Executive Summary
This is an exciting time in cancer science and medicine. Transformative research and technological innovations are driving unprecedented progress against the collection of diseases we call cancer. As the first and largest professional organization in the world dedicated to advancing all areas of cancer research and patient care, AACR has been and continues to be a catalyst for scientific breakthroughs that save and enhance the lives of cancer patients. It is also committed to increasing public understanding of cancer and advocating for increased federal funding for cancer research and related sciences.
The annual AACR Cancer Progress Report to Congress and the American public is a cornerstone of AACR’s educational efforts. This twelfth edition of the report highlights how research continues to extend and improve lives, including the lives of the five courageous individuals featured in the report and their family members who have shared their experiences with cancer. It also underscores how unwavering, bipartisan support from Congress, in the form of robust and sustained annual increases in funding for NIH, NCI, CDC, and FDA, is urgently needed if we are to realize our vision of eradicating cancer for all populations.
Cancer in 2022
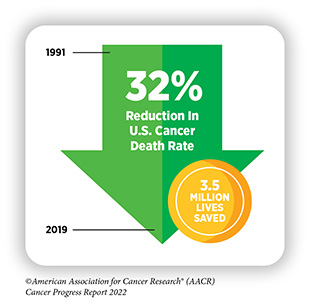
The remarkable progress being made against cancer—in particular, improvements in reducing smoking rates and developments in early detection and treatment—is resulting in a steady fall in cancer death rates, and a consistent rise in the number of people who live longer after a cancer diagnosis. In fact, in the United States, the age-adjusted overall cancer death rate has been declining since the 1990s, with the reductions between 1991 and 2019 translating into nearly 3.5 million cancer deaths avoided. Additionally, the number of cancer survivors living in the United States has exceeded 18 million as of January 1, 2022.
Unfortunately, certain U.S. populations, including racial and ethnic minorities and several other medically underserved groups, have not benefited equally from the advances against cancer. Complex and interrelated factors referred to as social determinants of health have contributed to cancer health disparities in the United States. It is imperative that all stakeholders work together to eradicate the systemic and structural injustices that are barriers to health equity.
Cancer exacts an immense toll because of the number of lives it affects each year and its significant economic impact. The direct medical costs of cancer care are one measure of the financial impact of cancer, and in the United States alone they were estimated to be $183 billion in 2015, the last year for which these data are currently available; this cost is projected to increase to $246 billion by 2030. The burden of cancer and its economic toll, both on individuals and the U.S. health care system, are expected to rise in the coming decades, highlighting the urgent need for more research to accelerate the pace of progress against cancer.
Understanding How Cancer Develops
Discoveries across the spectrum of cancer research, from basic to translational, clinical, and population science, have led to our current understanding of how cancer develops. We now understand that cancer is a collection of diseases characterized by the inability of a cell to respond to normal biological cues that regulate processes including cell division, identity, and life span. This happens primarily through alterations in the genetic material of normal cells. The identity of genetic alterations and the order and speed at which a cell acquires them determine the length of time it takes a particular cancer to develop.
Inherited mutations play a role in about 10 percent of cancer cases, but most cancers are caused by mutations acquired over an individual’s lifetime. Some mutations are acquired during normal cell division; others are acquired because of persistent exposure to substances that damage genetic material, such as carcinogens in tobacco smoke and ultraviolet (UV) radiation from the sun among other cancer risk factors; and yet other mutations are associated with underlying medical conditions, such as chronic inflammation.
Although genetic alterations underpin cancer initiation and progression in most cases, interactions between cancer cells and their environment, known as the tumor microenvironment, play an important role in disease progression.
Preventing Cancer: Identifying Risk Factors
Decades of research have led to the identification of numerous factors that increase a person’s risk of developing cancer. Given that exposure to many of these factors can be eliminated or reduced, many cases of cancer can be prevented. In fact, it is estimated that about 40 percent of cancer cases in the United States are attributable to preventable causes.
