.. Researchers are currently evaluating how the microbiome enhances the effectiveness of ICIs and exploring strategies to either mimic these effects through therapies or by modifying the microbiome using probiotics, antibiotics, or fecal transplants to make patients more responsive to ICIs.

Researchers are also actively testing combinations of different ICIs, pairing ICIs with other forms of immunotherapy, and combining them with other treatment approaches, such as molecularly targeted therapies, to improve effectiveness, safety, and overcoming resistance to cancer treatment.
As one example, two recent clinical trials found that adding a JAK inhibitor, a type of molecularly targeted therapeutic, to ICIs helped improve outcomes for patients with advanced NSCLC and Hodgkin lymphoma, even those who had not responded to previous ICI treatments (642)Gadina M, et al. (2024) Science, 384: 1303.. Researchers showed that the combination worked by reactivating exhausted and inactive immune cells, allowing them to better fight the cancer and leading to stronger and longer-lasting responses. In a separate set of clinical studies, researchers showed that combining ICIs and a type of targeted therapy called angiogenesis inhibitors (see Sidebar 34) with a standard procedure that involves using a catheter to deliver chemotherapy directly to the liver significantly delayed cancer progression in people with intermediate-stage liver cancer, offering a promising new treatment option (643)Kudo M, et al. (2025) Lancet, 405: 203.(644)Sangro B, et al. (2025) Lancet, 405: 216..
ICIs have transformed the clinical care of patients with a diverse array of cancer types, including historically intractable diseases, such as metastatic melanoma, lung cancer, and kidney cancer (see Figure 19). While their use was initially limited to people with very advanced cancers that were no longer responding to standard treatments, ICIs are now being approved as first-line, or initial, treatments for patients. Researchers are also evaluating how to best integrate the use of ICIs in combination with standard treatments such as surgery, radiation therapy, and/or chemotherapy in patients with early-stage cancers.
One area of extensive research is the use of these therapeutics before initial surgery, known as neoadjuvant treatment (see Sidebar 32), in people with locally advanced cancers that are largely restricted to the tissue of origin (645)Awada G, et al. (2025) Nat Cancer, 6: 967.. In this regard, a recent clinical trial showed that adding the ICI pembrolizumab before and after standard treatment (surgery and radiation) helped patients with advanced head and neck cancer live longer without the cancer coming back (646)Uppaluri R, et al. (2025) Cancer Research, 85: CT001.. Based on these data, in June 2025, FDA approved pembrolizumab as neoadjuvant treatment and as a combination with radiotherapy, with or without chemotherapy, after surgery for certain patients with head and neck cancers.
In another groundbreaking clinical trial among patients with early-stage cancers that have a specific genetic feature, treatment with the ICI dostarlimab before surgery led to tumor disappearance in most cases, allowing those patients to safely avoid surgery while maintaining excellent outcomes (533)Cercek A, et al. (2025) N Engl J Med, 392: 2297.. In the study, 103 patients with different types of cancer were treated with dostarlimab. Tumors disappeared in most patients—including all 49 with rectal cancer and many with cancers of the stomach, esophagus, liver, endometrium, urinary tract, and prostate—and after five years, only five experienced a recurrence, all of whom responded well to further treatment.
Boosting the Cancer-killing Power of Immune Cells
Research has shown that immune cells, such as T cells, are naturally capable of destroying cancer cells. It has also shown that in patients with cancer, often the numbers of cancer-killing T cells are insufficient, and that the cancer-killing T cells that are present are unable to find or destroy the cancer cells for one of several reasons. This knowledge has led researchers to identify several ways to boost the ability of T cells to eliminate cancer cells.
Adoptive cell therapy (ACT), also called cellular immunotherapy, is designed to dramatically increase the number of cancer-killing immune cells a patient has, thereby boosting the immune system’s ability to seek and destroy cancer cells (647)Rohaan MW, et al. (2019) Virchows Arch, 474: 449.. While many of the adoptive cell therapies currently in late-stage clinical development, and all that are approved by FDA, utilize patient-derived T cells, ongoing research is looking to harness the cancer-killing power of other types of immune cells, including natural killer (NK) cells and macrophages (see Sidebar 41).
Chimeric antigen receptor (CAR) T-cell therapy is one type of ACT that has generated enormous excitement in recent years. Like ICIs, CAR T-cell therapy is the culmination of decades of basic, translational, and clinical research utilizing knowledge of the cellular and molecular components of the immune system, genetic engineering, and the biological underpinnings of blood cancers. It works by collecting a patient’s own immune cells (T cells) and genetically modifying them to produce a special receptor, called a CAR, on their surface. This receptor enables the T cells to recognize and attack cancer cells. After being multiplied into millions in the laboratory, these engineered cells are infused back into the patient to target and destroy the cancer.
