Envisioning the Future of Cancer Science and Medicine
In this section, you will learn:
- The unprecedented advances against cancer in recent decades stem from breakthrough discoveries and technological advances across medicine.
- Radiotheranostics, a promising technique for detecting and treating cancer using radioisotopes, has shown remarkable success in treating multiple types of cancers simultaneously, marking a significant advancement in cancer treatment.
- Advances in noninvasive cancer imaging are revolutionizing visualization of tumor metabolism and assessment and monitoring of treatment response.
- Cancer engineering is emerging as a powerful interdisciplinary approach for understanding the complex nature of cancer to improve health outcomes.
The pace of progress against cancer has accelerated tremendously in recent years, as underscored throughout this report. Breakthrough discoveries and technological advances across the fields of science and medicine have substantially increased the understanding of cancer initiation and progression, providing the foundational knowledge for better strategies to reduce the risk of developing cancer, detect cancer at the earliest possible stage, and treat cancer effectively and more precisely. As a result, cancer deaths are declining, and cancer survivors are living longer and fuller lives.
The breadth of advances against cancer and their impact on saving and improving lives are a source of great optimism for cancer scientists, including the AACR president, 2024–2025, Patricia M. LoRusso, DO, PhD (hc), FAACR, who firmly believe that the fast-paced trajectory of progress against cancer can be further accelerated through sustained and predictable funding for cancer research.
Below, we highlight some of the most exciting areas in cancer science and medicine that are poised to transform the future of cancer research and patient care.
Cancer Engineering: An Interdisciplinary Approach to Drive Progress Against Cancer
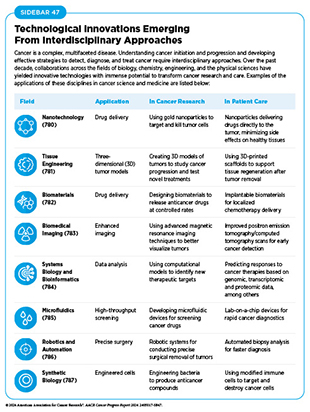
Cancer engineering is an interdisciplinary field that combines principles from engineering, biology, and medicine to develop innovative approaches for the prevention, detection, diagnosis, and treatment of cancer (779)Flores ER, et al. (2024) Cancer Cell, 42: 1133. DOI: 10.1016/j.ccell.2024.05.017.. It involves the application of engineering techniques and technologies to understand cancer biology and to create new tools and methods for combating the disease (see Sidebar 47).
One of the most promising applications of cancer engineering is the precise delivery of targeted therapeutics to tumors. For example, nanotechnology, a cornerstone of cancer engineering, enables the creation of nanoparticles that can deliver drugs directly to cancer cells, sparing healthy tissues (780)Zhang P, et al. (2023) Med, 4: 147. DOI: 10.1016/j.medj.2022.12.001.. Another way in which cancer engineering is accelerating the pace of progress against cancer is the development of advanced imaging techniques (see A New Wave of Imaging Technologies). These imaging approaches can detect cancer at earlier stages and monitor treatment responses more accurately (783)Schwenck J, et al. (2023) Nat Rev Cancer, 23: 474. DOI: 10.1038/s41568-023-00576-4.. Similarly, tissue engineering and biomaterials are revolutionizing cancer treatment by enabling the development of three-dimensional tumor models and implantable devices (781)Unnikrishnan K, et al. (2021) Front Oncol, 11: 733652. DOI: 10.3389/fonc.2021.733652.(782)Ashworth JC, et al. (2024) Nat Rev Cancer, 24: 461. DOI: 10.1038/s41568-024-00704-8.. These technologies allow researchers to study cancer in an environment that closely mimics the human body, facilitating the testing of new drugs and therapies. In the clinic, biomaterials can be used to create scaffolds that support tissue regeneration after tumor removal, improving patient recovery.
Because of the immense promise of cancer engineering, researchers have proposed conceptual frameworks to encourage interdisciplinary collaborations at institutional levels and accelerate further the pace of progress against cancer (779)Flores ER, et al. (2024) Cancer Cell, 42: 1133. DOI: 10.1016/j.ccell.2024.05.017.. It is important to note that realizing the promise of cancer engineering requires overcoming significant challenges, including financial and logistical barriers, as well as regulatory hurdles. Researchers are emphasizing the importance of education and training in cancer engineering to prepare a new generation of scientists with engineering expertise and a fundamental understanding of cancer biology to transform clinical cancer care (788)Keshari KR, et al. (2024) Cancer Cell, 42: 1138. DOI: 10.1016/j.ccell.2024.04.013.. Continued interdisciplinary collaboration, investment in medical research, and a commitment to patient-centered research and care will be essential to harness the full potential of cancer engineering and improve outcomes for cancer patients.
