Looking to the Future
In this section you will learn:
- Cutting-edge technologies that fuel the full spectrum of cancer science from bench to bedside will accelerate the pace at which we increase our understanding of cancer biology while transforming the future of clinical practice.
- As researchers accumulate large quantities of patient data, artificial intelligence approaches, such as machine learning programs have the potential to help us analyze these vast amounts of health care information to derive meaningful insights we previously could not have realized.
- Liquid biopsies have the potential to transform early detection, diagnosis, and treatment of cancer in the future.
- Our scientific understanding of the immune system and how it interacts with cancer cells is rapidly increasing, and numerous clinical trials are underway that are testing many novel immunotherapeutics and new ways to use those immunotherapeutics that we already have.
This is an incredibly exciting time for cancer science and medicine. Increasing public awareness of cancer prevention and early detection coupled with the development and approval of a range of novel anti-cancer therapeutics has led to dramatic reductions in overall cancer death rates for all Americans. Continued advances in the fields of cancer genomics and immunology are driving remarkable progress in the newest treatments in cancer care—molecularly targeted therapy and immunotherapy—which are benefiting many patients with a range of cancer types. The pace of progress in these research areas is expected to accelerate in the coming years for the benefit of patients with cancer.
Despite these advances, cancer continues to be an enormous public health challenge in the United States and worldwide. In fact, it is predicted that more than 606,520 people in the United States will die from some type of cancer in 2020. Furthermore, the medical research community has been inundated with numerous challenges due to the recent COVID-19 pandemic which has dampened the ongoing momentum in cancer research. However, many researchers, including AACR President, 2020–2021, Antoni Ribas, MD, PhD, are extremely hopeful about the future because they are confident that through collaborative and innovative research we will be able to overcome the public health crisis caused by COVID-19 while we continue to power more advances against cancer. The new wave of scientific and technological innovations discussed in this chapter has the potential to transform patient care in the years to come.
Artificial Intelligence
According to the NCI, artificial intelligence (AI) is defined as the ability of a computer to perform functions that are usually thought of as intelligent human behavior, such as learning, reasoning, problem solving, and decision-making. As researchers accumulate large quantities of cancer-related data ranging from tumor images from scans and pathological slides, cancer and patient genome profiles, and electronic health records to clinical outcomes, AI can analyze this information to derive meaningful insights that we previously could not have realized (486)Topol EJ. High-performance medicine: the convergence of human and artificial intelligence. Nat Med [Internet]. Springer US; 2019;25:44–56.. Machine learning is an application of AI that focuses on the development of computer programs that can access and learn from data, identify patterns, and make decisions without explicit human intervention. Deep learning is a subset of machine learning that utilizes neural networks to make decisions. The applications of AI in cancer science and medicine are vast and rapidly expanding. Some recent advances in the field are described below.
AI in Cancer Imaging
One of the most exciting areas of cancer research where AI is already showing great promise is cancer imaging. Analysis of images from normal tissue, precancerous lesions, or cancers derived from various means including clinical photographs (from endoscopy, colonoscopy, etc.), radiological images (from mammography, lung CT, etc.), or histological images (from tumor pathology), is a critical step in cancer detection and diagnosis. Traditionally, interpretation of these images is carried out by expert physicians through a process that is both laborious and time consuming. Several recent studies indicate that image analysis using AI has the potential to streamline processes that are necessary for accurate interpretation of images from numerous sources routinely used in cancer medicine. Notably, these reports highlight that AI is capable of spotting cancers with similar accuracy to, and in cases, higher accuracy than human experts, which allows for faster clinical decision-making for those with life-threatening cancers. Thereby, AI can also expand access to quality care in underserved regions where qualified clinical staff are lacking or scarce by taking over some of the diagnostic duties typically allocated to expert health care professionals.
Analyzing Clinical Photographs
The utility of AI in detecting cancerous polyps by analyzing digital photographs of the GI tract taken during routine endoscopy or colonoscopy procedures is an area of extensive investigation and in fact shows great promise in the detection of both gastric and colorectal cancers (487)AI Medical Service Inc. announces FDA Breakthrough Device Designation for endoscopic AI system [Internet]. [cited 2020 Jul 2].(488)P W, X X, JR GB, TM B, M T, F X, et al. Development and Validation of a Deep-Learning Algorithm for the Detection of Polyps During Colonoscopy. Nat Biomed Eng [Internet]. Nat Biomed Eng; 2018 [cited 2020 Jul 2];2.. Furthermore, according to a recent report, a machine learning approach outperformed human experts in detecting precancerous changes in cervical images obtained from volunteers who took part in a cancer screening study conducted in Costa Rica over two decades ago (489)L H, D B, S A, Z X, K Y, MP H, et al. An Observational Study of Deep Learning and Automated Evaluation of Cervical Images for Cancer Screening. J Natl Cancer Inst [Internet]. J Natl Cancer Inst; 2019 [cited 2020 Jul 2];111.. Ongoing research is testing whether such an approach may be utilized for generating high-quality photos of the cervix using smartphones during a routine pelvic exam since such low-cost, mobile methods could provide a valuable new tool to help reduce the burden of cervical cancer especially among underserved populations both in the U.S. and around the globe (490)Francis Collins. Using Artificial Intelligence to Detect Cervical Cancer – NIH Director’s Blog [Internet]. 2019 [cited 2020 Jul 2]..
Investigating Radiology and Pathology Images
Further examples of the use of AI in cancer imaging include radiological imaging analysis and pathology testing results determination, both of which are critical in diagnosing cancer. Traditionally, the former involves a radiologist scanning images by visually searching for signs of cancer while pathology testing involves a pathologist viewing a slide on which there is a slice of the abnormal tissue under a conventional light microscope to determine the presence of cancerous cells. Current methods of analyzing scans and slides are time consuming and can sometimes miss signs of cancer (false negative) or detect cancers that turn out to be imaging artifacts (false positives).