Treatment with CAR T cells has demonstrated unprecedented efficacy in certain patients with very advanced leukemia, lymphoma, and multiple myeloma (see A Decade of Progress: Transformative Advances in Blood Cancer). As of June 30, 2025, seven CAR T-cell therapies have been approved by FDA for treating a range of blood cancers.
CAR T-cell therapies can cause significant side effects, some of which, such as cytokine release syndrome (CRS), a condition characterized by excessive immune activation leading to organ toxicity, and immune effector cell–associated neurotoxicity syndrome (ICANS), a neurologic condition leading to confusion, speech difficulties, and seizure, can be potentially life-threatening. Researchers are actively working to identify biomarkers that can predict side effects and to mitigate the significant immune-related toxicities associated with CAR T-cell therapy (648)Mulvey A, et al. (2025) Nat Rev Drug Discov, 24: 379.. Strategies under investigation include the development of therapeutic interventions to manage the adverse effects and engineering next-generation CAR T cells such as those with safety switches that can be turned off in real time to fine-tune CAR T-cell activation and reduce toxicity (648)Mulvey A, et al. (2025) Nat Rev Drug Discov, 24: 379..
Additionally, efforts are underway to develop CAR T-cell therapies against solid tumors, but there are several challenges to overcome. These challenges include identifying tumor-specific cell surface proteins that are present in the tumor but absent in healthy tissue, infiltration of CAR T cells to the inside of solid tumors, and the immune suppressive nature of the tumor microenvironment. However, there are promising new data showing efficacy of CAR T-cell therapy in a number of solid tumors, including glioblastoma, gastrointestinal cancers, and advanced neuroblastoma (see Sidebar 42) (649)Bagley SJ, et al. (2025) Nat Med.(650)Choi BD, et al. (2024) N Engl J Med, 390: 1290.(651)Li CH, et al. (2025) Nat Med, 31: 1125.(652)Qi C, et al. (2025) Lancet, 405: 2049.. In addition, researchers are exploring whether other types of engineered immune cells, such as CAR macrophages, can effectively treat solid tumors (653)Reiss KA, et al. (2025) Nat Med, 31: 1171..
Developing simpler, safer, and faster ways to bring the promise of CAR T-cell therapies to more patients with different types of cancer is an area of ongoing investigation. One area of active research is using T cells collected from healthy donors instead of patients so that these “off-the-shelf” CAR T cells could be readily available for use rather than manufactured for each patient.
Although clinical research has long investigated the effectiveness of the three types of adoptive T-cell therapies, only CAR T-cell therapy had been approved by FDA before 2024. This changed with the approval of the first tumor-infiltrating lymphocytes (TILs), lifileucel (Amtagvi), in February 2024 (discussed in AACR Cancer Progress Report 2024), followed by the approval of the first T-cell receptor (TCR) T-cell therapy, afamitresgene autoleucel (Tecelra), in August 2024.
FDA approval of afamitresgene autoleucel, the first-ever TCR T-cell therapy approved for any cancer, marks a major milestone in cancer treatment. Like CAR T cells, TCR T cells are manufactured using a patient’s own T cells. However, unlike CAR T cells, which use synthetic receptors to recognize proteins on the surface of cancer cells, TCR T cells use modified versions of natural receptors to identify fragments of cancer-related intracellular proteins that are brought to the cell surface by molecules called human leukocyte antigens (HLAs). HLAs are proteins that are found on the surface of most cells and play a crucial role in helping the immune system detect and destroy cancer cells.
Afamitresgene autoleucel was approved for adults with inoperable or metastatic synovial sarcoma whose tumors test positive for a specific protein called MAGE-A4, and who carry certain HLA types. It is approved for patients who have already received chemotherapy and have no other curative treatment options.
Synovial sarcoma is a rare and aggressive cancer that usually develops in the soft tissues near joints, such as the arms or legs, and primarily affects young adults like Quinn Johnsen. Current treatments include surgery, chemotherapy, and radiation. However, people with advanced or metastatic disease often have limited treatment options and poor outcomes. Only about 8 percent to 15 percent of patients with metastatic cancer live for five years or more after diagnosis (655)D’Angelo SP, et al. (2024) Lancet, 403: 1460.. Therefore, there is an urgent need for more effective therapies.
Research has shown that synovial sarcoma cells have high levels of the cancer-related protein MAGE-A4, which is the target of the newly approved TCR T cells. HLA proteins display fragments of the MAGE-A4 protein on the surface of tumor cells, helping TCR T cells to recognize and eliminate cancer cells.
FDA approval was based on results from a phase II clinical trial (655)D’Angelo SP, et al. (2024) Lancet, 403: 1460. in which over 43 percent of patients with advanced synovial sarcoma who received a single infusion of afamitresgene autoleucel saw their tumors shrink, with some responses lasting more than a year.