A New Age of Radiation Therapy
Radiotherapy uses high-energy rays or particles to control the growth of and/or eradicate cancer cells by damaging their DNA (see Advances in Radiation-based Approaches to Cancer Care). About 50 percent of all cancer patients in the United States receive radiotherapy as part of their treatment regimens (789)Jaffray DA, et al. Radiation Therapy for Cancer. In: Gelband H, Jha P, Sankaranarayanan R, Horton S, editors. Cancer: Disease Control Priorities, Third Edition (Volume 3). Washington (DC) 2015.. The number of cancer survivors who have received radiotherapy is projected to increase from 3.38 million in 2020 to 4.17 million in 2030 (422)Kratz CP, et al. (2017) Clin Cancer Res, 23: e38.. Continued innovation and advances in radiotherapy are needed to minimize the adverse effects of the treatment while maximizing the benefits for patients.
Advances in Intraventricular Compartmental Radioimmunotherapy
Intraventricular compartmental radioimmunotherapy (cRIT) is a specialized form of radiotherapy for the treatment of certain types of cancer, particularly central nervous system tumors that are difficult to reach using other therapies. cRIT involves the development of antibodies that specifically target proteins present on the surface of cancer cells. These antibodies are labeled with a radioactive isotope and injected directly into the cerebrospinal fluid within the brain’s ventricles using a specialized surgical instrument (790)Kramer K, et al. (2014) Pediatr Blood Cancer, 61: 1590. DOI: 10.1002/pbc.25080.. Once injected, the radiolabeled antibodies bind to the cancer cells, and the radioactive isotopes attached to the antibodies help destroy cancer cells. Because the radiation emitted by the isotopes is localized to cancer cells, the radiation exposure to healthy tissue is minimal.
One of the cancer types for which cRIT has shown considerable effectiveness is medulloblastoma. Medulloblastoma is a type of brain tumor that originates in the cerebellum, the part of the brain located at the base of the skull. It arises in cells that are involved in motor control and coordination (791)Choi JY (2023) Brain Tumor Res Treat, 11: 28. DOI: 10.14791/btrt.2022.0046.. Medulloblastoma is the most common type of malignant brain tumor in children, with a 10-year survival rate of 70 percent (792)Ostrom QT, et al. (2023) Neuro Oncol, 25: iv1. DOI: 10.1093/neuonc/noad149.. Treatment options are limited to surgical removal of as much tissue as is safe, chemotherapy, and radiation of the entire brain and spinal cord; all of these options carry significant side effects (791)Choi JY (2023) Brain Tumor Res Treat, 11: 28. DOI: 10.14791/btrt.2022.0046..
Research has shown that the surface of cancer cells in patients with medulloblastoma (among other cancer types) carries a protein called B7-H3, which functions as a “brake” on the immune system (see Sidebar 47) (793)Yang S, et al. (2020) Int J Biol Sci, 16: 1767. DOI: 10.7150/ijbs.41105.. The B7-H3 protein is an attractive target for drug development, and researchers have developed several antibodies directed against it (793)Yang S, et al. (2020) Int J Biol Sci, 16: 1767. DOI: 10.7150/ijbs.41105.. One such antibody, omburtamab, is chemically linked with the radioactive form of iodine (131I) to make a cRT agent, called 131I-Omburtamab (794). In a recent study, 20 patients with medulloblastoma were treated with cRIT. Injections of 131I-Omburtamab once or twice monthly were associated with improved overall survival and survival without disease progression (795)Tringale KR, et al. (2023) J Neurooncol, 162: 69. DOI: 10.1007/s11060-022-04235-w.. Furthermore, six patients were alive with no evidence of disease 3 years after the treatment (795)Tringale KR, et al. (2023) J Neurooncol, 162: 69. DOI: 10.1007/s11060-022-04235-w.. This example highlights the potential of cRIT in improving health outcomes for patients with cancers.
Despite its immense promise, cRIT presents some challenges. For example, cRIT requires precise delivery of radiolabeled antibodies through a complex surgical procedure. Consequently, a multidisciplinary team of oncologists, neurosurgeons, and radiologists is needed for the procedure and for careful monitoring of radiation doses. Furthermore, even though cRIT is a highly precise targeted therapy, the treatment can still cause neurotoxicity, or other complications related to radiation. Additional research is needed to further optimize cRIT for the benefit of patients with cancer.