Emerging data highlight that AI can play a critical role in increasing the efficiency and accuracy of both radiology and pathology image analyses. For instance, recent studies have demonstrated that AI tools can better detect breast or lung cancers from mammograms or CT scans, respectively, compared with radiologists, resulting in fewer cases of false positives and false negatives (278)McKinney SM, Sieniek M, Godbole V, Godwin J, Antropova N, Ashrafian H, et al. International evaluation of an AI system for breast cancer screening. Nature [Internet]. Nature Publishing Group; 2020 [cited 2020 Jul 1];577:89–94.(491)Ardila D, Kiraly AP, Bharadwaj S, Choi B, Reicher JJ, Peng L, et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat Med [Internet]. Nature Publishing Group; 2019 [cited 2020 Jul 2];25:954–61.. AI systems have also been shown to detect and characterize abnormality in tumors from prostate cancer biopsies at an efficiency that is comparable to that of pathologists (492). Yet another remarkable use of AI, as documented in a recent report, is to rapidly provide surgeons with accurate, real-time information about the type of brain tumor a patient has while the patient is being operated on. The researchers found that AI could analyze pathology images from a biopsy sample obtained during surgery to accurately diagnose the type of brain tumor in fewer than 3 minutes, a process that traditionally takes about 40 minutes (493). The approach was also able to accurately distinguish tumor from surrounding healthy tissue, which can refine surgery and may result in major improvements in long-term patient outcomes. In addition to its role in cancer diagnosis, AI methods may help researchers accurately predict the presence of certain biomarkers (e.g., genetic mutations or proteins) in tumors by analyzing pathology images (494)G S, Y B, R S, I D, Z G, R K. Artificial Intelligence Algorithms to Assess Hormonal Status From Tissue Microarrays in Patients With Breast Cancer. JAMA Netw open [Internet]. JAMA Netw Open; 2019 [cited 2020 Jul 2];2.(495)Coudray N, Ocampo PS, Sakellaropoulos T, Narula N, Snuderl M, Fenyö D, et al. Classification and mutation prediction from non–small cell lung cancer histopathology images using deep learning. Nat Med [Internet]. Nature Publishing Group; 2018 [cited 2020 Jul 2];24:1559–67.. Such AI-based approaches could potentially be faster and less expensive compared with traditional techniques of biomarker detection, may allow for simultaneous profiling of multiple biomarkers in cancer tissues, and could transform the future of precision medicine.
AI in Drug Development
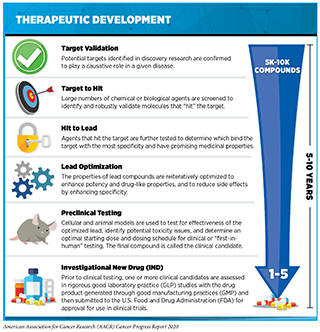
Researchers are harnessing the power of AI in many ways to accelerate cancer drug discovery (496). While some efforts are aimed toward making basic research investigations more effective, others have the goal of streamlining clinical trials to make them more efficient. In fact, the use of AI can augment each step of the drug development process (see sidebar on Therapeutic Development). For instance, AI can harness massive amounts of information from the scientific literature, clinical databases, and patient-derived data to identify potential new drug targets, e.g., proteins that are vital for cancer growth; to design new therapeutics that target such proteins; and to help evaluate the safety and effectiveness of those therapeutics (497)Vamathevan J, Clark D, Czodrowski P, Dunham I, Ferran E, Lee G, et al. Applications of machine learning in drug discovery and development. Nat Rev Drug Discov [Internet]. NIH Public Access; 2019 [cited 2020 Jul 29];18:463–77.. In this regard, one initiative currently underway is utilizing AI to identify novel ways to inhibit the activity of an altered KRAS protein, one of the most frequent alterations in cancers (498)T B, T B, JH D, YA E, EJ G, AL G, et al. AI Meets Exascale Computing: Advancing Cancer Research With Large-Scale High Performance Computing. Front Oncol [Internet]. Front Oncol; 2019 [cited 2020 Jul 2];9..
In clinical research, AI platforms including machine learning can accelerate cancer drug discovery by using biomarkers to accurately select patient populations in which to test new therapeutics while preventing serious adverse events by identifying high-risk individuals prior to patient enrolment in clinical trials. Furthermore, AI has the potential to improve clinical trial efficiency by incorporating information from historical control arms or real-world data, predicting effective combinations of drug targets that may improve patient outcomes. An area of urgent research focus is the expansion of datasets used to train AI platforms from primarily Caucasian populations to include racial and ethnic minorities and other underserved groups.
AI in Patient Care
Beyond its role in rapidly advancing the entire continuum of cancer research, it is anticipated that AI may also play a crucial role in patient care in the near future. AI has the potential to aid in clinical decision-making such as in deciding on the best treatment options for patients or in identifying responses to therapy, among other applications. As an example, in a recent study, researchers utilized data from computed tomography scans from patients with lung cancer to create an AI model that was able to analyze patterns within the tomography scans to predict how patients may respond to chemotherapy, targeted therapy or immunotherapy (499)Dercle L, Fronheiser M, Lu L, Du S, Hayes W, Leung DK, et al. Identification of Non–Small Cell Lung Cancer Sensitive to Systemic Cancer Therapies Using Radiomics. Clin Cancer Res [Internet]. American Association for Cancer Research; 2020 [cited 2020 Jul 2];. This model offers a promising approach to guiding clinical decisions and forecasting patient outcomes.
