- Investing in Research to Achieve a Healthier Future
- A Diverse Cancer Research and Care Workforce Drives Innovation
- Applying Regulatory Science to Ensure Safe and Effective Cancer Therapies
- Rapidly Delivering Safe and Effective Therapies to Patients
- Advancing Policies to Strengthen Cancer Prevention and Screening Programs
- Mitigating Preventable Risk Factors
- Leveraging Policy to Reduce Tobacco-related Illness
- Addressing Cancer Disparities and Improving Patient Outcomes
Advancing Cancer Science and Medical Research Through Evidence-Based Policies
In this section, you will learn:
- Robust investment in federal agencies, including NCI, is essential to advancing cancer screening, treatment, and survivorship.
- Support for education and training programs is vital to developing a medical and scientific workforce that will lead the next generation of cancer research and care.
- FDA plays a pivotal role in accelerating access to safe and effective cancer therapies by encouraging innovative clinical trials that promote broad participation of patients across the United States.
- Federal agencies, including NIH, FDA, CDC, and VA, are implementing evidence-based policies and programs to reduce the risk of cancer and lessen the disparities in cancer care that currently exist in certain populations.
The remarkable progress against cancer has been driven by sustained federal investments in biomedical research. Key agencies, including the National Institutes of Health (NIH), National Cancer Institute (NCI), US Food and Drug Administration (FDA), and Centers for Disease Control and Prevention (CDC) play vital roles in cancer prevention, early detection, treatment, and survivorship. For decades, these agencies have served as a catalyst for advancing cancer science and improving patient care.
Strategic investments in research have led to groundbreaking discoveries and more targeted treatments (see Basic Research Decoding Cancer’s Complexities), while efforts to strengthen the cancer workforce, modernize regulatory science, and expand access to prevention and screening have accelerated progress across the cancer continuum. As cancer research and care become increasingly complex, sustained funding for federal agencies and aligning public policy with the latest scientific evidence will remain essential to improving outcomes for all patients with cancer and ensuring a healthier future for them.
Investing in Research to Achieve a Healthier Future
Decades of investment and support by the United States (US) government have been critical to the development of new cancer treatments and the decline in the cancer mortality rate. Among the Department of Health and Human Services (HHS) agencies responsible for supporting this progress is NIH, the world’s largest public funder of biomedical research (1105)National Institutes of Health. Grants & Funding. Accessed: June 12, 2025.. Of the 27 institutes and centers (ICs) at NIH, NCI advances cancer research by providing individual grant funding for accomplished scientists and supporting broader clinical trials, while also training the next generation of researchers (1106)National Institutes of Health. National Cancer Institute. Accessed: June 12, 2025..
An additional component of NIH that is separate from the 27 ICs is the Advanced Research Projects Agency for Health (ARPA-H), which funds high-potential, high-impact biomedical research projects that cannot be realized through traditional commercial or research means (1107)National Institutes of Health. Advanced Research Projects Agency for Health (ARPA-H). Accessed: June 12, 2025.. Since the creation of ARPA-H in March 2022, the agency has launched multiple projects intended to improve cancer treatment, detection, and patient outcomes (1108)ARPAH Programs. Accessed: June 1, 2025..
FDA is another HHS agency that plays a major role in supporting efforts to prevent and treat cancer. Among FDA centers contributing to this mission are the Oncology Center of Excellence (OCE), which convenes experts to conduct expedited reviews of oncologic medical products (1109)US Food & Drug Administration. OCE Programs and Projects Overview. Accessed: June 12, 2025.; and the Center for Tobacco Products (CTP) (1110)US Food & Drug Administration. Center for Tobacco Products. Accessed: June 12, 2025., which regulates the sale of tobacco products and educates the public about the dangers of tobacco use.
As the primary public health agency in the United States, CDC is crucial to cancer prevention efforts. Through its Division of Cancer Prevention and Control (DCPC), CDC provides funding and support for the National Program of Cancer Registries, the largest disease surveillance system in the United States, which aims to collect, analyze, and disseminate data on cancer incidence to inform prevention and control efforts. Additional DCPC programs include the National Breast and Cervical Cancer Early Detection Program (NBCCEDP) and the Colorectal Cancer Control Program (CRCCP), which encourage cancer screenings and support various cancer prevention initiatives (1111)Centers for Disease Control and Prevention. Colorectal Cancer Control Program. Award Recipient Highlights. Accessed: March 17, 2025.(1112)Agency for Healthcare Research and Quality. Research Training and Education. Accessed: June 2, 2025..