The main preventable causes of cancer are tobacco use, obesity, poor diet, lack of physical activity, alcohol consumption, exposure to UV light from the sun or tanning devices, and failure to use interventions that treat or prevent infection with cancer- associated pathogens, such as cancer-causing strains of the human papillomavirus (HPV).
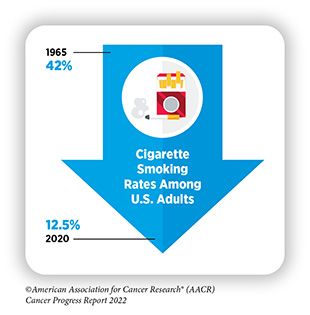
The development and implementation of public education and policy initiatives designed to eliminate or reduce exposure to preventable causes of cancer have reduced cancer incidence, morbidity, and mortality in the United States. Thanks to such initiatives, cigarette smoking rates among U.S. adults have been declining for more than five decades. Unfortunately, the current popularity of electronic cigarettes (e-cigarettes) among U.S. youth and young adults threatens to reverse the significant progress against tobacco use. In addition, the prevalence of obesity, another major risk factor that is linked to 15 types of cancer, continues to rise among U.S. adults and children. These troubling trends have the potential to slow the steady decline in cancer death rates that we have seen in recent years. Therefore, it is essential that all stakeholders work together to enhance the dissemination of our current knowledge of cancer prevention and implement evidence-based policies and programs to minimize the incidence, morbidity, and mortality of cancers attributable to preventable causes.
Screening for Early Detection
The purpose of cancer screening is to determine whether a person has precancerous lesions or cancer before any signs or symptoms of the disease appear, with the overarching goal of reducing the burden of cancer at the population level. The decision of whether an individual should be screened for cancer is determined by several factors including age; whether or not a person has a particular organ; smoking history; an all-negative prior screening history; life expectancy; family history of cancer; and/or race and ethnicity.
Currently, the U.S. Preventive Services Task Force (USPSTF)—an independent, volunteer panel of national experts in disease prevention and evidence-based medicine—has guidelines for five different types of cancer, four of which apply to individuals who are at an average risk of developing breast, colorectal, prostate, or cervical cancer. Guidelines for lung cancer apply to former or current smokers, i.e., individuals who are at a high risk of developing the disease because of tobacco use.
One area of rapid progress is the use of artificial intelligence (AI)-driven software systems for early detection. As just two examples of the advances in this area, during the 12 months covered by this report, FDA approved Lunit INSIGHT MMG to identify breast lesions suspected of being cancerous, and EndoScreener to identify potentially precancerous polyps during a colonoscopy.
Despite the many benefits, uptake of cancer screening among eligible individuals remains suboptimal. Underuse of routine cancer screening, as well as use of screening tests beyond the recommended age is common. There are also disparities among racial and ethnic minorities and medically underserved U.S. populations in adherence to cancer screening guidelines. Stakeholders across the cancer care continuum are working together to educate the public about the importance of cancer screening and to increase adherence to cancer screening in the general population.
Some strategies that have proven effective to achieve this goal include comprehensive public health campaigns; increased access to health insurance; community engagement and culturally tailored interventions; reduced structural barriers; and improved patient–provider communication.
Decoding Cancer Complexity. Integrating Science.
Transforming Patient Outcomes.
Researchers are harnessing the knowledge gleaned from the molecular underpinnings of cancer initiation and progression to develop safer and more effective treatments for cancer. Advances in novel and innovative approaches to surgery, radiotherapy, chemotherapy, molecularly targeted therapy, and immunotherapy—the five pillars of cancer treatment— are saving and improving lives. From August 1, 2021, to July 31, 2022, FDA has approved two imaging agents and eight new anticancer therapeutics, and has expanded the use of 10 previously approved anticancer therapeutics to treat additional cancer types. Many of the approvals are groundbreaking.