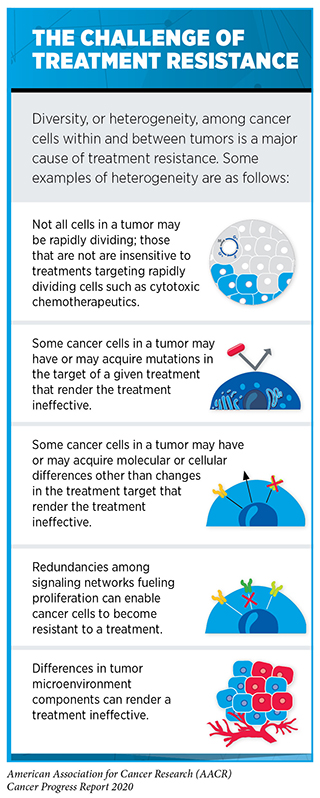
Another area in patient care where AI may play a pivotal role is in addressing the challenges of treatment resistance (see sidebar on The Challenge of Treatment Resistance). Treatment resistance arises when certain cancer cells deploy necessary mechanisms to overcome the toxic effects of the treatment, continue to multiply, and eventually outnumber the drug-sensitive cells to repopulate a tumor. Some researchers now believe that using high doses of therapeutics to eliminate the maximum number of cancer cells may accelerate the emergence of resistant cell populations (500)Thomas F, Donnadieu E, Charriere GM, Jacqueline C, Tasiemski A, Pujol P, et al. Is adaptive therapy natural? PLoS Biol [Internet]. Public Library of Science; 2018 [cited 2020 Aug 3];16:e2007066.(496). Therefore, an area of extensive investigation in AI is the application of mathematical models to identify optimal dosing regimens of therapeutics that will maintain a persistent population of drug-sensitive cancer cells in a tumor (500)Thomas F, Donnadieu E, Charriere GM, Jacqueline C, Tasiemski A, Pujol P, et al. Is adaptive therapy natural? PLoS Biol [Internet]. Public Library of Science; 2018 [cited 2020 Aug 3];16:e2007066.(501)Gatenby RA, Silva AS, Gillies RJ, Frieden BR. Adaptive therapy. Cancer Res [Internet]. NIH Public Access; 2009 [cited 2020 Aug 3];69:4894–903.. Researchers hypothesize that maintaining a threshold level of drug-sensitive cells that compete for growth with resistant populations will prevent or slow the multiplication of resistant cell populations.
Across the continuum of cancer care there is growing interest in utilizing AI coupled with patient data and treatment guidelines to guide cancer management, although the full potential of such approaches remains to be determined. For instance, in a recent study, AI was able to utilize data from electronic health records to identify patients with cancer who are at high risk of short-term mortality, allowing health care providers to engage in more timely conversations regarding patients’ goals and values (502)RB P, C M, C C, SH R, J B, ME D, et al. Machine Learning Approaches to Predict 6-Month Mortality Among Patients With Cancer. JAMA Netw open [Internet]. JAMA Netw Open; 2019 [cited 2020 Jul 2];2.. Collectively, these reports emphasize the incredible potential of AI in the future of clinical cancer care. However, as mentioned above, an area where researchers must pay close attention is the inclusion of diverse datasets that are representative of the U.S. population in the development of AI platforms. Lack of diversity in the data that are used to train AI or machine learning systems may incorporate racial/ethnic or other biases within AI applications and limit their generalizability for all patients who need to benefit from these state-of-the-art technologies (503)Vyas DA, Eisenstein LG, Jones DS. Hidden in Plain Sight — Reconsidering the Use of Race Correction in Clinical Algorithms. Malina D, editor. N Engl J Med [Internet]. Massachusetts Medical Society; 2020 [cited 2020 Jul 2];NEJMms2004740.(504)Obermeyer Z, Powers B, Vogeli C, Mullainathan S. Dissecting racial bias in an algorithm used to manage the health of populations. Science [Internet]. American Association for the Advancement of Science; 2019 [cited 2019 Dec 18];366:447–53..
Minimally Invasive Testing Using Liquid Biopsies
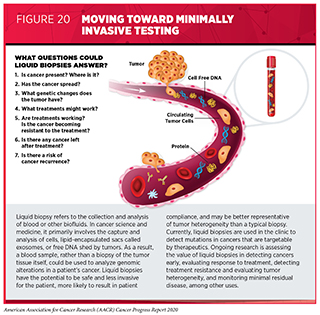
A biopsy is the removal of cells or tissues from a patient for testing to help physicians diagnose a condition such as cancer or monitor how it changes in response to treatment. Traditionally, biopsies are invasive procedures. However, research has shown that during cancer development and treatment, tumors routinely shed detectable cells, lipid encapsulated sacs called exosomes, and free DNA into a patient’s blood or cerebrospinal fluid. Recent studies have also shown that it is possible to use a blood or another biofluid sample, or “liquid biopsy,” rather than a traditional tissue biopsy, to obtain material that can be analyzed to provide valuable information such as the molecular alterations associated with a patient’s cancer (505). Liquid biopsies, therefore, provide a less invasive means to detect or track the status of cancer. There is much excitement in the cancer field that, as opposed to traditional biopsies which only provide a snapshot of the tumor characteristics at one specific timepoint, liquid biopsy approaches may generate a more complete picture of an individual’s cancer by allowing for the monitoring of disease progression and its response to treatments in real time.
