- Investments in Research for a Healthier Future
- A Diverse Cancer Research and Care Workforce Drives Innovation
- Programs to Expand and Diversify the Scientific Research Workforce
- Programs to Strengthen and Expand the Health Care Workforce
- Ensuring Safe and Effective Cancer Therapies Through Regulatory Science
- Diversifying and Decentralizing Trials
- Rapidly Delivering Safe and Effective Therapies to Patients
- Addressing Cancer Drug Shortages
- Advancing Policies to Strengthen Cancer Prevention and Screening Programs
- Leveraging Policy to Reduce Tobacco-related Illness
- Spotlight: Accelerating Progress Against Childhood Cancer
- Addressing Cancer Disparities and Improving Patient Outcomes
Advancing Cancer Research and Patient Care Through Evidence-based Policies
In this section, you will learn:
- Robust investment in federal agencies, including NIH and NCI, is vital to making further progress, including improvements in cancer screening, treatment, and survivorship.
- Support is needed for education and training programs to ensure that the United States has a strong medical research and clinical workforce that is broadly representative of society.
- FDA plays a central role in expediting the availability of safe and effective cancer therapies, including through the expansion and diversification of clinical trials.
- Federal agencies, including NIH, CDC, AHRQ, and EPA, and many of their programs are crucial for reducing the risk of cancer and eliminating cancer disparities.
The tremendous progress we have made against cancer over the past several decades has depended on strong federal investments in medical and public health research. Important federal programs in cancer prevention, early detection, and treatment are funded and managed by many different agencies, including the National Institutes of Health (NIH), the National Cancer Institute (NCI), the US Food and Drug Administration (FDA), the Centers for Disease Control and Prevention (CDC), the Agency for Healthcare Research and Quality (AHRQ), the Department of Veterans Affairs (VA), and the Environmental Protection Agency (EPA).
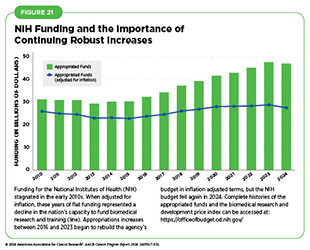
Despite the importance of these federal efforts, the 2023 Fiscal Responsibility Act places strict caps on federal domestic spending (including funding for research and health programs) in fiscal year (FY) 2024 and FY 2025 (831)Congress.gov. H.R.2882 – 118th Congress (2023-2024): Further Consolidated Appropriations Act, 2024. Accessed: July 12, 2024.. These funding constraints are already curtailing important scientific progress (832)National Cancer Institute. Fiscal Year 2024 Appropriation Brings Clarity and Difficult Choices. Accessed: July 12, 2024.. It will therefore be critical that Congress lift these spending caps and provide robust, sustained, and predictable funding for the federal agencies that are crucial for the fight against cancer.
Investments in Research for a Healthier Future
The US Department of Health and Human Services (HHS) contains a rich ecosystem of agencies, such as NIH, dedicated to advancing medical research. As the world’s largest public funder of medical science (833)National Institutes of Health. Grants & Funding. Accessed: July 12, 2024., NIH provides funding for research projects and clinical trials across the nation that aim to promote the prevention, diagnosis, and treatment of various medical conditions.
The largest of the 27 institutes and centers within NIH is NCI, whose main focus is to support cancer research and help train cancer researchers (834)National Institutes of Health. National Cancer Institute. Accessed: July 12, 2024.. An additional component of NIH, the Advanced Research Projects Agency for Health (ARPA-H), funds and empowers high-potential, high-impact medical research projects that cannot be realized through traditional commercial or research means (835)National Institutes of Health. Advanced Research Projects Agency for Health (ARPA-H). Accessed: July 12, 2024..
FDA is another HHS agency that plays a major role in supporting efforts to prevent and treat cancer. Two key FDA centers assist in this mission: the Oncology Center for Excellence (OCE), which brings together leading researchers to conduct expedited reviews of cancer-related medical products (836)US Food & Drug Administration. Oncology Center of Excellence. Accessed: July 12, 2024.; and the Center for Tobacco Products (CTP) (837)US Food & Drug Administration. Center for Tobacco Products. Accessed: July 12, 2024., which enforces laws and regulations on tobacco products that cause cancer.
Also a part of HHS, AHRQ supports health care access and research by collecting survey data and assisting with the development of guidelines for screening and preventative services. AHRQ additionally offers intramural and extramural predoctoral and postdoctoral grants to improve education and career development opportunities for health services researchers (838)Agency for Healthcare Research and Quality. Research Training and Education. Accessed: August 2, 2024..
As the nation’s leading public health agency, CDC plays an essential role in cancer prevention and research to promote public health. Its Division of Cancer Prevention and Control leads efforts to collect data on cancer cases, promote cancer screenings, and fund cancer prevention programs (839)Centers for Disease Control and Prevention. About the Division of Cancer Prevention and Control | National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP). Accessed: July 12, 2024.. CDC funds the North American Association of Central Cancer Registries (NAACCR) program, a core cancer surveillance system across the United States. This coordinated data collection effort is vital to advance cancer research and develop more effective public health interventions (840)North American Association of Central Cancer Registries. About NAACCR. Accessed:. Beyond cancer-specific programs, CDC funds public health capacity and workforce training across the nation through grants to state and local public health departments (841)Centers for Disease Control and Prevention. About the Public Health Infrastructure Center. Accessed: August 2, 2024.. These investments also increase access to screening and prevention programs and improve data collection in the fight against cancer. As alluded to by Congresswoman Madeleine Dean, these investments also increase access to screening and prevention programs and improve data collection in the fight against cancer.
Continued investment in these agencies that advance medical research and public health is of vital importance to the nation. These investments have improved health outcomes for patients with cancer, resulting in a drop in the cancer mortality rate. As of 2021, the age-adjusted cancer death rate has declined by 33 percent since reaching its peak in 1991 (2)American Cancer Society. Cancer Facts and Figures 2024. Accessed: July 10, 2024.. This decline would not have been possible without the development of life-saving cancer treatments. For example, between 2010 and 2019, NIH funding contributed to 354 of 356 new FDA-approved drugs, including 86 first in class products to treat cancer and other diseases. Furthermore, the National Breast and Cervical Cancer Early Detection and Screening Program (NBCCEDP), the Colorectal Cancer Control Program, and other programs have contributed to the downward trend in cancer deaths by allowing people who are uninsured or underinsured to access free screening services.
Despite significant progress in improving cancer-related health outcomes, there are concerning trends related to cancer incidence that underscore the need to continue prioritizing investments in medical research. Six common cancers—breast, prostate, endometrial, pancreatic, kidney, and melanoma— have seen diagnoses increase in recent years, which can be attributed to an aging and a growing population. There are also alarming increases in the incidence and mortality of certain cancers in younger people, notably those of colorectal cancer in people between ages 18 and 49 (842)Loomans-Kropp HA, et al. (2019) J Cancer Epidemiol, 2019: 9841295. DOI: 10.1155/2019/9841295.. As a result, the number of new cancer diagnoses in the United States is projected to surpass two million in 2024, the first time that such a threshold has been reached (2)American Cancer Society. Cancer Facts and Figures 2024. Accessed: July 10, 2024.. Thus, continued investment in medical research will only become more important as the number of Americans diagnosed with cancer continues to rise.
Additionally, investment in medical research immensely benefits the US economy. NIH-awarded funding for extramural research supports the purchase of services, goods, and materials across the nation, which helps to generate new employment opportunities and economic growth. In FY 2023, NIH awarded $37.81 billion to investigators in all 50 states and the District of Columbia to conduct extramural research. This funding directly and indirectly supported 412,041 new jobs and yielded $92.89 billion in economic activity. Furthermore, robust funding for NIH helps ensure that the United States continues to be a global leader in medical research and innovation (843)United For Medical Research. NIH’s Role in Sustaining the U.S. Economy. Every State Benefits. Accessed: July 12, 2024..