Emergence of Radiotheranostics
Radiotheranostics, also known as radiopharmaceutical therapy (RPT), refers to the combined imaging and precise delivery of radiopharmaceuticals to the tumor (796)Bodei L, et al. (2022) Nat Rev Clin Oncol, 19: 534. DOI: 10.1038/s41571-022-00652-y.(797)Lapi SE, et al. (2024) Lancet Oncol, 25: e236. DOI: 10.1016/S1470-2045(24)00030-5.. Briefly, in RPT, cancer is visualized by single-photon emission computed tomography (SPECT) or positron emission tomography (PET) imaging using molecules that are linked to diagnostic radionuclides and bind to specific proteins on the surface of cancer cells or tumor microenvironment. Once the presence of cancer is confirmed, the same targeting agents—labeled with more potent therapeutic radioisotopes—are then used to kill cancer cells (798)Duan H, et al. (2022) Nanotheranostics, 6: 103. DOI: 10.7150/ntno.64141..
RPT has shown great promise in the clinic in treating various types of cancer, as evident from improved outcomes, lower side effects, and better quality of life compared to alternative therapies. The utility of RPT in cancer treatment is further underscored by the approval of several radiopharmaceuticals by the US Food and Drug Administration (FDA) in recent years, including a first-in-class combination of a radiodiagnostic and a radiotherapeutic agent to treat metastatic prostate cancer (84)American Association for Cancer Research. AACR Cancer Progress Report 2022. Accessed: July 5, 2023..
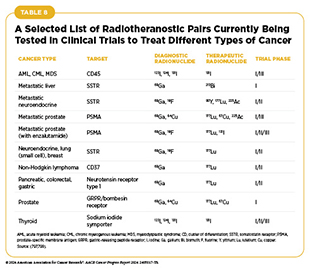
In addition to using RPT to treat advanced-stage cancers, researchers are testing RPT approaches as initial treatments and to treat earlier stages of the disease (see Advances in Radiation-based Approaches to Cancer Care) (799)Aboagye EO, et al. (2023) CA Cancer J Clin, 73: 255. DOI: 10.3322/caac.21768.. For example, findings from a phase I/II clinical trial show that when given before surgery to patients with prostate cancer whose tumor had not metastasized, RPT safely and precisely delivered radiation to tumors and, in about half of the patients, decreased the levels of prostate-specific antigen, a biomarker for prostate cancer, by half. Importantly, patients experienced minimal treatment-related adverse events, and surgery was safe with a low rate of complications (800). As researchers develop new and more sophisticated ways to deliver an expanding array of diagnostic and therapeutic radioisotopes, a number of clinical trials are underway to test the efficacy and safety of radiotheranostic pairs, alone or in combination with other types of cancer treatments, for treating multiple cancer types (see Table 8) (797)Lapi SE, et al. (2024) Lancet Oncol, 25: e236. DOI: 10.1016/S1470-2045(24)00030-5.(799)Aboagye EO, et al. (2023) CA Cancer J Clin, 73: 255. DOI: 10.3322/caac.21768.. While advances in RPT offer exciting new frontiers for progress against cancer, some challenges remain, such as production and supply of radiotheranostic agents, patients’ access to RPT, and training of the workforce to administer this highly specialized form of cancer treatment, among others (797)Lapi SE, et al. (2024) Lancet Oncol, 25: e236. DOI: 10.1016/S1470-2045(24)00030-5.(801)Korde A, et al. (2024) EJNMMI Radiopharm Chem, 9: 2. DOI: 10.1186/s41181-023-00230-2.(802)Giammarile F, et al. (2024) Lancet Oncol, 25: e260. DOI: 10.1016/S1470-2045(24)00041-X.(803)Scott AM, et al. (2024) Lancet Oncol, 25: e250. DOI: 10.1016/S1470-2045(24)00037-8..
A New Wave of Imaging Technologies
Imaging has revolutionized cancer diagnosis and treatment by providing noninvasive methods to visualize tumors, monitor their progression, and guide therapeutic interventions. Some of the most used imaging approaches in cancer care include X-ray imaging, SPECT, PET, computed tomography (CT) and magnetic resonance imaging (MRI) (796)Bodei L, et al. (2022) Nat Rev Clin Oncol, 19: 534. DOI: 10.1038/s41571-022-00652-y.. These techniques enable early detection, accurate staging, and monitoring of treatment response, significantly improving patient outcomes.