Ongoing research is evaluating multiple liquid biopsy approaches that may transform the future landscape of cancer screening, early detection, treatment, and monitoring of treatment responses (see Figure 20). Some liquid biopsy platforms analyze blood samples to identify specific genetic or epigenetic alterations in the DNA that are associated with certain cancer types, while others look more broadly at the patterns of fragmentation of the shredded cell-free DNA in the blood, and yet others aim to detect tumor-associated proteins in the blood (506)Shen SY, Singhania R, Fehringer G, Chakravarthy A, Roehrl MHA, Chadwick D, et al. Sensitive tumour detection and classification using plasma cell-free DNA methylomes. Nature [Internet]. Nature Publishing Group; 2018 [cited 2019 Jun 3];563:579–83.(507)Cristiano S, Leal A, Phallen J, Fiksel J, Adleff V, Bruhm DC, et al. Genome-wide cell-free DNA fragmentation in patients with cancer. Nature [Internet]. 2019 [cited 2019 Jun 20];570:385–9.(508)Panditharatna E, Kilburn LB, Aboian MS, Kambhampati M, Gordish-Dressman H, Magge SN, et al. Clinically Relevant and Minimally Invasive Tumor Surveillance of Pediatric Diffuse Midline Gliomas Using Patient-Derived Liquid Biopsy. Clin Cancer Res [Internet]. 2018 [cited 2019 Jun 20];24:5850–9.(509)Chen X, Gole J, Gore A, He Q, Lu M, Min J, et al. Non-invasive early detection of cancer four years before conventional diagnosis using a blood test. Nat Commun [Internet]. Nature Publishing Group; 2020 [cited 2020 Aug 3];11:3475.. Early clinical data indicate that liquid biopsies have the potential to transform early detection, interception, diagnosis, treatment, and surveillance of cancer by identifying markers of disease, therapeutic response, resistance, and recurrence (501)Gatenby RA, Silva AS, Gillies RJ, Frieden BR. Adaptive therapy. Cancer Res [Internet]. NIH Public Access; 2009 [cited 2020 Aug 3];69:4894–903.(511)Wang D-S, Liu Z-X, Lu Y-X, Bao H, Wu X, Zeng Z-L, et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut [Internet]. 2019 [cited 2019 Jun 20];68:1152–61.(512)Leighl NB, Page RD, Raymond VM, Daniel DB, Divers SG, Reckamp KL, et al. Clinical Utility of Comprehensive Cell-free DNA Analysis to Identify Genomic Biomarkers in Patients with Newly Diagnosed Metastatic Non-small Cell Lung Cancer. Clin Cancer Res [Internet]. American Association for Cancer Research; 2019 [cited 2019 Jun 20];. Selected examples of recent research examining the role of liquid biopsies across the spectrum of cancer research are presented here.
Detecting Cancers Early
In a recent study, researchers were able to utilize a blood test combined with imaging techniques to detect cancers in women without any prior history or symptom (280)Lennon AM, Buchanan AH, Kinde I, Warren A, Honushefsky A, Cohain AT, et al. Feasibility of blood testing combined with PET-CT to screen for cancer and guide intervention. Science (80- ) [Internet]. American Association for the Advancement of Science; 2020 [cited 2020 Jul 1];. The test identified breast, lung, and colorectal cancers for which there are recommended screening tests, but also seven additional cancer types such as ovarian, uterine, and kidney cancer for which there are no screening tests available at the current time. Notably, some of the cancers were detected at an early stage, when interventions are most likely to be effective. A second liquid biopsy platform utilized innovative DNA sequencing methods to analyze specific patterns in the circulating DNA to detect more than 50 different types of cancer (281). An added benefit of this platform was that it was able to identify the tissues in which the cancer originated. While these reports are very promising, additional research is needed to determine whether cancer detection using liquid biopsies can ultimately reduce the number of deaths from cancer, before such tests can be introduced to the clinic. Furthermore, potential harms of detecting slow-growing cancers that would have never caused serious harms during an individual’s lifetime, a phenomenon known as overdiagnosis, and of unnecessary invasive interventions known as overtreatment, need to be weighed against potential benefits.
Making Treatment Decisions
While molecularly targeted therapies have transformed the landscape of cancer treatment for many diseases such as lung or breast cancer, it is often difficult using traditional biopsies to test for all genetic alterations in the cancer that may be therapeutically targetable. Underlying reasons may vary ranging from lack of adequate biospecimen or lack of quality biospecimen, to compliance and bioethical issues. In this regard liquid biopsies provide a great alternative. They are potentially safer and less invasive for the patient and may better represent the tumor heterogeneity than a typical biopsy. Therefore, one of the biggest appeals and the only FDA-approved use of this technology is as a companion diagnostic in identifying cancer-causing mutations to make treatment decisions for patients. Thus far, two liquid biopsy companion diagnostic tests have been approved by the FDA. In June 2016, the FDA approved the first for identifying whether a patient with metastatic NSCLC is eligible for treatment with the EGFR-targeted therapeutic erlotinib. In May 2019, the FDA approved the second liquid biopsy companion diagnostic test which detects PIK3CA mutations in individuals with HER2-negative, advanced or metastatic breast cancer. Ongoing research is underway to develop and validate numerous new liquid biopsy platforms that can simultaneously detect multiple targetable genetic alterations using blood or other biofluid samples from patients with cancer (510). It remains to be determined whether such tests can detect therapeutically targetable mutations with the same accuracy as traditional biopsies and whether treatments based on liquid biopsy-derived information result in comparable long-term outcomes for patients with cancer.