Continued investment in medical research and public health is also a matter of national security. Infectious diseases, such as COVID-19, can threaten military readiness due to their potential to temporarily incapacitate or disable both current and potential armed services personnel (844)University of Illinois Chicago. A National Security Case for Public Health Infrastructure and Universal Healthcare | School of Public Health. Accessed: July 12, 2024.. Fortunately, decades of NIH-supported research, including NCI-supported research on the immune system, contributed to the development of COVID-19 vaccines (24)American Association for Cancer Research. AACR Report on the Impact of COVID-19 on Cancer Research and Patient Care. Accessed: June 30, 2022.(845)Wherry EJ, et al. (2021) Clin Cancer Res, 27: 2136.(846)National Institutes of Health. Decades in the Making: mRNA COVID-19 Vaccines. Accessed: July 12, 2024., which are effective in limiting the spread of SARS-CoV-2 infections, hospitalizations and deaths (847)Damijan JP, et al. (2022) Vaccines (Basel), 10: 678. DOI: 10.3390/vaccines10050678., and infection-associated chronic conditions known as long COVID (848)Scientific American. Vaccination Dramatically Lowers Long COVID Risk. Accessed: July 12, 2024.. Therefore, support for NIH funding is an asset for national security.
Beyond vaccine development, investment in public health infrastructure is essential to ensure that threats to public health can be swiftly addressed. Currently, CDC supports two preparedness programs that provide states, localities, and territories with funding to support a skilled public health workforce and physical infrastructure, such as laboratories. These programs played a key role in deploying vaccines during the COVID-19 pandemic.
In recognition of these benefits, the Biden administration supports additional investments in medical research in fiscal year (FY) 2025. Released in March 2024, the president’s FY 2025 Budget Request calls for $48.3 billion for the base NIH budget, which amounts to a $1.2 billion or 2.7 percent increase over FY 2024 funding. Additionally, the budget proposes $7.8 billion in FY 2025 funding for NCI, a $615 million increase over the FY 2024 level. Also under the NIH umbrella, ARPA-H would receive $1.5 billion per the administration’s FY 2025 request, which is the same amount the agency received in FY 2024.
The White House budget would reinvest in the Cancer Moonshot, proposing more than $2 billion for programs aimed at cutting the cancer death rate by at least 50 percent over the next 25 years (849)The White House. Budget of the U.S. Government FISCAL YEAR 2025. Accessed: July 12, 2024.. Initially launched in 2016, funding for the Cancer Moonshot expired in 2023. This initiative has provided significant additional funding for NCI to support more cancer research opportunities (850)National Cancer Institute. Cancer Moonshot. Accessed: July 12, 2024.. For example, Moonshot-supported programs such as the Immuno-Oncology Translational Network and the Pancreatic Cancer Microenvironment Network have contributed to progress against cancer by facilitating the discovery of new immune system targets for cancer therapies.
Despite support from the White House, medical research funding faces a difficult budgetary environment as the FY 2025 appropriations process unfolds. Finalized in March 2024, the Consolidated Appropriations Act 2024, provided NIH and NCI with $47.1 billion and $7.2 billion, respectively, amounting to reductions of $378 million and $96 million compared to FY 2023 levels (831)Congress.gov. H.R.2882 – 118th Congress (2023-2024): Further Consolidated Appropriations Act, 2024. Accessed: July 12, 2024.. This marks the first occasion since FY 2014 that Congress did not appropriate additional funds for NIH (851)Congressional Research Service. National Institutes of Health (NIH) Funding: FY1996-FY2023. Accessed: July 12, 2024..
The primary reason for the lack of a funding increase for NIH in FY 2024 was the establishment of caps on discretionary defense and nondefense spending imposed in June 2023 as part of an agreement to raise the debt ceiling (852)Congress.gov. H.R.3746 – 118th Congress (2023-2024): Fiscal Responsibility Act of 2023. Accessed: July 12, 2024.. Combined with a decrease in funding from the 21st Century Cures Act (including the expiration of the Cancer Moonshot funding), these spending caps resulted in the decrease in NIH appropriations for the current fiscal year.
Flat or declining funding for medical research can set a precedent for lower funding in coming years because the FY 2026 budget will be built on the FY 2025 final budget as a baseline. Additionally, flat funding is further damaging to science due to the increased costs of conducting research brought about by inflation (see Figure 21).
The caps on discretionary nondefense spending do not expire until September 30, 2025, which means they will continue to put downward pressure on investments in NIH and other discretionary nondefense priorities that support medical research and health. Adding to the difficulties facing the FY 2025 appropriations process is the 2024 general election on November 5, 2024, which will determine control of the presidency, the House of Representatives, and the Senate. As a result, Congress is likely to delay final decisions on FY 2025 appropriations bills until after federal election results have been determined.
Furthermore, partisan differences over federal appropriations could cloud prospects for greater investments in medical research in FY 2025. Senate Appropriations Committee Chair Patty Murray (D-WA) and Ranking Member Susan Collins (R-ME) remain committed to a bipartisan approach to FY 2025 appropriations to ensure that investments in NIH and NCI continue to grow. However, some House Republicans are demanding strong adherence to the spending caps as well as additional cuts to discretionary nondefense spending. As a result, advocates for stronger investments in medical research are likely to encounter a contentious appropriations environment in FY 2025.
A Diverse Cancer Research and Care Workforce Drives Innovation
Further progress in cancer research and patient care will require a robust and diverse scientific and clinical workforce. Diversity strengthens scientific collaborations, and progress toward health equity depends on educating a health care workforce that is broadly representative of society (853)Freeman RB, et al. (2014) Nature, 513: 305. DOI: 10.1038/513305a.(854)AlShebli BK, et al. (2018) Nat Commun, 9: 5163. DOI: 10.1038/s41467-018-07634-8.(855)Pittman P, et al. (2021) Med Care, 59: S405. DOI: 10.1097/MLR.0000000000001609.(856)Jackson CS, et al. (2014) Public Health Rep, 129 Suppl 2: 57. DOI: 10.1177/00333549141291S211.. However, many structural barriers remain that lead to a lack of diversity and representation in research, medicine, and other health care fields, as highlighted in the AACR Cancer Disparities Progress Report 2024 (29)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2024. Accessed: June 14, 2024..
Programs to Expand and Diversify the Scientific Research Workforce
In recent years, many federal initiatives have been undertaken to further broaden participation in Science, Technology, Engineering, Mathematics, and Medicine (STEMM). The National Science Foundation (NSF), an independent federal agency that does not fall under any cabinet within the executive branch, funds a broad portfolio of basic and applied research programs as well as an extensive series of STEMM education and training programs, including support for K–12 education, undergraduate and graduate students, postdoctoral fellows, faculty, and other members of the scientific and technical workforce (857)US National Science Foundation. About NSF. Accessed: July 12, 2024.(858)US National Science Foundation. Directorate for STEM Education (EDU). Accessed: July 12, 2024.. Broadening representation and participation in the sciences, engineering, and medicine is a major part of the NSF mission (859)US National Science Foundation. About Equity for Excellence in STEM (EES). Accessed: July 12, 2024..
Several NSF programs aimed at diversifying the scientific workforce were either created or enhanced by provisions of the 2022 Creating Helpful Incentives to Produce Semiconductors (CHIPS) and Science Act, including new initiatives to bolster programs at Historically Black Colleges and Universities (HBCUs) and other minority-serving institutions (MSIs), create new research and educational opportunities across diverse geographies, and combat sexual and gender harassment in research settings (860)US National Science Foundation. CHIPS and Science. Accessed: July 12, 2024.(861)The White House. FACT SHEET: CHIPS and Science Act Will Lower Costs, Create Jobs, Strengthen Supply Chains, and Counter China | The White House. Accessed: March 30, 2024.. Despite the scale and ambition of these initiatives, Congress has funded them far below the levels authorized by the CHIPS and Science Act (862)Brookings. The Bold Vision of the CHIPS and Science Act Isn’t Getting the Funding it Needs. Accessed: July 12, 2024..