CRS was a common immediate side effect seen in 70 percent of patients following treatment with afamitresgene autoleucel. CRS has also been associated with other adoptive T-cell therapies and is characterized by an excessive immune reaction that leads to widespread organ dysfunction that can become life-threatening. While afamitresgene autoleucel–related CRS was usually mild and treatable, experts recommend that this therapy be given at specialized cancer centers with trained health care professionals who are experienced in managing cell-based treatments.

Directing the Immune System to Cancer Cells
An immune cell must find a cancer cell before it can attack and eliminate it. Many therapeutics approved by FDA for treating cancer work, at least in part, by helping immune cells find cancer cells. Bispecific T-cell engagers (BiTEs) are one such class of therapeutic antibodies that are moving rapidly from the laboratory to clinical practice. Using two or more arms that are engineered into these antibody molecules, these immunotherapeutics bind to T cells and cancer cells simultaneously. By acting as a connector, BiTEs bring cancer cells in close proximity to the immune cells, which are then activated and eliminate the cancer cells.
The first of these agents, blinatumomab (Blincyto), was approved by FDA in December 2014 for treating certain patients with a type of acute lymphoblastic leukemia (ALL) called B-cell ALL (see Redirecting T Cells to Eradicate Blood Cancers) (656)Baselga J, et al. (2015) Clin Cancer Res, 21: S1.. Unprecedented advances in genetic engineering, molecular biology, and immunology over the past decade have led to rapid growth in this innovative new area of cancer medicine. As of June 30, 2025, FDA has approved eight BiTEs for the treatment of cancer (657)Tian Z, et al. (2021) J Hematol Oncol, 14: 75.. Many of these groundbreaking therapeutics are approved for patients with advanced cancer that has returned following several prior treatments or is resistant to therapy.
Like ICIs and CAR T cells, BiTEs may cause serious adverse side effects, some of which could be life-threatening if not managed immediately and appropriately by trained medical professionals. While ongoing research is needed to identify ways to minimize the adverse effects, BiTEs are now offering new hope to many patients with advanced cancer who previously were without options and urgently needed effective treatments for their rapidly progressing disease.
Researchers are currently investigating additional approaches to enhance the efficacy of BiTEs and evaluating multispecific (e.g., trispecific and tetraspecific) immune cell engagers designed to direct different types of immune cells, such as NK cells or macrophages, in addition to T cells, toward attacking cancer. Preclinical studies have shown that these innovative approaches can improve immune cell infiltration within tumors, enhance antitumor responses, and when used in combinations may overcome resistance to single-agent therapies, offering hope for more durable and effective cancer treatments (658)Rolin C, et al. (2024) Cell Mol Immunol, 21: 643..
Another group of therapeutic antibodies that mark cancer cells for elimination by the immune system uses a natural process called antibody-dependent cellular cytotoxicity. When the immune system detects a pathogen or damaged cells, B cells produce antibodies that flag unwanted cells or organisms, which are then recognized and killed by immune cells such as NK cells (see Sidebar 39) (659)Zahavi D, et al. (2018) Antib Ther, 1: 7.. Researchers are using this knowledge to develop antibodies that bind to specific targets on cancer cells and invoke antibody-dependent cellular cytotoxicity to kill them.
Many therapeutic antibodies that work this way have been approved by FDA so far. The most recent, zolbetuximab-clzb (Vyloy), was approved in October 2024, in combination with chemotherapy, for the initial treatment of adults with locally advanced or metastatic gastric or gastroesophageal junction (GEJ) cancers that cannot be removed surgically and that test positive for the protein claudin 18.2 but not HER2. FDA also approved the VENTANA CLDN18 RxDx Assay as a companion diagnostic device to identify patients with gastric or GEJ cancer who are eligible for treatment with zolbetuximab-clzb.
Claudin 18.2 is normally found only in the healthy lining of the stomach, where it is buried within tightly connected cells. In many stomach and gastroesophageal cancers, claudin 18.2 becomes exposed and accessible on the surface of cancer cells, making it easier for antibody-based therapies to find and attack the cancer cells. Hence, claudin 18.2 is a promising new target for cancer treatment.
Zolbetuximab-clzb is the first FDA-approved therapy that targets claudin 18.2, which is now benefiting patients such as Greg Myers. By attaching to this protein, zolbetuximab-clzb helps the immune system recognize and kill cancer cells through natural defense mechanisms, including antibody-dependent cellular cytotoxicity.
FDA approval was based on results from two phase III clinical trials. In the first trial, patients who received zolbetuximab-clzb with chemotherapy lived about 10.6 months without their cancer getting worse, compared to 8.7 months for those who received chemotherapy alone (660)Shitara K, et al. (2023) Lancet, 401: 1655.. In the second trial, patients who received zolbetuximab-clzb with chemotherapy lived about 8.2 months before their cancer worsened, compared to 6.8 months with chemotherapy alone (660)Shitara K, et al. (2023) Lancet, 401: 1655..
Next Section: Transformative Advances in Blood Cancer Previous Section: Cancer Screening for Early Detection