Predicting Cancer Resistance and Recurrence
Liquid biopsies may also provide researchers with important clues as to whether a patient’s cancer has the potential to spread, grow resistant to treatments, or relapse. For instance, ctDNA analysis in patients with gastric cancer expressing the protein HER2 and treated with the HER2-targeted therapeutic trastuzumab allowed researchers to gain novel insights into the genetic alterations that contribute to resistance to the targeted therapeutic (511)Wang D-S, Liu Z-X, Lu Y-X, Bao H, Wu X, Zeng Z-L, et al. Liquid biopsies to track trastuzumab resistance in metastatic HER2-positive gastric cancer. Gut [Internet]. 2019 [cited 2019 Jun 20];68:1152–61.. According to another recent report, the prevalence of circulating tumor cells and the presence of certain genetic alterations within those cells detected during surgery of early-stage NSCLCs could predict the recurrence of metastatic cancer (513)Chemi F, Rothwell DG, McGranahan N, Gulati S, Abbosh C, Pearce SP, et al. Pulmonary venous circulating tumor cell dissemination before tumor resection and disease relapse. Nat Med [Internet]. Nature Publishing Group; 2019 [cited 2020 Jul 2];25:1534–9.. Similar data have emerged in colon cancer where the detection of circulating tumor DNA after surgery or after adjuvant chemotherapy was associated with excess risk for disease recurrence during a 3-year follow-up (514)Tie J, Cohen JD, Wang Y, Christie M, Simons K, Lee M, et al. Circulating Tumor DNA Analyses as Markers of Recurrence Risk and Benefit of Adjuvant Therapy for Stage III Colon Cancer. JAMA Oncol [Internet]. American Medical Association; 2019 [cited 2020 Jul 2];5:1710.. In addition, ongoing research is underway to evaluate the clinical utility of circulating tumor DNA detection in determining the risk of recurrence of a particularly intractable form of breast cancer known as triple-negative breast cancer (515)Radovich M, Jiang G, Hancock BA, Chitambar C, Nanda R, Falkson C, et al. Association of Circulating Tumor DNA and Circulating Tumor Cells After Neoadjuvant Chemotherapy With Disease Recurrence in Patients With Triple-Negative Breast Cancer: Preplanned Secondary Analysis of the BRE12-158 Randomized Clinical Trial. JAMA Oncol [Internet]. JAMA Oncol; 2020 [cited 2020 Aug 3];.
New Wave of Innovations in Cancer Immunotherapy
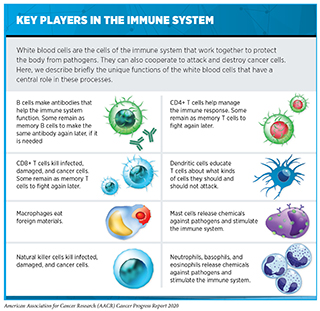
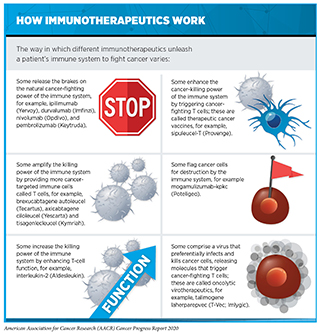
Cancer immunology and immunotherapy are some of the most exciting areas of cancer research. In the past decade, immunotherapeutics have revolutionized the landscape of cancer treatment. As described in Treatment with Immunotherapeutics, these therapeutics work in many ways (see sidebar on How Immunotherapeutics Work). However, thus far, immunotherapeutics have been successful in treating only a small fraction of patients with cancer. Furthermore, many patients who respond initially may develop resistance after a period of period. Researchers are working diligently to increase the number of patients who benefit from these groundbreaking treatments. Novel avenues that are being pursued include identifying ways to select the right patients who have a higher probability of responding to existing immunotherapies as well as designing new immunotherapeutics that may benefit additional patient populations. While most immunotherapeutics approved by the FDA to date have focused on the anticancer effects of a type of immune cells called T cells, researchers are now harnessing the power of many additional types of immune cells with distinct functions to attack and kill cancer (see sidebar on Key Players in the Immune System). In the following sections we describe some of the exciting new approaches that are being investigated.
Expanding the Scope of Checkpoint Inhibitors
Among the most promising anticancer therapeutics that have emerged in the last decade are checkpoint inhibitors. These molecules work by releasing certain brakes on the natural cancer-fighting power of the immune system (see Releasing the Brakes on the Immune System) and have transformed the care of many aggressive cancers including melanoma and NSCLC. Unfortunately, only a fraction of patients responds to checkpoint inhibitors, and many who do respond initially develop resistance after a while. Identifying the right patients who are most likely to have durable responses is key to guiding treatment decisions and is an area of active research. In order to select the right patients, it is important to understand the cellular and molecular features of the tumors that influence response to checkpoint inhibitors. Many approaches are being pursued to characterize these features including state-of-the-art imaging techniques and quantitation of measurable tumor characteristics referred to as biomarkers which can predict treatment outcomes.