NIH has also taken a multi-faceted approach in its efforts to create a more inclusive medical workforce. The NIH UNITE initiative is an agency-wide effort to dismantle structural racism within the NIH and across the medical research community. The initiative has four focus areas: expanding health disparities research, promoting equity and inclusion within NIH, promoting equity in the extramural medical community, and improving the collection and dissemination of racial and ethnic equity data (863)National Institutes of Health. Ending Structural Racism. UNITE. Accessed: March 17, 2024.. The Office of the Chief Officer for Scientific Workforce Diversity (COSWD) plays a key role in the advancement of scientific training, diversity, and inclusion in the medical research workforce.
COSWD programs include administrative supplements to existing NIH awards to support Diversity, Equity, Inclusion, and Accessibility (DEIA) mentorship activities; a prize competition for institutions implementing novel DEIA programs; and the NIH Distinguished Scholars Program, designed to promote and enhance DEIA within the NIH intramural program (864)National Institutes of Health. Office of the Director. Chief Officer for Scientific Workforce Diversity. Act. Accessed: July 12, 2024.. Each Institute and Center (IC) within NIH, including NCI, also has a wide range of education and career development programs aimed at enhancing workforce training specific to the disciplinary focus of each respective IC (865)National Institutes of Health. NIH Institute/Center Research Training and Career Development Information. Accessed: July 12, 2024..
To expand and diversify the cancer research workforce, NCI implements and manages a range of policies and programs (866)National Cancer Institute. Funding for Cancer Training. Accessed: July 12, 2024.. Beginning in 2021, NCI began requiring a Plan to Enhance Diversity (PED) as a core component of the application for a Cancer Center Support Grant, the major source of support for NCI-Designated Cancer Centers (867)National Institutes of Health. Cancer Center Support Grants (CCSGs) for NCI-designated Cancer Centers (P30 Clinical Trial Optional). Accessed: July 12, 2024.. As part of their PEDs, NCI-designated centers are directed to implement plans that boost diversity and representation among cancer center leadership; foster the careers of diverse junior, early, and mid-career scientists; and establish criteria for measuring progress. The establishment of the PED requirement has been important for the creation of new recruitment, training, and mentoring initiatives at cancer institutes; however, more work and resources will be needed to maximize the effectiveness of PEDs (868)Li CI, et al. (2024) J Natl Cancer Inst, 00: djae100. DOI: 10.1093/jnci/djae100..
Within NCI, the Center for Cancer Health Equity (CCHE) leads initiatives to improve diversity and representation in cancer research. It funds a broad portfolio of programs that support education and mentoring of students and trainees at all levels; fosters opportunities for scientists from diverse backgrounds to become independent scientific investigators; and partners with academic institutions serving populations subjected to health disparities and underrepresented students (see Sidebar 48) (29)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2024. Accessed: June 14, 2024.(869)National Cancer Institute. CRCHD Diversity Training. Accessed: July 12, 2024..
Unfortunately, the Supreme Court decision in June 2023, which struck down affirmative action policies in university admissions, will likely have a detrimental effect on broadening participation in scientific and medical training. Prior to the June 2023 ruling, state bans on the use of race in academic admission had caused enrollment of members of racial minorities to drop at public universities (870)Ly DP, et al. (2022) Ann Intern Med, 175: 873. DOI: 10.7326/M21-4312.. Similar outcomes are expected as universities shift their policies to align with the Court’s ruling (871)Pereira RI, et al. (2024) Lancet, 403: 332. DOI: 10.1016/S0140-6736(23)02700-9..
Another area of concern for the future health of the cancer research enterprise is the decline in the number of postdoctoral fellows, particularly in the biological and medical sciences (872)National Science Foundation. Graduate Enrollment in Science, Engineering, and Health Continues to Increase among Foreign Nationals, while Postdoctoral Appointment Trends Vary across Fields. Accessed: July 12, 2024.. Postdoctoral researchers are an essential part of the biomedical workforce, and postdoctoral experience is typical for life scientists who go on to have careers in academic research. However, postdoctoral fellows typically receive low compensation relative to their professional training, and often face job insecurity and uneven opportunities for mentorship and career advancement; these issues are further exacerbated for postdoctoral fellows from historically underrepresented or marginalized groups (873)National Institutes of Health. NIH Advisory Committee To The Director Working Group On Re-Envisioning NIH-Supported Postdoctoral Training. Accessed: July 12, 2024.. Given these problems, many biomedical scientists who might have pursued postdoctoral training are choosing alternative career opportunities in the pharmaceutical or biotechnology industries (874)Science Magazine. Fewer U.S. Scientists Are Pursuing Postdoc Positions, New Data Show. Accessed:. Scientists in industry make important contributions to the advancement of new cancer therapeutics and other technologies, but the health of the biomedical workforce and the well-being of individual scientists will depend on reforms to the postdoctoral system. To this end, NIH has adopted recommendations from an advisory committee on ways to improve financial support, working environments, career advancement, and other aspects of the postdoctoral experience (875)National Institutes of Health. ACD Working Group on Re-envisioning NIH-Supported Postdoctoral Training. Accessed: July 12, 2024..
Programs to Strengthen and Expand the Health Care Workforce
Breakthroughs in medical and public health research can further benefit the populace if there is a robust medical and health care workforce that reflects the diversity of society. However, population groups underrepresented in the biomedical workforce face many barriers to educational opportunities and, in turn, full and equitable participation in health care professions, including physicians, physician-scientists, and nurses (29)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2024. Accessed: June 14, 2024..
Many of the NIH and NCI programs described in this chapter are also working to address shortcomings in cancer care (particularly regarding medical professionals who conduct basic, translational, and clinical research), though more robust federal support is needed. Importantly, Medicare is the largest source of funding in the United States for graduate medical education (MD or the equivalent), supporting approximately 98,000 medical residency positions (876)Congressional Research Service. Medicare Graduate Medical Education Payments: An Overview. Accessed: March 17, 2024.(877)American Medical Association. 2023 Compendium of Graduate Medical Education Initiatives Report. Accessed: March 17, 2024.. Under current law, however, the number of residency positions is capped at approximately the same number as in 1996 (980). The same cap on Medicare funding for residencies also applies to medical fellowships, limiting the number of publicly funded specialty training opportunities for physicians (878)Congressional Research Service. Federal Support for Graduate Medical Education: An Overview. Accessed: August 2, 2024.. Given the growth of the US population and the increased demand for medical care, this cap is a significant obstacle, especially given the shortages of physicians in rural and underserved communities (879)Rains J, et al. (2023) JAMA, 330: 968. DOI: 10.1001/jama.2023.14452..
More support is also needed for cancer prevention training. Programs such as the NCI Cancer Prevention Fellowship Program (CPFP) provide opportunities for fellows to pursue research projects related to cancer prevention (880)National Cancer Institute. Cancer Prevention Fellowship Program. Accessed: July 12, 2024., and many universities have academic programs focused on prevention (881)MD Anderson Cancer Center. Cancer Prevention Research Training Program. Accessed: July 12, 2024.(882)Robert H. Lurie Comprehensive Cancer Center of Northwestern University. Cancer Prevention & Control. Accessed: July 12, 2024.(883)VCU Massey Comprehensive Cancer Center. Cancer Prevention & Control Program. Accessed: July 12, 2024.(884)Yale Cancer Center. Yale Cancer Prevention and Control (CPC) Training Program. Accessed: July 12, 2024.. However, these efforts are somewhat limited in scale. Initiatives such as partnerships between Health Resources and Services Administration (HRSA)– funded community health centers and NCI-designated Cancer Centers may provide opportunities to leverage federal resources to expand the training of health care professionals focused on cancer prevention (885)US Department of Health and Human Services. HHS Announces Health Resources and Services Administration-Funded Health Centers Partnering With National Cancer Institute-Designated Cancer Centers to Improve Equity in Cancer Screening. Accessed: July 12, 2024..