Thanks to the investments and support provided by these federal agencies, the United States has seen tremendous progress against cancer, most prominently a drop in the cancer mortality rate. Since reaching its highest point in 1991, the overall age-adjusted cancer death rate has declined by 34 percent (6)American Cancer Society. Cancer Facts and Figures. Accessed: June 13, 2025.. This decrease would not have been achievable without the advancement of lifesaving cancer therapies. For instance, NIH funding played a central role in the development of 354 out of 356 drugs approved by FDA between 2010 and 2019, including 126 first-in-class products to treat cancer and other diseases (70)Galkina Cleary E, et al. (2023) JAMA Health Forum, 4: e230511..
Although cancer-related health outcomes have improved significantly, cancer remains the second-leading cause of death in the United States, and the need for additional investment in cancer research persists. An estimated two million new cancer diagnoses are projected in the United States in 2025, with a total of 618,000 people expected to die from the disease (6)American Cancer Society. Cancer Facts and Figures. Accessed: June 13, 2025.. Additionally, between 2010 and 2019, incidence rates of 14 cancer types increased among people under the age of 50 (see The Growing Population Burden of Cancer). Among these, cancers of the female breast, colon and rectum, kidney, and uterus contributed to the majority of the additional early-onset cancer diagnoses in 2019 (44)Shiels MS, et al. (2025) Cancer Discov: OF1..
At the same time, improvements in early detection and treatment have led to a growing population of cancer survivors who require continued care and innovation. There were an estimated 18.6 million cancer survivors in the United States in 2025, and this number is projected to exceed 22 million by 2035 (9)Wagle NS, et al. (2025) CA Cancer J Clin.. This underscores the growing importance of sustained investment in medical research.
Federal funding is crucial for training the next generation of cancer researchers, ensuring a robust scientific workforce capable of tackling complex challenges and driving advancements in cancer prevention, detection, and treatment. NIH provides vital funding for training programs, fellowships, and career development opportunities that are instrumental for early-career researchers. Funding cuts will impact the training pathway of graduate students, postdoctoral fellows, and early-career researchers, slowing down the development of new cancer therapies and imperiling progress. This includes a decline in physicians pursuing additional research training to become physician-scientists, many of whom have contributed to pivotal discoveries of novel therapies (1113)Vyas JM, et al. (2024) JAMA Netw Open, 7: e2433123.. Federally funded training awards and programs are a vital component of training and retention for the next generation of independent cancer researchers (1114)Schmidt M, et al. (2024) J Cancer Educ, 39: 490.. In addition, these training programs are uniquely positioned to improve equity in cancer outcomes and have a track record of helping diversify the biomedical research workforce and reduce cancer health disparities (1115)Ferguson MA, et al. (2025) J Cancer Educ, 40: 359.. Disruptions to NIH-funded training programs have already reduced the number of students pursuing careers in cancer research, limited opportunities for early-career scientists, and stymied the development of a knowledgeable workforce (1116)Garisto D (2025) Nature.(1117)The Transmitter. Exclusive: NIH nixes funds for pre-and postdoctoral training programs. Accessed: June 30, 2025.(1118)CalMatters. Another casualty of Trump cuts? California students pursuing science research careers. Accessed: June 30, 2025..