Advanced Imaging to Guide Immunotherapy
Immune cells called T cells are naturally capable of destroying cancer cells and are also the targets of checkpoint inhibitors which release certain brakes on T cells to mobilize them to kill cancer cells. Imaging T-cell localization in a patient’s tumor may provide information about how the tumor is responding to checkpoint inhibitors. Positron emission tomography (PET) offers an attractive approach for imaging tumors. In a traditional PET scan the whole body is imaged by intravenously administering a radioactive molecule such as 18F-fluorodeoxyglucose to a patient, which helps to visualize multiple tumors at once. To visualize the immune cells, researchers have developed an alternative method called immuno-PET, which uses radiolabeled antibodies that bind to specific proteins on the T-cell surface providing information about their localization (516)Tavaré R, McCracken MN, Zettlitz KA, Knowles SM, Salazar FB, Olafsen T, et al. Engineered antibody fragments for immuno-PET imaging of endogenous CD8+ T cells in vivo. Proc Natl Acad Sci U S A [Internet]. National Academy of Sciences; 2014 [cited 2020 Jul 2];111:1108.. While conventional antibodies are large molecules and only achieve low levels of penetration in the tumor tissue, researchers have devised smaller molecules called “nanobodies,” which contain fragments of an antibody and could potentially be more easily taken up by the tumor tissue (517)M R, MW L, VL V, A D, Y Z, TH N, et al. Immuno-PET Identifies the Myeloid Compartment as a Key Contributor to the Outcome of the Antitumor Response Under PD-1 Blockade. Proc Natl Acad Sci U S A [Internet]. Proc Natl Acad Sci U S A; 2019 [cited 2020 Jul 2];116.. Using nanobodies that bind to a cell-surface protein CD8, which is found on cancer-killing T cells, researchers were able to visualize the trafficking of T cells into the tumor microenvironment. In animal models of colorectal cancer it was shown that tumors that respond to checkpoint inhibitors have a higher degree of CD8-expressing T cell infiltration throughout their core (517)M R, MW L, VL V, A D, Y Z, TH N, et al. Immuno-PET Identifies the Myeloid Compartment as a Key Contributor to the Outcome of the Antitumor Response Under PD-1 Blockade. Proc Natl Acad Sci U S A [Internet]. Proc Natl Acad Sci U S A; 2019 [cited 2020 Jul 2];116.. Ongoing research is underway to determine whether this technique could be used in the clinic to help predict which patients will respond to immunotherapeutic regimens.
Using Biomarkers to Predict Responses to Immune Checkpoint Inhibitors
Cellular or molecular characteristics of tumors, referred to as biomarkers, can sometimes help researchers to predict clinical outcomes and to stratify patients as likely responders or non-responders to therapeutics, including immune checkpoint inhibitors. Currently, three biomarkers are approved by the FDA to predict response to checkpoint inhibitors: the amount of checkpoint protein PD-L1 in the tumor tissue, and two different genetic characteristics of tumors—mismatch repair deficiency/microsatellite instability, and high tumor mutational burden. However, there has only been modest success in using these biomarkers to predict response, and current methods to measure them can be invasive, revealing the need for more accurate and noninvasive biomarkers. For instance, determination of PD-L1 levels correlates only moderately with patient survival and response to anti-PD-L1 checkpoint inhibitor treatment. Heterogeneity of PD-L1 expression within and across tumors in a patient might limit the predictive value of the current methods that are used to quantify PD-L1 levels using tissue pathology. Notably, according to recent reports, tumor PD-L1 and PD-1 expression in patients with NSCLC can be quantified noninvasively through PET scanning using radiolabeled molecules that bind to PD1 or PD-L1 (518)Donnelly DJ, Smith RA, Morin P, Lipovšek D, Gokemeijer J, Cohen D, et al. Synthesis and Biologic Evaluation of a Novel 18F-Labeled Adnectin as a PET Radioligand for Imaging PD-L1 Expression. J Nucl Med [Internet]. Society of Nuclear Medicine; 2018 [cited 2020 Jul 2];59:529–35.(519)Niemeijer AN, Leung D, Huisman MC, Bahce I, Hoekstra OS, van Dongen GAMS, et al. Whole body PD-1 and PD-L1 positron emission tomography in patients with non-small-cell lung cancer. Nat Commun [Internet]. Nature Publishing Group; 2018 [cited 2020 Jul 2];9:4664.. The researchers found that a lack of response to checkpoint blockade corresponded with low PD-L1 expression, indicating a prognostic utility for these advanced PET techniques. Noninvasive imaging methods such as these can evaluate multiple tumors simultaneously, as opposed to traditional methods using a tissue biopsy, and may address the issues of heterogeneity. Combination with key additional clinical information such as tumor genetics, as well as other novel biomarkers relevant to checkpoint inhibition, for example, genetic information derived from abnormal immune-related tissue formations called tertiary lymphoid structures found in cancer (520)Sautès-Fridman C, Petitprez F, Calderaro J, Fridman WH. Tertiary lymphoid structures in the era of cancer immunotherapy. Nat Rev Cancer [Internet]. Nature Publishing Group; 2019 [cited 2020 Jul 2];19:307–25.(521)Cabrita R, Lauss M, Sanna A, Donia M, Skaarup Larsen M, Mitra S, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature [Internet]. Nature Publishing Group; 2020 [cited 2020 Jul 2];577:561–5., may help improve outcomes for patients.
Combining Therapeutics to Address Treatment Resistance
In order to overcome treatment resistance to immune-checkpoint inhibitors, researchers are currently investigating the underlying mechanisms of such resistance. The goal is to identify potential approaches to bypassing or overcoming the cellular and molecular pathways that lead to treatment resistance. In this regard, one approach that is currently being evaluated is combining checkpoint inhibitors with a range of therapeutic modalities, including molecularly targeted therapeutics, a separate checkpoint inhibitor, as well as other types of immunotherapeutics. For instance, a recent preclinical study identified the underlying mechanisms by which mutations in three proteins JAK1, JAK2, and beta-2-microglobulin (B2M), all of which regulate key immune-activating pathways, render patients with melanoma resistant to treatment with immune checkpoint inhibitors (522)Torrejon DY, Abril-Rodriguez G, Champhekar AS, Tsoi J, Campbell KM, Kalbasi A, et al. Overcoming genetically-based resistance mechanisms to PD-1 blockade. Cancer Discov [Internet]. American Association for Cancer Research; 2020 [cited 2020 Jul 2];. The researchers proposed that a combination therapy using a molecule, bempegaldesleukin (BEMPEG), which can reverse the effect of B2M mutation, may be able to restore responses to checkpoint inhibitors. Notably, an early clinical trial that examined a combination of BEMPEG with the anti-PD-1 checkpoint inhibitor nivolumab in patients with various solid tumors, including melanoma, found that the combination led to encouraging clinical responses that are worth further exploration (523)Diab A, Tannir NM, Bentebibel S-E, Hwu P, Papadimitrakopoulou V, Haymaker C, et al. Bempegaldesleukin (NKTR-214) plus Nivolumab in Patients with Advanced Solid Tumors: Phase I Dose-Escalation Study of Safety, Efficacy, and Immune Activation (PIVOT-02). Cancer Discov [Internet]. American Association for Cancer Research; 2020 [cited 2020 Jul 2];.