Ensuring Safe and Effective Cancer Therapies Through Regulatory Science
FDA is the principal agency responsible for ensuring that medicines are safe and effective. Its regulatory oversight spans the entire process of drug development—from translational laboratory studies to clinical trials and post-marketing evaluation. In recent years, drug development, particularly for cancer, has become increasingly complex and often involves multiple stakeholders across the globe. To streamline the process, FDA’s Oncology Center of Excellence (OCE) was established in 2017 under the 21st Century Cures Act and collaborates with three FDA product centers: the Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), and Center for Devices and Radiological Health (CDRH). This multi-center effort seeks to expedite the availability of new cancer therapies by integrating reviews and advancing regulatory science and policy. Since OCE’s first drug approval in 2017, 174 new anticancer therapies for solid tumors have been approved. In comparison, the agency only approved 71 therapies for adults with solid tumors from 2002 to 2016 (886)Goldberg KB, et al. (2018) Experimental Biology and Medicine, 243: 308. DOI: 10.1177/1535370217740861.. FDA relies on funding from Congress and congressionally authorized user fees paid by the pharmaceutical industry to implement these initiatives. This funding is crucial to support FDA’s mission, keep pace with evolving regulatory science, and advance progress in cancer research and treatment.
Diversifying and Decentralizing Trials
In recent decades, clinical trials have played a pivotal role in driving medical and scientific progress by introducing innovative treatments and deepening our understanding of cancer. Although most patients express interest in participating in cancer clinical trials, a small percentage of people with cancer or at risk for cancer participate in clinical trials today. Only 8 percent of adults with cancer participate in clinical trials (489)Unger JM, et al. (2024) J Clin Oncol, 42: 2139. DOI: 10.1200/JCO.23.01030.. These percentages are even lower for many groups historically underrepresented in clinical research (887)Pittell H, et al. (2023) JAMA Netw Open, 6: e2322515. DOI: 10.1001/jamanetworkopen.2023.22515.. Low participation rates can be attributed to structural barriers, which include narrow eligibility criteria and inaccessibility, study burden, distrust, lack of awareness, and fear (888)NIH Office of Research on Women’s Health. Review of the Literature: Primary Barriers and Facilitators to Participation in Clinical Research. Accessed: March 17, 2024..
The COVID-19 pandemic exacerbated these disparities, which triggered the need to develop innovative ways to recruit and retain participants for clinical studies. Conducting virtual appointments between health care providers and patients was fundamental to providing continuous care and accelerated the transition to decentralized trials (889)Underhill C, et al. (2024) JAMA Oncol, 10: 526. DOI: 10.1001/jamaoncol.2023.6565.. Decentralizing clinical trial operations allows trial participation from home, which can be implemented using digital health technologies, including wearable devices, mobile health apps, and platforms for telemedicine.
FDA has taken additional steps to support decentralized clinical trials (DCTs) and in May 2023 released draft guidance providing recommendations for their use. In the guidance, FDA provides design considerations for DCTs, conduct of remote clinical trial visits, and the use of digital health technologies for remote data collection (890)US Food & Drug Administration. Decentralized Clinical Trials for Drugs, Biological Products, and Devices. Guidance for Industry, Investigators, and Other Stakeholders. Accessed: July 12, 2024.. Another key element in the guidance focuses on the trial sponsor’s responsibility to include diverse groups in their study populations—a longstanding commitment of the agency. FDA has developed numerous patient-centered initiatives, including Project Equity, which aims to improve access to cancer clinical trials for historically underrepresented populations, and Project Silver, which focuses on increasing representation of older adults in cancer research (891)US Food & Drug Administration. OCE Programs and Projects Overview. Accessed: July 12, 2024.. In addition, FDA has issued multiple guidance documents offering recommendations that may bolster diversity within cancer clinical studies. Most recently, FDA issued guidance on Diversity Action Plans in June 2024 aimed at enhancing the recruitment of participants from underrepresented populations in clinical studies. This new guidance replaces FDA’s previous draft from April 2022, fulfilling a mandate under the Food and Drug Omnibus Reform Act of 2022 (FDORA) to outline the structure and details of Diversity Action Plans (892)US Food & Drug Administration. Diversity Action Plans to Improve Enrollment of Participants from Underrepresented Populations in Clinical Studies Guidance for Industry. Accessed: July 12, 2024.(893)US Food and Drug Administration. Diversity Plans to Improve Enrollment of Participants from Underrepresented Racial and Ethnic Populations in Clinical Trials Guidance for Industry. Accessed: July 5, 2023.. The plan recommends inclusion of underrepresented populations that reflect different races and ethnicities, age groups, sexes at birth, genders, socioeconomic statuses, disabilities, pregnancy and lactation statuses, and comorbidities. When submitting the plans, clinical study sponsors should include enrollment goals for diverse participation and the rationale for selecting those goals; a plan of action to enroll and retain diverse participants; and the status of meeting enrollment goals throughout the duration of the study.
Narrow eligibility criteria may also limit patient access to clinical studies, which can result in study populations that inadequately reflect real-world patients. Historically, cancer clinical trials have employed restrictive eligibility criteria to define the study population to minimize risk to participants (894)Kim ES, et al. (2021) Clin Cancer Res, 27: 2394.. To address this issue, FDA launched Project Pragmatica, which aims to encourage simple cancer clinical studies that incorporate pragmatic design elements, including fewer eligibility criteria. The agency also released three draft guidance documents in April 2024 that provide recommendations for broadening eligibility criteria to increase participation and diversity in cancer clinical trials (895)US Food & Drug Administration. Cancer Clinical Trial Eligibility Criteria: Washout Periods and Concomitant Medications Guidance for Industry, IRBs, and Clinical Investigators. Accessed: July 12, 2024.(896)US Food & Drug Administration. Cancer Clinical Trial Eligibility Criteria: Laboratory Values Guidance for Industry, IRBs, and Clinical Investigators. Accessed: July 12, 2024.(897)US Food & Drug Administration. Cancer Clinical Trial Eligibility Criteria: Performance Status Guidance for Industry, IRBs, and Clinical Investigators. Accessed: July 12, 2024.. The draft guidance documents focus on three areas:
- appropriate use of therapeutic washout periods and concomitant medication exclusions;
- laboratory values to describe their appropriate use in determining eligibility to participate; and
- broadening performance status to achieve greater generalizability of results.
FDA continues to demonstrate its commitment to modernizing clinical trials. While some progress has been made, more work is needed holistically to enhance the clinical trial infrastructure. Developing meaningful solutions to increase participation in clinical trials is essential for advancing medical knowledge, improving patient outcomes, and ensuring equitable access to cutting-edge treatments for all individuals affected by cancer.
Rapidly Delivering Safe and Effective Therapies to Patients
Thanks to incredible advances in cancer treatment, many patients with common cancer types are living longer and fuller lives following diagnosis. Continued progress against cancer requires researchers to innovate how they measure whether a novel therapy is safe and effective in a timely manner. To this end, there has been an increasing shift from using the gold standard endpoint of overall survival (OS) (see Sidebar 28), to earlier endpoints like progression free survival, overall response rate, and duration of response to achieve an accelerated approval designation from FDA. As one example, at a recent Oncologic Drugs Advisory Committee of FDA, the committee unanimously voted in favor of using minimal residual disease (MRD) negativity as an early endpoint to support accelerated approval for multiple myeloma (898)The Cancer Letter. ODAC Unanimously Upholds MRD As Early Endpoint Across All Settings in Multiple Myeloma. Accessed: July 12, 2024..