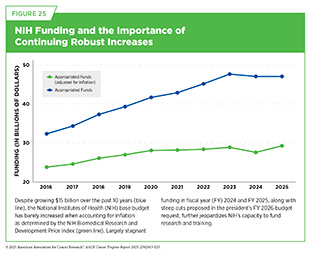
NIH has traditionally received strong bipartisan support from Congress and the White House. After more than a dozen years of stagnant funding, NIH received a budget increase every year between fiscal year (FY) 2016 and FY 2023 totaling $17.6 billion—a 57 percent increase from FY 2015. However, FY 2024 marked the first occasion in roughly a decade that Congress did not provide NIH with a funding increase, largely due to caps on discretionary non-defense spending imposed by the Fiscal Responsibility Act (1119)Congress.gov. H.R.3746 – 118th Congress (2023-2024): Fiscal Responsibility Act of 2023. Accessed: June 12, 2028.. For FY 2025, Congress kept NIH funding consistent with the FY 2024 level at approximately $47 billion. Importantly, the costs of biomedical research have been steadily increasing due to several factors, including increasing inflation, rising clinical trial complexity, and growing staffing needs (1120)Woolston C (2023) Nature, 613: 601.(1121)Harvard Gazette. How progress happens. Accessed: June 30, 2025.. Without federal funding increases to keep pace with both rising research costs and inflation, these escalating expenses will limit innovation, slow progress, and delay treatments for the patients with cancer, such as Richard Schlueter, who desperately need them.
Support for medical research also provides substantial economic benefits to the US economy. NIH funding for extramural research supports the purchase of goods, services, and materials, which contributes to job creation and economic growth in communities across the nation. In FY 2024, NIH awarded $36.94 billion to researchers across all 50 states and Washington, DC, supporting 407,782 jobs and generating $94.58 billion in economic activity (see Sidebar 5) (78)NIH’s Role in Sustraining the US Economy 2025 Update. Accessed: July 1, 2025.. Strong NIH funding also helps maintain the United States’ position as a global leader in medical research. As the United States’ peer nations continue to invest more of their gross domestic product into medical research, the need to continue to increase funding for NIH will become increasingly more important (1122)The State of the US Biomedical and Health Research Enterprise: Strategies for Achieving a Healthier America. Accessed: July 1, 2025..
Funding for NIH stagnated in the early 2010s. When adjusted for inflation, years of flat funding represented a decline in NIH’s ability to fund research and training. However, between FY 2016 and FY 2023, Congress provided NIH with robust annual budget increases, resulting in a 57 percent increase ($17.6 billion) during that time span. Unfortunately, the momentum stopped in FY 2024 and FY 2025 when NIH’s budget was held flat, potentially slowing progress in many areas of cancer research (see Figure 25).
In May 2025, the White House released the president’s budget request for FY 2026. It recommended $27 billion in funding for NIH, a proposed 40 percent decrease from its current allocation. If enacted, this drop in funding would severely impact NIH’s ability to support biomedical research and its lifesaving impact on all Americans. The FY 2026 budget request reflects just a small part of the Trump Administration’s proposals and policies that have impacted federal investment in research. In February 2025, the administration proposed a 15 percent cap on reimbursement of indirect costs for all NIH grants (1123)National Institutes of Health. NOT-OD-25-068: Supplemental Guidance to the 2024 NIH Grants Policy Statement: Indirect Cost Rates. Accessed: June 30, 2025.. Indirect costs refer to essential institutional expenses that support research, but are not tied to a specific project, such as facility maintenance, utilities, administrative support, and compliance with federal regulations. These costs are vital to keeping research programs operational. According to the United for Medical Research (UMR) 2024 Annual Economic Report, indirect costs account for approximately 24 percent of the total economic activity generated by NIH funding (78)NIH’s Role in Sustraining the US Economy 2025 Update. Accessed: July 1, 2025.. Under the direction of the administration, NIH has also significantly delayed new notices of grant awards and canceled more than 715 grants worth $815 million. Other directives include a reduction in workforce affecting more than 1,300 NIH personnel and the cancellation of numerous contracts (1124)STAT News. Trump administration orders NIH to eliminate $2.6 billion in federal contracts. Accessed: June 30, 2025..
While the president’s annual budget request is the first step in the appropriations process, ultimately, it is the responsibility of Congress to draft, negotiate, and enact spending bills that will determine the FY 2026 budget for NIH. During initial congressional hearings on the president’s FY 2026 budget request, lawmakers from both parties have criticized the administration’s proposed cuts to NIH’s budget, as well as the administration’s proposal to cap the reimbursement of indirect costs. However, many Republican lawmakers are also focused on advancing the president’s agenda to reduce federal spending. And with Republican control of both the Senate and the House of Representatives, the future of investment in medical research remains uncertain. Thus, it is imperative for the medical research community to remain actively engaged with Congress, requesting that they prioritize NIH funding and support the development of lifesaving treatments and therapies.