Visualizing Tumor Metabolism Better
PET is an imaging technique that provides detailed information about the metabolic and functional activities within the body. PET is performed by injecting a tracer molecule, typically a form of glucose labeled with a radioactive isotope, into a patient’s bloodstream. Because of higher metabolism, cancer cells absorb a higher amount of the tracer molecule than normal cells. The radioactive isotope interacts with electrons in the body to produce gamma rays that are detected by PET scanners. Using images captured by PET scanners, a detailed three-dimensional map of the patient’s body is constructed, with areas of high metabolism indicating the presence of tumors (804)Farwell MD, et al. (2014) Cancer, 120: 3433. DOI: 10.1002/cncr.28860.. PET is widely used in cancer diagnosis, staging, and monitoring treatment response. However, a drawback of PET is exposure of the patient to radiation, especially when repeated scans are necessary to accurately detect tumors, although the radiation dose is still less than that of diagnostic CT scans (804)Farwell MD, et al. (2014) Cancer, 120: 3433. DOI: 10.1002/cncr.28860..
Researchers are continually innovating PET to maximize its benefits for patients, while minimizing potential harm (783)Schwenck J, et al. (2023) Nat Rev Cancer, 23: 474. DOI: 10.1038/s41568-023-00576-4.. An important recent development in this regard is the development of PET scanners that can cover much larger portions of the body in a single scan (805)Alberts I, et al. (2023) Cancer Imaging, 23: 28. DOI: 10.1186/s40644-023-00540-3.(806)Daube-Witherspoon ME, et al. (2022) Br J Radiol, 95: 20220357. DOI: 10.1259/bjr.20220357.. This new generation of PET scanners can image the entire body or large sections of it simultaneously. This is beneficial for patients in several ways, including reduced radiation exposure, improved image quality, and decreased scan time (806)Daube-Witherspoon ME, et al. (2022) Br J Radiol, 95: 20220357. DOI: 10.1259/bjr.20220357.(807)Nadig V, et al. (2022) Eur J Nucl Med Mol Imaging, 49: 445. DOI: 10.1007/s00259-021-05536-4.(808)Cherry SR, et al. (2018) J Nucl Med, 59: 3. DOI: 10.2967/jnumed.116.184028.. Furthermore, the ability to image the entire body or large sections of it also allows researchers to study the efficacy of new and novel radiopharmaceutical agents quickly (805)Alberts I, et al. (2023) Cancer Imaging, 23: 28. DOI: 10.1186/s40644-023-00540-3.(808)Cherry SR, et al. (2018) J Nucl Med, 59: 3. DOI: 10.2967/jnumed.116.184028..
Because PET primarily captures metabolic activity or target expression with high accuracy, it is often combined with other imaging approaches, such as CT (809)Beyer T, et al. (2000) J Nucl Med, 41: 1369. PMID: 10945530 or MRI (810)Shao Y, et al. (1997) Phys Med Biol, 42: 1965. DOI: 10.1088/0031-9155/42/10/010.(811)Judenhofer MS, et al. (2008) Nat Med, 14: 459. DOI: 10.1038/nm1700., to simultaneously capture high-resolution anatomic information for enhanced diagnostic accuracy. Thus, combined PET-MRI scans help develop highly detailed and precise 3D maps of the body. This precision has led to exciting recent developments in cancer imaging in which researchers are using the combined PET-MRI scans for predicting outcomes in patients with lymphoma after CAR T-cell therapy (812)Sjoholm T, et al. (2022) Cancer Imaging, 22: 76. DOI: 10.1186/s40644-022-00513-y., identifying lesions inside the prostate gland (813)Sandgren K, et al. (2023) Nucl Med Commun, 44: 997. DOI: 10.1097/MNM.0000000000001743., and predicting overall survival in patients with glioma (814)Lombardi G, et al. (2022) Br J Radiol, 95: 20211018. DOI: 10.1259/bjr.20211018.. Another way researchers are exploring the utility of combined PET-CT is imaging tumors during surgery for a more precise assessment of tumor margins (815)Darr C, et al. (2023) Eur Urol Open Sci, 54: 28. DOI: 10.1016/j.euros.2023.05.017..
In addition to improved PET approaches discussed here, two other recent advances to capture dynamic changes in tumor metabolism are hyperpolarized MRI and deuterium metabolic imaging (817)Kaggie JD, et al. (2022) Neuroimage, 257: 119284. DOI: 10.1016/j.neuroimage.2022.119284.. Hyperpolarized MRI uses specialized imaging agents to significantly enhance the MRI signal, while deuterium metabolic imaging uses deuterium, a stable and non-radioactive form of hydrogen, to map and visualize metabolic processes within the body. Because of higher precision in detecting tumors and visualizing tumor metabolism, and because of the absence of ionizing radiation which can cause DNA damage, both approaches have the potential to revolutionize the assessment of cancer metabolism (see Sidebar 32) (818)Brindle KM (2024) npj Imaging, 2: 1. DOI: 10.1038/s44303-023-00004-0..