Unfortunately, early endpoints do not always correlate with OS, which causes uncertainty about the benefit of new drugs (899)Merino M, et al. (2023) J Clin Oncol, 41: 2706. DOI: 10.1200/Jco.23.00225.. To help advance novel early endpoints and improve the quality of benefit-risk analyses, FDA launched Project Endpoint in 2022. A recent article related to Project Endpoint detailed many considerations and new statistical methods to improve the use of limited OS data to evaluate for indications of harm (900)Rodriguez LR, et al. (2024) Clin Cancer Res.. Most importantly, the article encourages trial sponsors to plan ahead to collect and analyze all OS data for every late-stage trial.
One likely cause of the discrepancy between early endpoints and OS is that a new drug may be effective at shrinking a tumor but may also cause severe and delayed toxicities. Traditionally, doses of a cancer drug were determined by escalating the dose until the highest dose of the drug with acceptable side effects, called the maximum tolerated dose, was found. In the era of precision medicine and immunotherapies, increasing doses may not result in better efficacy, but often results in worse side effects (901)Fourie Zirkelbach J, et al. (2022) J Clin Oncol, 40: 3489. DOI: 10.1200/JCO.22.00371.. To encourage selection of optimal doses of new drugs, FDA issued a draft guidance, titled Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases, in January 2023 (902)US Food and Drug Administration. Optimizing the Dosage of Human Prescription Drugs and Biological Products for the Treatment of Oncologic Diseases. Accessed: July 5, 2023.. Additionally, two FDA-AACR workshops in 2024— Optimizing Dosages for Oncology Drug Products and How Much Is Enough? Trial Designs for Treatment Regimens with Multiple Phases—convened experts from academia, industry, government, and patient organizations to discuss improving dosage selection (903)American Association for Cancer Research. FDA-AACR Workshop: How Much is Enough? Trial Designs for Treatment Regimens with Multiple Phases. Accessed: July 12, 2024.(904)American Association for Cancer Research. FDA-AACR Public Workshop: Optimizing Dosages for Oncology Drug Products. Accessed: July 12, 2024..
Addressing Cancer Drug Shortages
Many cancer drugs are in short supply, and there has recently been momentum to address cancer drug shortages (905)National Comprehensive Cancer Network. New Survey from NCCN Finds Cancer Drug Shortage Management Remains a Moving Target, Impacting Clinical Trials. Accessed: July 31, 2024.. FDA plays a crucial role in addressing and managing drug shortages, including those affecting cancer treatments. Despite the agency’s efforts, cancer drug shortages have reached a record high with approximately 16 commonly used cancer drugs in limited supply (906)Medscape. ‘Nothing Rivaled This’: Navigating the Cancer Drug Shortage. Accessed: July 12, 2024.. Increased demand and limited supply along with manufacturing capacity and market dynamics are among the major factors contributing to cancer drug shortages (907)Breastcancer.org. Chemotherapy Drug Shortage: What You Need to Know. Accessed: July 12.. Ten percent of people living with cancer have been adversely impacted by the shortage, with the majority citing treatment delays and difficulty finding alternative therapies (908)American Cancer Society Cancer Action Network. Survivor Views: Drug Shortages, Telehealth, & Biomarker Testing. Accessed: July 31, 2024.. FDA continually provides updated drug shortage information and is working closely with drug manufacturers to reduce the impact of shortages, mitigate supply disruption and develop preventative methods to avoid cancer drug shortages (909)US Food & Drug Administration. Drug Shortages. Accessed: July 12, 2024.. The agency’s efforts have increased the US supply of cisplatin, a widely used cancer chemotherapeutic, to approximately its pre-shortage levels (910)The White House. Strengthening the supply chain for cancer drugs. Accessed: July 12, 2024..
In addition to FDA’s work, the US Senate Finance Committee proposed a voluntary Medicare Drug Shortage Prevention and Mitigation Program in May 2024 that aims to encourage transparent purchasing practices across supply chains and drug manufacturers (911)United States Senate Committee on Finance. Senate Finance Committee Discussion Draft: Preventing & Mitigating Generic Drug Shortages. Accessed: July 12, 2024.. The Centers for Medicare & Medicaid Services (CMS) has also proposed and implemented several policies to help address drug shortages (see Sidebar 49). Addressing cancer drug shortages remains a critical issue that will require a multifaceted approach involving health care providers, pharmaceutical companies, policymakers, and regulatory bodies to develop and implement solutions that can restore the limited supply of cancer therapies.
Advancing Policies to Strengthen Cancer Prevention and Screening Programs
Nearly 40 percent of cancer cases in the United States can be attributed to preventable risk factors, such as tobacco use, dietary factors, and ultraviolet (UV) exposure (see Reducing the Risk of Cancer Development). Research has shown that routine screening using evidence-based approaches to detect common cancers and cancer warning signs is essential for improving treatment options and chances of survival (see Screening for Early Detection). However, inequities in access to screenings and follow-up care for numerous populations contribute to delayed diagnoses and lower chances of survival. Innovative investments in inclusive screening practices, from both health care providers and policymakers, are necessary to improve overall prevention and survival rates. Further expansion of health insurance through Medicaid expansion and growth of the Patient Care and Affordable Care Act (ACA) marketplace plans will also improve access to cancer screening and preventive services.
Human papillomavirus (HPV) can cause several cancers, including nearly all cases of cervical cancer (see Prevent and Eliminate Infection From Cancer-causing Pathogens). There are effective strategies to prevent HPV infection and its associated cancers, including HPV vaccination, timely screening, and follow-up care. HPV vaccination rates have increased among adolescents, though there is still more to be done to reach the Healthy People 2030 goal of an 80 percent vaccination rate among adolescents (914)Pingali C, et al. (2022) MMWR Morb Mortal Wkly Rep, 71: 1101. DOI: 10.15585/mmwr.mm7135a1.. In May 2024, FDA approved the use of cervical self-sampling by patients, an important step in expanding access to cervical cancer screening (915)US Food & Drug Administration. FDA Roundup: May 17, 2024. Accessed: July 12, 2024..
For health care providers, addressing the needs of patients in rural areas, where screening rates are lower than in urban environments (916)National Cancer Institute. Rural-Urban Disparities in Cancer. Accessed: July 12, 2024., can require adapting to the limitations faced by these populations. Having lower insurance rates, increased distance from health care centers, and lack of public transportation options are all factors faced by women in these areas, resulting in lower breast, cervical, and colorectal cancer screenings (917)Champion VL, et al. (2023) JAMA Netw Open, 6: e2311004. DOI: 10.1001/jamanetworkopen.2023.11004.. However, tools like simultaneous screenings and increased patient navigation services are one way to increase cancer screening for rural populations (917)Champion VL, et al. (2023) JAMA Netw Open, 6: e2311004. DOI: 10.1001/jamanetworkopen.2023.11004..
CDC Screening Programs
The Centers for Disease Control and Prevention (CDC) is a key federal public health agency that works to increase access to cancer screenings; both CDC programs and CDC-sponsored external organizations work to address cancer screening disparities. Continued investments in programs like CDC’s NBCCEDP and support for bills like S.1840 – Screening for Communities to Receive Early and Equitable Needed Services for Cancer Act of 2023, introduced by Senator Collins (R-ME) and Senator Baldwin (D-WI) (918)Congress.gov. Text – S.1840 – 118th Congress (2023-2024): Screening for Communities to Receive Early and Equitable Needed Services for Cancer Act of 2023. Accessed: July 12, 2024., are necessary tools to increase overall screening rates.