A Diverse Cancer Research and Care Workforce Drives Innovation
While the administration’s position has been to eliminate programs and grants related to diversity, equity, and inclusion, the American Association for Cancer Research (AACR) strongly believes that to continue building on the monumental progress that has been made in cancer research and patient care during the past few decades, a diverse workforce is essential.
A diverse scientific workforce is more likely to ask different questions, develop varied study designs, and adopt many different approaches to data collection and interpretation, ultimately expanding the scope of scientific knowledge. Research and health care are strengthened when individuals with unique perspectives and educational backgrounds work together (12)American Association for Cancer Research®. AACR Cancer Disparities Progress Report 2024. Accessed: June 14, 2025.(1125)Gomez LE, et al. (2019) J Natl Med Assoc, 111: 383..
Applying Regulatory Science to Ensure Safe and Effective Cancer Therapies
FDA plays a central role in safeguarding public health by approving medical products, including cancer therapies, that are safe and effective. The agency provides comprehensive oversight across every stage of the drug development process, including from early-stage laboratory research through clinical trials and long-term post-approval monitoring.
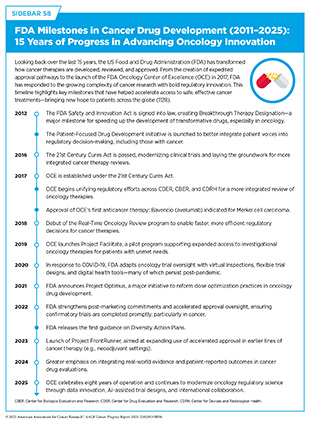
Over the past few decades, the cancer research landscape has significantly evolved, marked by increasing global collaboration and growing complexity in scientific methods, data integration, and regulatory challenges. In response, FDA has modernized its approach to keep pace with rapid scientific advancements. A major milestone in this evolution was the establishment of OCE in 2017, which was included as part of the 21st Century Cures Act (see Sidebar 58). This cross-center initiative brings together expertise from the Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER), and Center for Devices and Radiological Health (CDRH) to streamline the review and approval of anticancer therapies and devices. Since its launch in 2017, OCE has supported over 400 approvals for anticancer therapies, significantly surpassing the 239 approvals in the nine years prior (2008–2016) (1126)Zhou J, et al. (2019) J Natl Cancer Inst, 111: 449..
This progress has been made possible through sustained funding from Congress and user fees authorized by the federal law, which support the infrastructure needed to adapt regulatory science to new frontiers in precision medicine, immunotherapy, and patient-focused care. As the cancer research landscape continues to evolve, FDA remains committed to modernizing the drug development process, expanding access to innovation, and accelerating the delivery of safe and effective treatments to those who need them most.
Increasing Access to and Decentralizing Trials
Clinical trials have been a cornerstone of progress against cancer, ushering in breakthrough therapies and deepening our understanding of cancer biology (see Innovations in Cancer Clinical Trials). However, access to these trials has historically been limited. Most were conducted at large academic medical centers and primarily accessible to patients with the resources, proximity, and health status required for enrollment. Over the past 15 years, cancer clinical trials have undergone a significant transformation—from rigid, centralized models to more inclusive, patient-centered approaches. This evolution has been driven by the urgent need to expand access, improve participation, and ensure that the benefits of scientific discovery are equitably distributed across all populations affected by cancer.
The COVID-19 pandemic marked an inflection point, accelerating the adoption of innovations designed to make clinical trials more accessible. The rapid integration of remote and virtual care paved the way for decentralized clinical trials, a model that allows participants to engage from home through digital tools such as wearable devices, mobile health apps, and telemedicine platforms. These technologies have proven to be effective mechanisms to reduce access barriers and enhance participant diversity.