In August 2021, FDA approved belzutifan (Welireg) for adults, such as Alexandra Vitale, who have von Hippel- Lindau (VHL) disease, which is a rare and inherited disorder that increases the risk of developing certain types of cancer. In October 2021, FDA approved the molecularly targeted therapeutic, asciminib (Scemblix), to treat patients with chronic myeloid leukemia whose cancer cells carry a specific genetic alteration. In March 2022, FDA approved the first combination of a radiodiagnostic agent and a radiotherapeutic agent to visualize and eradicate prostate cancer.
Another significant leap forward in treating cancers is the approval of an immune checkpoint inhibitor against a novel target, the first such approval in more than eight years. The immunotherapeutic, relatlimab-rmbw (Opdualag), approved in March 2022 to treat melanoma, has already benefited patients, such as Johnny Borgstrom, who has been cancer free for more than two years since his treatment. FDA approval in June 2022 of a combination of two molecularly targeted therapeutics—dabrafenib (Tafinlar) and trametinib (Mekinist)—vastly expands the treatment options for adult and pediatric patients, such as Tyler Richards, who have a solid tumor harboring a specific genetic alteration.
While these exciting new advances have the potential to transform patient care, much work is needed to ensure equitable access to these treatments for all populations.
Supporting Cancer Patients and Survivors
Research-fueled advances in cancer detection, diagnosis, and treatment are helping more people to survive longer and lead fuller lives after a diagnosis of cancer. According to the latest estimates, more than five percent of the U.S. population is living with a history of a cancer diagnosis, equating to more than 18 million people; three out of four U.S. families have at least one member who has experienced a cancer diagnosis. This is in stark contrast to 50 years ago, when cancer survivors constituted only 1.4 percent of the U.S. population. Researchers predict that there will be 26 million survivors in the U.S. by 2040.
Rapid advances across the continuum of cancer research and patient care have highlighted the current gaps in our knowledge that require additional investigation. We have learned that because of their disease and treatment, survivors of cancer may face serious and persistent adverse outcomes, including physical, emotional, and psychosocial challenges. These challenges can also extend to friends and family members who often act as informal caregivers.
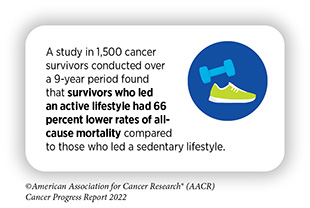
Researchers are exploring ways to utilize health behaviors, palliative care, psycho-oncology, and other evidence-based strategies to improve quality of life for survivors of cancer. As one example, it has been indicated that an active lifestyle can help mitigate the numerous physical, mental, and emotional challenges that survivors of cancer may experience.
Ongoing research is investigating the potential of new technologies and innovative intervention strategies for coordinated care that improves the quality of life and meets the personalized needs of cancer survivors and caregivers from all age groups.
Looking to the Future of Cancer Science and Medicine
Research drives progress against cancers because it provides us with a deeper understanding of cancer biology which leads to advances in prevention, detection, diagnosis, treatment and to the deployment of evidence-based policies for improving public health.
As we look to the future, many researchers, including AACR President, 2022-2023, Lisa M. Coussens, MD (hc), PhD, FAACR, are confident that we can accelerate the pace of progress against cancer by facilitating synergistic collaborations across disciplines and by assembling and supporting a diverse workforce. The new wave of innovation driven by advances in discovery science will enable researchers to gain a greater insight into the mechanisms underlying cancer development, and to identify novel ways to target and eradicate cancer cells. In addition, incorporation of cutting-edge technologies, such as liquid biopsies and AI, will allow us to achieve the full potential of precision medicine by addressing a wide range of unresolved clinical questions across the spectrum of cancer research and patient care.
Impacting the Future of Cancer Research and Patient Care Through Evidence-Based Policies
Steady declines in U.S. cancer incidence and mortality during the past three decades have been fueled by scientific discoveries and initiatives supported by federal investments in NIH, NCI, FDA, and CDC. The enormous excitement in cancer science and medicine has led to a surge in the number of grant applications from researchers, but increases to NCI funding levels have not kept pace to support the same level of innovation experienced in the 1990s.