NBCCEDP also does targeted outreach to medically underserved populations, including creating flyers about breast cancer screening for Amish populations and caregiver kits and modified booklets for women with learning disabilities (919)Centers for Disease Control and Prevention. Meeting the Needs of Special Populations. Accessed: July 12, 2024.. CDC has also created educational materials for populations with a higher rate of breast cancer. This includes the Bring Your Brave campaign, informing Ashkenazi Jewish women about their increased risk of breast cancer due to a higher prevalence of BRCA gene mutations in this population (920)Centers for Disease Control and Prevention. Jewish Women and BRCA Gene Mutations. Bring Your Brave Campaign. Accessed: July 12, 2024..
EPA Cancer Moonshot Programs
Agencies like EPA work to assess exposures to carcinogens in the environment and to reduce cancer risks. Within EPA, offices like the Office of Air and Radiation (OAR) create policies that reduce exposure to cancer-causing pollutants (921)US Environmental Protection Agency. EPA Efforts to Reduce Exposure to Carcinogens and Prevent Cancer. Accessed: March 17, 2024.. Initiatives like the Clean School Bus Program (922)US Environmental Protection Agency. Clean School Bus Program. Accessed: July 12, 2024. that have allocated funds to replace school buses with zero-emission and low-emission updated models, are not only saving funds with reduced fuel costs, but also lowering overall pollutant emission exposures in children. EPA is also responsible for emergency responses to hazardous events, including oil spills, radiologic releases, and large-scale national emergencies (923)US Environmental Protection Agency. Emergency Response. Accessed: July 12, 2024.. Work by the agency to keep cancer-causing agents, like arsenic, benzene, perchloroethylene (PCE), and trichloroethylene (TCE) out of public land and drinking water is essential for overall public health and reduction of cancer rates (921)US Environmental Protection Agency. EPA Efforts to Reduce Exposure to Carcinogens and Prevent Cancer. Accessed: March 17, 2024..
Leveraging Policy to Reduce Tobacco-related Illness
Effective tobacco control policies and awareness campaigns have led to historically low smoking rates in the United States. In 2021, 18.7 percent of US adults regularly used any tobacco product (49)Cornelius ME, et al. (2023) MMWR Morb Mortal Wkly Rep, 72: 475. DOI: 10.15585/mmwr.mm7218a1., and 11.5 percent of adults regularly smoked cigarettes. Progress is also evident with tobacco use among middle and high school students. At the peak of the e-cigarette epidemic in 2019, 31 percent of high school students and 12.5 percent of middle school students regularly used any tobacco product (924)Wang TW, et al. (2019) MMWR Surveill Summ, 68: 1. DOI: 10.15585/mmwr.ss6812a1.. In 2023, those rates were reduced by about half to 12.6 percent and 6.6 percent, respectively, the vast majority of which were illicit flavored e-cigarettes (185)Birdsey J, et al. (2023) MMWR Morb Mortal Wkly Rep, 72: 1173. DOI: 10.15585/mmwr.mm7244a1.. Advancing new tobacco control policies is necessary to continue progress on reducing tobacco-related cancers.
Flavors increase the addictiveness and appeal of tobacco products, particularly for youth. Menthol cigarettes alone were estimated to have caused 378,000 premature deaths in the United States between 1980 and 2018, disproportionately among Black adults (925)Le TT, et al. (2021) Tob Control, 00: tobaccocontrol. DOI: 10.1136/tobaccocontrol-2020-056256.. In 2022, FDA proposed draft regulations that would prohibit menthol cigarettes and flavored cigars (926)US Food & Drug Administration. FDA Proposes Rules Prohibiting Menthol Cigarettes and Flavored Cigars to Prevent Youth Initiation, Significantly Reduce Tobacco-Related Disease and Death. Accessed: July 12, 2024.. Unfortunately, HHS Secretary Becerra announced these regulations would be delayed for an indefinite time (927)US Department of Health and Human Services. Secretary Becerra Statement on the Proposed Menthol Cigarette Rule. Accessed: July 12, 2024.. E-cigarettes and other novel tobacco products require proactive authorization from FDA prior to being sold legally in stores. While there are notable examples of fines and seizures of illegal products totaling millions of dollars, these efforts have not yet substantially impacted the multibillion-dollar illicit market (928)US Food & Drug Administration. Joint Federal Operation Results in Seizure of More Than $18 Million in Illegal E-Cigarettes. Accessed: July 12, 2024.(929)US Food & Drug Administration. FDA Seeks $20K+ Fines Against Retailers Selling Unauthorized Youth-Appealing E-Cigarettes. Accessed: July 12, 2024.. In June 2024, FDA announced the formation of a new multi-agency task force to improve enforcement against distributors and importers of these illegal products (930)US Food & Drug Administration. Justice Department and FDA Announce Federal Multi-Agency Task Force to Curb the Distribution and Sale of Illegal E-Cigarettes. Accessed: July 12, 2024..
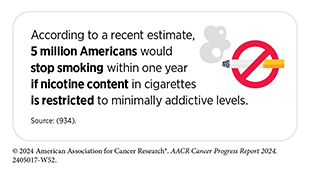
Additionally, FDA has expressed interest in proposing a regulation to limit nicotine to minimally addictive concentrations in combustible tobacco products (931)The New York Times. F.D.A. Aims to Cut Down on Smoking by Slashing Nicotine Levels in Cigarettes. Accessed: July 12, 2024.(932)Federal Register. Tobacco Product Standard for Nicotine Level of Combusted Cigarettes. Accessed: July 12, 2024.. Several high-quality clinical trials have demonstrated that reducing nicotine levels by 95 percent in cigarettes significantly increases smoking cessation attempts and decreases the number of cigarettes smoked by trial participants (933)Donny EC, et al. (2022) Int J Drug Policy, 99: 103436. DOI: 10.1016/j.drugpo.2021.103436..
Reducing the addictiveness of tobacco products by prohibiting flavors and minimizing nicotine concentrations would save millions of lives in the coming decades and help achieve the goals of the Cancer Moonshot. Additional policies that could reduce tobacco-related illness include improved insurance coverage of evidence-based smoking cessation therapies; further restrictions on tobacco product advertising and promotions; and increased funding for FDA, NCI, and CDC smoking awareness and cessation programs.

Accelerating Progress Against Childhood Cancer
From 2015 to 2019, the overall cancer death rate for children ages 0 to 14 years decreased by 1.5 percent per year due to several factors including improved treatments, increased clinical trial participation, and earlier detection (935)Cronin KA, et al. (2022) Cancer, 128: 4251. DOI: 10.1002/cncr.34479.. However, continued investment in childhood cancer research remains crucial as NCI estimates that 14,910 children and adolescents ages 0 to 19 years will be diagnosed with cancer in 2024 (936)National Cancer Institute. Cancer Among Adolescents and Young Adults (AYAs) – Cancer Stat Facts. Accessed: Jul 5, 2023..
Childhood cancers are considered “rare diseases,” making clinical trials more difficult to complete and limiting the incentive for drug sponsors to invest in approvals for this vulnerable patient population. In some cases, drugs are studied and approved in adults, then data from the trials are extrapolated to determine appropriate usage for childhood cancers. To enhance incentives for pharmaceutical companies to develop new drugs for childhood cancers, Congress passed the Pediatric Research Equity Act (PREA) in 2003, which authorizes FDA to require studies with children for therapies developed for adults. However, certain exemptions under PREA allowed sponsors to avoid conducting these studies. The Research to Accelerate Cures and Equity (RACE) for Children Act, passed in 2017 and implemented in 2020, was designed to eliminate these exemptions and allow FDA to require sponsors to study the effectiveness of drugs in children when the molecular target of their drug is relevant. Early results from a report by the US Government Accountability Office analyzing the effectiveness of the RACE Act indicate an increased number of planned studies to test certain cancer therapeutics used in adults in children with cancer, though it is still too soon to know if these efforts will result in an increase in drug approval for childhood cancers (937)US Government Accountability Office. Pediatric Cancer Studies: Early Results of the Research to Accelerate Cures and Equity for Children Act. Accessed: July 12, 2024..