FDA has played a pivotal role in advancing equity in clinical research. Over the past decade, the agency has developed cancer-specific guidance aimed at increasing the inclusion of representative populations throughout all phases of drug development. Most recently, FDA released a landmark guidance in June 2024 titled Diversity Action Plans to Improve Enrollment of Participants from Underrepresented Populations in Clinical Studies (489)US Food and Drug Administration. Diversity Action Plans to Improve Enrollment of Participants from Underrepresented Populations in Clinical Studies. Accessed:. This guidance fulfills a mandate of the Food and Drug Omnibus Reform Act of 2022 and builds upon earlier drafts by offering a more detailed and actionable framework. It required sponsors of certain clinical trials to submit Diversity Action Plans outlining specific enrollment goals for underrepresented populations and the strategies to achieve and track those goals. Complementing these efforts, Project Pragmatica promotes simplified trial designs that minimize participant burden and complexity while maintaining scientific rigor. These pragmatic trials offer more generalizable findings and support broader participation (1129)US Food and Drug Administration. Project Pragmatica. Accessed: June 30, 2025..
Reflecting on the past 15 years reveals that equity in clinical trial participation has evolved from a conceptual goal to a foundational principle embedded in cancer drug development. Still, persistent gaps remain. Continued modernization of the clinical trial ecosystem is essential to ensure that all individuals affected by cancer, regardless of race, age, geography, or socioeconomic status, can access and benefit from clinical research. As the cancer research community looks to the future, building a truly inclusive and patient-centered clinical trial infrastructure remains both a scientific necessity and an ethical imperative.
Rapidly Delivering Safe and Effective Therapies to Patients
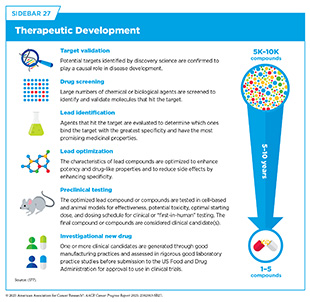
Cancer drug development continues to deliver therapeutics that are more effective and less toxic than previous treatments (see Sidebar 27). As a result, many patients with cancer are living longer and fuller lives after diagnosis. A key concept that has facilitated this progress is an increasing focus on personalized medicine, whereby knowledge of patient characteristics is leveraged to ensure maximal safety and efficacy for a specific drug in a specific patient (see Figure 7).
Biomarker testing has fueled this personalized medicine revolution by identifying patient-specific characteristics that inform therapeutic selection. Precision therapies, or drugs requiring pre-treatment biomarker testing to select patients most likely to benefit, account for over 50 percent of all oncology drugs approved between 1998 and 2024, with the percentage increasing to over 61 percent in the past 5 years (1130)Suehnholz SP, et al. (2024) Journal of Clinical Oncology, 42: e13507.. This percentage is likely to increase as more potential drug targets are identified, and more drugs are developed against known targets.
There are many recent examples of biomarkers being centered in drug development for the benefit of patients. For example, multiple meetings of FDA’s Oncology Drug Advisory Committee (ODAC) this year have highlighted the use of biomarkers to select patients who will benefit most from specific treatments. Recent decisions have recommended against expanding the indication of certain therapeutics from biomarker-defined patient populations to all patients with certain cancers or have recommended the restriction of therapeutics initially approved for biomarker-agnostic populations towards biomarker-defined ones (1131)US Food and Drug Administration. September 26, 2024: Meeting of the Oncologic Drugs Advisory Committee Meeting Announcement – 09/26/2024. Accessed: June 30, 2025.(1132)US Food and Drug Administration. May 20-21, 2025: Meeting of the Oncologic Drugs Advisory Committee – 05/20/2025. Accessed: June 30, 2025..