Monitoring Treatment Response Effectively
Cancer diagnosis and treatment have evolved significantly over the past decades, driven by advances in molecular biology and imaging technologies. One of the most promising developments in this field is the use of molecular imaging agents as companion diagnostics. These agents can be visualized using PET, MRI, single-photon emission computed tomography (SPECT), and optical imaging, and provide critical insights into the molecular characteristics of tumors, helping to identify patients who are most likely to benefit from specific therapies, monitor treatment responses, and detect resistance mechanisms.
A key advantage of molecular imaging agents is the dynamic assessment of the effectiveness of treatment by measuring changes in tumor metabolism, proliferation, immune response, and presence or abundance of certain proteins on the surface of cancer cells. Over the past decade, researchers have developed many new molecular imaging agents that are being tested in preclinical research models. One example of such molecular imaging agents is the fibroblast activation protein inhibitor (FAPI) (819)Mori Y, et al. (2023) Radiology, 306: e220749. DOI: 10.1148/radiol.220749., which binds to FAP, a protein that is abundantly present on the surface of cells present in the tumor microenvironment of more than 90 percent of epithelial tumors, and on the cell surfaces of some tumors, such as sarcomas (820)Altmann A, et al. (2021) J Nucl Med, 62: 160. DOI: 10.2967/jnumed.120.244806.. In cancer imaging, researchers are using versions of FAPI conjugated with radioisotopes (e.g., 68Ga) to evaluate characteristics of a number of cancer types, including pancreatic, colorectal, and breast cancer (821)Koerber SA, et al. (2023) J Nucl Med, 64: 1712. DOI: 10.2967/jnumed.123.266046.(822)Chandekar KR, et al. (2023) Diagnostics (Basel), 13: 2018. DOI: 10.3390/diagnostics13122018.(823)Kratochwil C, et al. (2019) J Nucl Med, 60: 801. DOI: 10.2967/jnumed.119.227967.. Another group of molecules being used for molecular imaging as companion diagnostics consists of antibodies against proteins present on the surface of cancer cells and linked with imaging agents (824)Arroyo A, et al. (2024) Methods Mol Biol, 2729: 117. DOI: 10.1007/978-1-0716-3499-8_8..
The integration of molecular imaging agents as companion diagnostics is the next frontier in cancer imaging that is helping researchers visualize tumors noninvasively. In the clinic, the use of these agents is guiding treatment decisions and monitoring patients’ responses to treatments. For example, SC16.56 is an antibody directed against a protein called delta-like ligand 3 (D-exprLL3), which is abundantly present in certain types of tumors, including cancers of lung, liver, and breast and neuroendocrine tumors (825)Xiu MX, et al. (2020) Onco Targets Ther, 13: 3881. DOI: 10.2147/OTT.S244860.. When chemically linked with a radioactive form of zirconium, the resulting imaging agent, [89Zr]Zr-DFO-SC16.56, can detect DLL3essing tumors (826)Korsen JA, et al. (2022) J Nucl Med, 63: 1401. DOI: 10.2967/jnumed.121.263221.. In a recent study, researchers successfully used [89Zr]Zr-DFO-SC16.56 for the first time to noninvasively image DLL3-expressing neuroendocrine tumors inside the human body (827)Tendler S, et al. (2024) medRxiv: 2024.01.10.24301109. DOI: 10.1101/2024.01.10.24301109.. This advance can significantly help develop approaches to select patients who are eligible for certain treatments and test the efficacy of new treatments targeted against DLL3-expressing tumors using a precision-medicine approach, which is an intense area of focus (828)Rudin CM, et al. (2023) J Hematol Oncol, 16: 66. DOI: 10.1186/s13045-023-01464-y.(829)Yao J, et al. (2022) Oncologist, 27: 940. DOI: 10.1093/oncolo/oyac161..
As the clinical use of radiolabeled imaging agents as companion diagnostics gains traction, it is important to note that the development and approval of these agents in the clinic require extensive validation. Furthermore, the need for a specialized infrastructure and workforce can significantly limit their availability and utilization, similar to other specialized imaging techniques (e.g., MRI), and may contribute to cancer disparities. Finally, because these agents are radioactive, similar to CT scans, repeated imaging may pose potential risks, especially for children, adolescent, and young adult patients, and their use should be balanced with the benefits gained with the information obtained (830)Liao S, et al. (2023) iScience, 26: 107277. DOI: 10.1016/j.isci.2023.107277..
Next Section: Advancing Cancer Research and Patient Care Through Evidence-based Policies Previous Section: Supporting Cancer Patients and Survivors