Combination therapies have been shown to be incredibly effective in treating many cancers, as they help target the variability of cancer cells in a tumor. Unfortunately, combination therapies were not explicitly outlined in the PREA or the RACE Act. In May 2024, the House Energy & Commerce Health Subcommittee advanced the Give Kids a Chance Act that would authorize FDA to direct companies to study targeted combinations of cancer therapies in pediatric trials, should it become law (938)Energy and Commerce. Health Subcommittee Markup Recap: E&C Advances Legislation to Strengthen America’s Health Care System. Accessed: July 12, 2024..
Advances in cancer therapy rely on basic scientific understanding, and there is still much to be understood about the underlying mechanisms that lead to childhood cancer. To address this, Congress implemented the Gabriella Miller Kids First Pediatric Research Program (Kids First) in 2015. This program at NIH has two primary initiatives: identifying children with childhood cancer and birth defects, and their families, for whole genome sequencing; and developing a database of clinical and genetic data from patients with childhood cancers to help lead to the discovery of new implicated genetic pathways (939)National Institutes of Health. Gabriella Miller Kids First Pediatric Research (Kids First). Accessed: July 5, 2023.. Gabriella Miller Kids First Research Act 2.0 has been introduced in both the House and the Senate in the 118th Congress, demonstrating continued support for this important initiative. As of June 30, 2024, the bill has passed the House but has not yet passed the Senate.
The Childhood Cancer STAR Reauthorization Act was signed into law in January 2023, reauthorizing the program for an additional 5 years at its fully authorized level of $30 million. This legislation aims to enhance research on the late effects of childhood cancers and establishes a new pilot program to begin to explore innovative models of care for childhood cancer survivors. This reauthorization also supports the Childhood Cancer Data Initiative (CCDI) at $50 million. CCDI is an effort to collect, share, and analyze clinical care and research data on childhood cancers to increase our understanding of childhood cancer and subsequent survivorship. The CCDI has three foundational goals (940)National Cancer Institute. Childhood Cancer Data Initiative (CCDI). Accessed: July 12, 2024.:
- gather data from every child, adolescent, and young adult diagnosed with a childhood cancer, regardless of where they receive their care;
- create a national strategy of appropriate clinical and molecular characterization to speed diagnosis and inform treatment for all types of childhood cancers; and
- develop a platform and tools to bring together clinical care and research data that will improve preventive measures, treatment, quality of life, and survivorship for childhood cancers.
Of note, the Childhood Cancer STAR Reauthorization Act only provides legal authorization for these programs to continue but Congress must fully fund these efforts during the FY 2025 appropriations process.
On April 1, 2023, emergency coverage protections for Medicaid enrollees afforded during the pandemic ended, with the federal government allowing state Medicaid agencies up to 14 months to redetermine the eligibility of enrollees, threatening the coverage of 6.7 million children (941)Georgetown University Health Policy Initiative. Millions of Children May Lose Medicaid: What Can Be Done to Help Prevent Them From Becoming Uninsured? Accessed: July 5, 2023.. Earlier this year, CMS released a final rule regarding the simplification of the eligibility and enrollment processes for Medicaid, the Children’s Health Insurance Program (CHIP), and the Basic Health Program (BHP) (942)Centers for Medicare & Medicaid Services. Streamlining the Medicaid, Children’s Health Insurance Program, and Basic Health Program Application, Eligibility Determination, Enrollment, and Renewal Processes Final Rule Fact Sheet. Accessed: July 12, 2024.. This final regulation simplifies the process for eligible people to enroll and stay enrolled in CHIP coverage, keeping eligible individuals, including children, covered and ensuring equitable access to coverage.
Addressing Cancer Disparities and Improving Patient Outcomes
As described in the AACR Cancer Disparities Progress Report 2024 (29)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2024. Accessed: June 14, 2024., cancer and other health disparities are driven by a complex set of interrelated causes, including social, economic, and environmental factors; collectively, these factors are called social determinants or social drivers of health (SDOH) (see Figure 3) (39)Warnecke RB, et al. (2008) Am J Public Health, 98: 1608. DOI: 10.2105/AJPH.2006.102525.(40)Asare M, et al. (2017) Oncol Nurs Forum, 44: 20. DOI: 10.1188/17.ONF.20-23.. Policies that were designed to discriminate against racial and ethnic minority communities, commonly known as systemic or structural racism, continue to produce and reinforce the negative impact of SDOH, and in turn, exacerbate cancer disparities and other health, economic, and social disparities (29)American Association for Cancer Research. AACR Cancer Disparities Progress Report 2024. Accessed: June 14, 2024.. Addressing the negative impact of SDOH is essential for achieving health equity and will continue to require multifaceted solutions, including new policies at the federal, state, and local levels, as well as collaboration between policymakers, health care providers, and patients.
At the federal level, agencies across the government have diverse programs seeking to further understand and improve health equity. For example, the National Institute on Minority Health and Health Disparities (NIMHD) within NIH is furthering health equity research and practice, including efforts to reduce cancer disparities (943)National Institute of Minority Health and Health Disparities. Research Interest Areas. Accessed: March 17, 2024.. The CCHE within NCI also has a significant portfolio of programs to conduct research on cancer disparities, broaden opportunities for scientific training, and pilot new initiatives to improve cancer screening (944)National Cancer Institute. NCI Center to Reduce Cancer Health Disparities. Accessed: March 17, 2024..
CDC, as the nation’s frontline public health agency, has many cancer prevention programs, including efforts to reduce cancer disparities. The CDC’s Division of Cancer Prevention and Control provides funding and partners with state and local governments and other organizations to broaden access to screening and other health care services. These actions include the use of tools like mobile mammography vans at worksites and culturally tailored care (839)Centers for Disease Control and Prevention. About the Division of Cancer Prevention and Control | National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP). Accessed: July 12, 2024.(945)Centers for Disease Control and Prevention. What CDC Is Doing to Achieve Equity in Cancer Control. Accessed: March 17, 2024.(946)Centers for Disease Control and Prevention. Offering Flexible Hours and Locations | NBCCEDP. Accessed: July 12, 2024.. CDC has launched its CORE Commitment to Health Equity, managed by the CDC Office of Health Equity, a strategic framework for collaboration across multiple sectors to improve public health for all populations and reduce health disparities (947)Centers for Disease Control and Prevention. CDC’s CORE Commitment to Health Equity. Accessed: March 17, 2024. (see Sidebar 50).
Achieving health equity and eliminating cancer disparities will also require policy interventions beyond medical and public health programs. Many of the structural changes proposed in the Health Equity for All Act (HEAA), including expansion of Medicaid coverage, would facilitate access to care for underserved populations across the United States (948)Congress.gov. H.R.7585 – 117th Congress (2021-2022): Health Equity and Accountability Act of 2022. Accessed: July 12, 2024.. Expanding insurance coverage for cancer-related services, including coverage for comprehensive tobacco cessation programs, is critical. The White House recently announced steps to expand coverage for cancer patient navigation services (949)The White House. FACT SHEET: Biden Cancer Moonshot Announces Commitments from Leading Health Insurers and Oncology Providers to Make Navigation Services Accessible to More than 150 Million Americans. Accessed: July 12, 2024..
Inflation Reduction Act
Policymakers recognize the vital role health systems have in supporting vulnerable populations and have introduced several pieces of legislation seeking to protect innovation for these groups. On August 16, 2022, President Biden signed the Inflation Reduction Act into law – a significant piece of legislation that aims to reduce the federal government budget deficit, lower prescription drug prices, and invest in domestic energy production while promoting clean energy.
Patients with cancer are currently benefiting from specific provisions in the law, most notably the cap that is now in place on out-of-pocket prescription drug costs for older adults. Medicare beneficiaries are receiving better financial protection through this provision that is capping out-of-pocket costs for prescription drugs at $2,000 annually. Additionally, the law is making health coverage more affordable for 13 million people because it extends enhanced ACA marketplace subsidies through 2025.