Robust, sustained, and predictable annual budget increases for NIH and NCI are paramount for maintaining the positive momentum against cancer. Congress’s ongoing commitment for supporting FDA also helps ensure that anticancer therapeutics continue to be safe and effective. Additionally, federal support for CDC’s cancer prevention and control programs helps bring lifesaving preventive services to those who need them the most. Federal investments are vital for diversifying the cancer research and care workforce, advancing regulatory science initiatives, and pursuing policies that improve cancer prevention, early detection, and control for everyone.
AACR Call to Action
Cancer continues to be the second leading cause of death in the United States, thus there is an urgent need for more research to accelerate the pace of progress against this disease that touches so many lives. Remarkable bipartisan, bicameral efforts in Congress have increased NIH funding by $14.9 billion, or roughly 49 percent, from FY 2015 to FY 2022. These significant investments have made it possible for researchers to discover scientific breakthroughs against cancer and many other diseases.
AACR deeply appreciates the commitment of Congress to expediting progress against cancer and other diseases through robust funding increases for NIH, as well as its support of the critical regulatory science work at FDA and public health initiatives at CDC. These investments and initiatives will transform cancer care, increase survivorship, and maintain the United States’ position as a global leader in science and cancer research.
Therefore, AACR strongly encourages Congress and stakeholders committed to eradicating cancer to:
- Continue to support robust, sustained, and predictable funding growth for NIH and NCI by providing increases to the FY 2023 base budget, including $49.1 billion in base budget authority for NIH, representing an increase of $4.1 billion, and $7.766 billion for NCI, which is an increase of $853 million and is consistent with the NCI Director’s Professional Judgment Budget.
- Fully fund initiatives authorized in the 21st Century Cures Act, including the National Cancer Moonshot, and ensure that Moonshot funding supplements rather than supplants NIH funding in FY 2023.
- Reauthorize the Childhood Cancer STAR Act and provide no less than $30 million for STAR Act implementation, as well as $50 million for the Childhood Cancer Data Initiative, which seeks to better understand cancer biology specific to pediatric patients and improve prevention, treatment, quality of life, and survivorship.
- Invest in vital initiatives of the CDC Division of Cancer Prevention and Control by providing at least $462.6 million to support comprehensive cancer control, central cancer registries, and screening and public awareness programs for specific cancers.
- Increase funding for FDA’s critical regulatory science initiatives that advance the development and regulation of oncology products, by providing an increase of at least
- $318 million, for a total of $3.653 billion in discretionary budget authority in FY 2023, as recommended in President Biden’s budget.
- Ensure that patients with cancer have equitable access to quality, affordable health care by expanding Medicaid and enacting the Accelerating Kids’ Access to Care Act, which would reduce barriers to care for children on Medicaid who receive specialist care from an out-of-state pediatric provider.
- Increase participation and diversity of cancer clinical trials by reducing barriers for patient enrollment and encouraging diverse representation in clinical trials, as contained in the Diversifying Investigations Via Equitable Research Studies for Everyone (DIVERSE) Trials Act and the Diverse and Equitable Participation in Clinical Trials (DEPICT) Act, respectively.
- Encourage research institutions to recruit, support, and retain a robust cancer research workforce that reflects the diversity of our society, and support NCI initiatives such as the NCI Equity and Inclusion Program that strive to build a more inclusive and equitable workforce and markedly reduce cancer disparities.
- Reduce cancer incidence and mortality by addressing nicotine addiction through expanded coverage of tobacco cessation services, removing flavored tobacco products including menthol from the market, and limiting nicotine concentration in tobacco products.
- Expand tax policies to encourage philanthropic giving so that nonprofit cancer research organizations can continue to fund high-risk, high-reward research proposals and accelerate the discovery of new treatments and cures.
The items contained in AACR Call to Action would fuel innovation and usher in a new era of cancer science, reduce cancer disparities, improve cancer prevention and detection, and bring lifesaving cures to millions of people whose lives are touched by cancer.
Next Section: A Message from the AACR Previous Section: AACR Call to Action