Targeting Novel Immune Checkpoints
Thus far the FDA has approved seven immune checkpoint inhibitors that inhibit proteins PD-1, PD-L1, and CTLA-4, for the treatment of numerous cancer types. However, given that many patients do not respond to the currently approved inhibitors while many others develop resistance after initial response, the identification of new checkpoint pathways to target therapeutically is a key area of immunotherapy research. Two checkpoint proteins that are both expressed on T-cell surface and are currently being tested as potential targets for anticancer therapy are the poliovirus receptor-related immunoglobulin (PVRIG) and T cell immunoreceptor with Ig and ITIM domains (TIGIT). Similar to the interaction of PD-1 with PD-L1, when checkpoint proteins PVRIG or TIGIT on T cells interact with their counterparts which are often expressed on the surface of cancer cells, T cells are “turned off” and the cancer cell is able to evade the immune response. The clinical impact of inhibiting PVRIG and TIGIT, alone or in combination with PD-1/PD-L1 inhibition is currently being evaluated in the clinic (524)COM701 Shows Antitumor Activity, +/− Nivolumab. Cancer Discov [Internet]. American Association for Cancer Research; 2020 [cited 2020 Jul 2];(525)An Investigational Immuno-therapy Study to Evaluate the Safety and Effectiveness of Experimental Medication BMS-986207 by Itself and in Combination With Nivolumab in Solid Cancers That Are Advanced or Have Spread [Internet]. [cited 2020 Jul 2].. Targeting a third immune checkpoint protein, OX40, is another ongoing area of therapeutic investigation in immunotherapy. In contrast to the PD-1 and PD-L1 interaction, the interaction between OX40 protein on T cells and its binding partner on tumors enhances immune system function. In fact, there is growing evidence that OX40 activation can boost antitumor immune responses by modulating T-cell function (526)Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria J-C, Zitvogel L, Marabelle A. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer [Internet]. Elsevier; 2016 [cited 2020 Jul 2];52:50–66.(527)Peng W, Williams LJ, Xu C, Melendez B, McKenzie JA, Chen Y, et al. Anti-OX40 Antibody Directly Enhances The Function of Tumor-Reactive CD8 + T Cells and Synergizes with PI3Kβ Inhibition in PTEN Loss Melanoma. Clin Cancer Res [Internet]. American Association for Cancer Research; 2019 [cited 2020 Jul 2];25:6406–16.(528)Gaspar M, Pravin J, Rodrigues L, Uhlenbroich S, Everett KL, Wollerton F, et al. A CD137/OX40 Bispecific Antibody Induces Potent Antitumor Activity That Is Dependent on Target Co-Engagement. Cancer Immunol Res [Internet]. American Association for Cancer Research; 2020 [cited 2020 Jul 2];.
Next Generation of Adoptive Cell Therapies
Our increasing knowledge of the immune system and how it interacts with cancer cells is rapidly being harnessed to expand on the number of approaches to eradicating cancer by the immune system. An approach that has already garnered lot of attention and has immense future potential is through amplifying the killing power of the immune system by providing more cancer-targeted immune cells (see sidebar on How Immunotherapeutics Work).
One way to boost the killing power of immune cells called T cells is through adoptive T-cell therapy (see sidebar on Types of Adoptive T-Cell Therapy). The goal is to dramatically increase the number of functional cancer-killing T cells in a patient. Two of these new types of immunotherapy have been approved by the FDA, axicabtagene ciloleucel and tisagenlecleucel. Both are a type of CAR T-cell therapy approved for treating certain patients with hematological cancers. The treatment involves harvesting T cells from a patient’s blood, expanding them in number, and genetically modifying them to target and kill cancer cells when infused back into the patient. Currently CAR T-cell therapy involves a complex medical process that is customized for each individual patient. Many efforts are underway to facilitate the production of these therapeutics including the development of off-the-shelf and universal CAR T cells as well as to expand these treatments beyond blood cancers.
Researchers are currently investigating ways to make T-cell therapies more powerful and persistent. Some are looking to identify safe and effective uses of gene editing techniques such as CRISPR to knock out selected genes while also adding certain DNA into CAR T cells to make them better attack cancer cells and/or to enhance T-cell survival. For instance, one area of extensive investigation is the use of CRISPR to disrupt PD-1 in order to help T cells become more effective (529)C L, H M, Y H. Applications and Explorations of CRISPR/Cas9 in CAR T-cell Therapy. Brief Funct Genomics [Internet]. Brief Funct Genomics; 2020 [cited 2020 Jul 2];19.. This strategy is similar in concept to combining PD-1 checkpoint inhibition along with adoptive T cells and may improve the clinical effect of CAR T or other adoptive T-cell therapy. A recent report demonstrated the ability of the CRISPR technique to successfully perform multiple genetic edits to the T cells. The process enabled edited T cells to sustain their ability to attack and kill tumors while surviving in the patients’ body for several months (529)C L, H M, Y H. Applications and Explorations of CRISPR/Cas9 in CAR T-cell Therapy. Brief Funct Genomics [Internet]. Brief Funct Genomics; 2020 [cited 2020 Jul 2];19.. The clinical benefit of this method in terms of patient outcomes as well as long-term safety remains to be determined. Another exciting recent application of CRISPR in cancer immunotherapy has been in the genetic manipulation of cancers that leads to putting a tag on the tumor cells so that immune cells can find, attack, and eliminate them (531)Wang G, Chow RD, Bai Z, Zhu L, Errami Y, Dai X, et al. Multiplexed activation of endogenous genes by CRISPRa elicits potent antitumor immunity. Nat Immunol [Internet]. Nature Publishing Group; 2019 [cited 2020 Jul 2];20:1494–505..