However, with all major legislation, Congress has a responsibility to continually examine the provisions and identify areas where adjustments are needed. One such example involves the Optimizing Research Progress Hope and New (ORPHAN) Cures Act, which is a proposed bill that would amend the Inflation Reduction Act to ensure medicines that treat one or more rare diseases are excluded from Medicare price negotiations. The bipartisan legislation aims to safeguard existing incentives and advance the development of innovative therapies for the 30 million Americans affected by rare diseases, including 200,000 people living with a rare form of cancer.
Currently, under the Inflation Reduction Act, only orphan drugs that treat a single rare disease would be excluded from price negotiation. The provision could unintentionally discourage manufacturers from seeking additional indications, limiting treatment options for people with rare diseases. About one in five FDA-approve orphan drugs are also approved to treat additional diseases.
Exempting rare disease treatments with multiple approved uses from price negotiation, on the other hand, can help encourage manufacturers to continue investing in groundbreaking treatments for people living with rare diseases.
Environmental Racism and Environmental Justice
Environmental racism can be defined as environmental harm inflicted due to systemic racism (950)Inside Climate News. In Louisiana’s ‘Cancer Alley,’ Excitement Over New Emissions Rules Is Tempered By a Legal Challenge to Federal Environmental Justice Efforts – Inside Climate News. Accessed: July 12, 2024.. Conversely, environmental justice refers to efforts to ensure that populations are not subjected to disproportionate environmental harms, and that all people have equitable access to a sustainable and healthy environment. Racial and ethnic minorities have unjustly been disproportionately harmed by pollutants, including carcinogens (951)Collins MB, et al. (2016) Environ Res Lett, 11: 015004. DOI: 10.1088/1748-9326/11/1/015004.(952)Johnston J, et al. (2020) Curr Environ Health Rep, 7: 48. DOI: 10.1007/s40572-020-00263-8.. A glaring example of this is an 85-mile stretch of the Mississippi River from Baton Rouge to New Orleans known as “Cancer Alley” (953)Keele University. Land of the Free? Environmental Racism and Its Impact on Cancer Alley, Louisiana. Accessed:. Roughly 25 percent of the nation’s petrochemical production is located in this area, and the region has a larger share of Black residents than state or national averages (954)James W, et al. (2012) Int J Environ Res Public Health, 9: 4365. DOI: 10.3390/ijerph9124365.. EPA has called out Louisiana policymakers for neglecting the health needs of Black residents and being complicit in environmental racism (955)ProPublica. EPA Calls Out Environmental Racism in Louisiana’s Cancer Alley. Accessed: July 12, 2024., and the region has been called a “failure of state and federal authorities to properly regulate the [fossil fuel] industry” (956)Human Rights Watch. US: Louisiana’s ‘Cancer Alley’. Dire Health Crisis From Government Failure to Rein in Fossil Fuels. Accessed: July 12, 2024..
Environmental and grassroot activists, as well as EPA, play important roles in advocating for environmental protection and educating residents about the health ramifications of living near waste zones. EPA has a range of programs focused on environmental justice to help ensure that all people have access to a healthy and sustainable environment and are protected from disproportionate environmental harms(957)US Environmental Protection Agency. Environmental Justice. Accessed: March 17, 2024.. For communities living with high air carcinogens, tools like EPA’s air monitoring systems (958)US Environmental Protection Agency. Air Data: Air Quality Data Collected at Outdoor Monitors Across the US. Accessed: July 12, 2024. provide advocates and government officials with data important for regulatory and legal action against polluters. EPA recently issued stricter air pollution rules on chemical plans to address the severe health threats in locations like Cancer Alley (959)US Environmental Protection Agency. Biden-Harris Administration Finalizes Stronger Clean Air Standards for Chemical Plants, Lowering Cancer Risk and Advancing Environmental Justice. Accessed: July 12, 2024., and Congress has proposed comprehensive legislation to holistically expand environmental justice (960)Senate.gov. Booker, Grijalva, Lee, Duckworth Introduce the A. Donald McEachin Environmental Justice for All Act | U.S. Senator Cory Booker of New Jersey. Accessed: July 12, 2024..
FDA also has an important role in regulating exposures to carcinogens through its consumer protection activities. In 2022, Congress passed the Modernization of Cosmetics Regulation Act of 2022 (MoCRA). This law expands FDA’s existing authorities to regulate cosmetic products, including the power to recall cosmetic products in the event that they cause harm to the public (961)US Food & Drug Administration. Modernization of Cosmetics Regulation Act of 2022 (MoCRA). Accessed: July 12, 2024..
Improving the Use of Digital Information in Cancer Treatment and Management
To improve the delivery of care to patients and improve outcomes for survivors, it is critically important to expand the use of digital health information, including making health registries more robust, timely, and inclusive of information beyond what they currently collect. There is a concomitant need to standardize the collection, security, and dissemination of this information for patients, caregivers, clinicians, and researchers. Although there has been progress in the expansion of electronic health records and telemedicine, especially in the wake of the COVID-19 pandemic, there is much work to be done to maximize the benefits of digital healthcare technologies in oncology (962)Parikh RB, et al. (2022) J Natl Cancer Inst, 114: 1338. DOI: 10.1093/jnci/djac108.. For example, patient information is often stored across multiple databases, depending on the systems used by a patient’s health care providers, pharmacies, and insurers. Tracking patient data, including patient reported outcomes, is therefore incredibly difficult, hampering effective care (963)Bradley CJ, et al. (2022) J Natl Cancer Inst, 114: 1065. DOI: 10.1093/jnci/djac086.. There are also significant shortcomings in the collection and systematic organization of different types of relevant patient data, including the patient’s race and ethnicity, sexual orientation and gender identity, patient reported outcomes of cancer therapy, and diagnostic results (962)Parikh RB, et al. (2022) J Natl Cancer Inst, 114: 1338. DOI: 10.1093/jnci/djac108.(963)Bradley CJ, et al. (2022) J Natl Cancer Inst, 114: 1065. DOI: 10.1093/jnci/djac086..
Several policy interventions at the state and federal level (as well as actions by industry and health care providers) could help to advance the use of digital technologies in the treatment and management of cancer, while at the same time safeguarding protected health information. These include new rules and guidelines on data standardization and interoperability, the security and privacy of digital health data, new funding/reimbursement mechanisms for digital health technologies, and efforts to reduce bias and promote equity in data collection (962)Parikh RB, et al. (2022) J Natl Cancer Inst, 114: 1338. DOI: 10.1093/jnci/djac108.. The federal government has begun to take steps along these lines, for example, issuing new CMS rules on digital health care information interoperability, patient access, and security (964)Federal Register. Medicare and Medicaid Programs; Patient Protection and Affordable Care Act; Interoperability and Patient Access for Medicare Advantage Organization and Medicaid Managed Care Plans, State Medicaid Agencies, CHIP Agencies and CHIP Managed Care Entities, Issuers of Qualified Health Plans on the Federally-Facilitated Exchanges, and Health Care Providers. Accessed: Jul 12, 2024.(965)Federal Register. Medicare and Medicaid Programs; Patient Protection and Affordable Care Act; Advancing Interoperability and Improving Prior Authorization Processes for Medicare Advantage Organizations, Medicaid Managed Care Plans, State Medicaid Agencies, Children’s Health Insurance Program (CHIP) Agencies and CHIP Managed Care Entities, Issuers of Qualified Health Plans on the Federally-Facilitated Exchanges, Merit-Based Incentive Payment System (MIPS) Eligible Clinicians, and Eligible Hospitals and Critical Access Hospitals in the Medicare Promoting Interoperability Program. Accessed: July 12, 2024..
Next Section: Conclusion Previous Section: Envisioning the Future of Cancer Science and Medicine