A second exciting approach to adoptive T-cell therapy is the use of tumor-infiltrating lymphocytes (TIL). Contrary to CAR T-cell therapy which uses circulating T cells in the blood, TIL therapy involves harvesting T cells from a patient’s tumor, expanding them, and infusing them back into the patient. Therefore, the TIL approach utilizes T cells that may have already been primed to recognize and target a patient’s tumor. The first evidence of the anticancer effects of TIL was demonstrated in the treatment of melanoma over three decades ago (532)Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, et al. Use of Tumor-Infiltrating Lymphocytes and Interleukin-2 in the Immunotherapy of Patients with Metastatic Melanoma. N Engl J Med [Internet]. Massachusetts Medical Society ; 1988 [cited 2020 Jul 2];319:1676–80.. Since then, the TIL approach has been shown to be effective in treating several other solid tumors including breast, colorectal, lung, and ovarian cancers (533)N Z, H C, M B, H X, YC L, Z Z, et al. Immune Recognition of Somatic Mutations Leading to Complete Durable Regression in Metastatic Breast Cancer. Nat Med [Internet]. Nat Med; 2018 [cited 2020 Jul 2];24.(534)E T, PF R, YC L, TD P, JJ G, L J, et al. T-Cell Transfer Therapy Targeting Mutant KRAS in Cancer. N Engl J Med [Internet]. N Engl J Med; 2016 [cited 2020 Jul 2];375.(535)Tumor-Infiltrating Lymphocytes Help Rein In NSCLC. Cancer Discov [Internet]. American Association for Cancer Research; 2020 [cited 2020 Jul 2];10:OF5–OF5.(536)DC D, A P, PF R, JJ G, TD P, BC P, et al. T-cell Responses to TP53 “Hotspot” Mutations and Unique Neoantigens Expressed by Human Ovarian Cancers. Clin Cancer Res [Internet]. Clin Cancer Res; 2018 [cited 2020 Jul 2];24.. Numerous clinical trials are currently underway to evaluate the long-term survival benefits of TIL therapy, alone or in combination with other immunotherapies.
The number of adoptive cell therapies against cancer in preclinical and clinical development globally is expanding rapidly and, in fact, constitutes the largest number of agents currently in development in immunotherapy (400)Yu JX, Upadhaya S, Tatake R, Barkalow F, Hubbard-Lucey VM. Cancer cell therapies: the clinical trial landscape. Nat Rev Drug Discov [Internet]. 2020 [cited 2020 Jul 2];. While a majority of these efforts are centered around T cells, many researchers are trying to harness the function of other immune cells such as macrophages, dendritic cells, or natural killer (NK) cells to eradicate cancer (see sidebar on Key Players in the Immune System) (537)Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature [Internet]. Nature Publishing Group; 2019 [cited 2020 Jul 2];572:392–6.(538)S H, VE K, BH H, C Z, MA B, BL K, et al. Dendritic Cell Paucity Leads to Dysfunctional Immune Surveillance in Pancreatic Cancer. Cancer Cell [Internet]. Cancer Cell; 2020 [cited 2020 Jul 2];37.(539). NK cell therapy approaches have garnered much attention recently due to several lines of promising early preclinical and clinical evidence. For instance, in a recent clinical study, patients with certain types of leukemia or lymphoma demonstrated significant clinical responses, including some who achieved complete remission of their cancers, when treated with CAR expressing NK cells (539). The CARs on these NK cells were engineered to target the same CD19 protein on cancer cells that is used in CAR T cell therapies. Notably, treatment with CAR NK cells did not cause some of the toxic side effects that are often associated with CAR T therapy highlighting a potential advantage of the NK cell therapy approach. Ongoing research is investigating additional NK cell therapies employing novel CARs that are directed against distinct antitumor proteins. One strategy using a genetically engineered version of the receptor NKG2D is especially exciting since this receptor can interact with eight different proteins located on the surface of cancer cell, simultaneously (540)Xiao L, Cen D, Gan H, Sun Y, Huang N, Xiong H, et al. Adoptive Transfer of NKG2D CAR mRNA-Engineered Natural Killer Cells in Colorectal Cancer Patients. Mol Ther [Internet]. Elsevier; 2019 [cited 2020 Jul 2];27:1114–25.. Such interactions may potentially enhance the specificity of NK cells toward cancer cells and thereby increase and their cancer-cell-killing ability.
Immunotherapeutics have yielded extraordinary benefits for patients with a diverse array of cancer types, but because these therapeutics work by unleashing the power of the immune system, they are often associated with adverse and sometimes severe side effects. The immune-related adverse events can affect any organ in the body and range from minor rash and local inflammation that can be treated with steroids and/or by temporarily discontinuing the treatment, to more severe adverse effects like thyroiditis and diabetes that need lifelong treatment with thyroid medications and insulin, respectively (541)June CH, Warshauer JT, Bluestone JA. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat Med [Internet]. 2017 [cited 2019 Jun 21];23:540–7.. Understanding the serious adverse events including autoimmune diabetes, cardiotoxicity, and how to mitigate them is a crucial step in order to ensure positive outcomes for all patients, and it remains an area of intensive research investigation.