Additionally, FDA has become increasingly aware of pharmacogenomic biomarker testing to increase patient safety by optimizing dosages and preventing adverse events. One long-standing area of interest for OCE was DPD deficiency (see Advances in Treatment With Chemotherapy). Patients with DPD deficiency have an increased risk of severe and fatal toxicities upon receipt of fluoropyrimidine chemotherapies, which led FDA to update the labels for the fluoropyrimidines, capecitabine and 5-fluorouracil, in 2022 and 2024, respectively, to encourage health care providers to counsel patients about testing for this deficiency prior to initiating treatment with fluoropyrimidines (1133)US Food and Drug Administration. FDA approves updated drug labeling including new indications and dosing regimens for capecitabine tablets under Project Renewal. Accessed: June 30, 2025.(1134)US Food and Drug Administration. FDA approves safety labeling changes regarding DPD deficiency for fluorouracil injection products. Accessed: June 30, 2025.. To encourage further awareness of this issue, FDA released a first-of-its-kind safety announcement highlighting the risks of treating patients with DPD deficiency with fluoropyrimidines (572)US Food and Drug Administration. Safety announcement: FDA highlights importance of DPD deficiency discussions with patients prior to capecitabine or 5FU treatment. Accessed: June 25, 2025.. FDA also issued a request for public comment and partnered with AACR to host a workshop focused on the data patients and health care providers should consider when deciding whether to undergo DPD testing, interpreting test results, and determining appropriate next steps (1135)American Association for Cancer Research. 2025 FDA-AACR Workshop: DPD Deficiency and Weighing Potential Harms. Accessed: June 30, 2025.(1136)Federal Registrar. Dihydropyrimidine Dehydrogenase Deficiency and the Use of Fluoropyrimidine Chemotherapy Drugs; Establishment of a Public Docket; Request for Comments. Accessed: June 30, 2025..
FDA has kept pace with innovation in oncology drug development, despite ongoing regulatory uncertainties in recent years. A 2025 analysis found that nearly 95 percent of novel oncology therapies received FDA approval before approval by the European Medicines Agency (EMA), reflecting both sponsors’ tendency to submit first to FDA and the agency’s significantly shorter average review timelines—by more than 200 days (1137)Friends of Cancer Research. 20 Years of FDA Leadership in Novel Cancer Drug Approvals. Accessed: June 30, 2025..
This efficiency stems from FDA’s long-standing priority to speed up the approval of treatments for serious conditions through expedited review programs, including its flagship Accelerated Approval pathway, established in 1992. This program allows for earlier approval of drugs that address unmet medical needs by relying on surrogate markers reasonably likely to predict clinical benefit (1138)US Food and Drug Administration. Accelerated Approval Program. Accessed: June 30, 2025.. 226 oncology drugs have been approved through the Accelerated Approval pathway since its inception, including notable drugs such as pembrolizumab, imatinib, and trastuzumab, thereby reaching patients and saving lives faster (1138)US Food and Drug Administration. Accelerated Approval Program. Accessed: June 30, 2025..
While the Accelerated Approval pathway increases patient access to innovative therapeutics—on average 3.1 years before their own approval through the traditional pathway—it also introduces uncertainty (1139)Mehta GU, et al. (2024) J Clin Oncol, 42: 3778.. Since drugs receiving accelerated approval do not have confirmed clinical benefit, there is a risk that patients are exposed to unsafe therapies that may ultimately not improve patient outcomes. This risk is mitigated by requiring subsequent large confirmatory clinical trials to validate their expected clinical benefit. Unfortunately, not all treatments approved through this pathway have shown real-world patient benefit, leading to their withdrawal from the market. In other cases, confirmatory trials are not initiated or completed in a timely manner, further increasing the associated uncertainties and potential risks.
To remedy this, the Food, Drug, and Cosmetic Act was amended in 2023 to grant FDA new authorities, including the ability to impose additional conditions on confirmatory studies and to expedite withdrawal of products that do not meet post-approval requirements. One key authority allows FDA to require that a confirmatory trial is underway at the time of accelerated approval, ensuring timely evaluation of a therapeutic’s benefit. In 2025, FDA issued a new draft guidance delineating how it would implement these new authorities (1140)US Food and Drug Administration. Accelerated Approval and Considerations for Determining Whether a Confirmatory Trial is Underway. Accessed: June 30, 2025.(1141)US Food and Drug Administration. Accelerated Approval – Expedited Program for Serious Conditions. Accessed: June 30, 2025.. Of particular note, FDA defined the term “underway” to mean that patient enrollment has begun, and the trial has a target completion date. In some cases, FDA may even require the trial to be fully enrolled.
Complementing these regulatory updates, FDA also announced the launch of the Commissioner’s National Priority Voucher (CNPV) program—a new initiative designed to incentivize the development of therapies that address urgent public health needs. By offering expedited review vouchers for qualifying products, the CNPV program seeks to accelerate access to high-priority treatments, while reinforcing the FDA’s commitment to protecting and advancing the health interests of the American people (1142)US Food and Drug Administration. FDA to Issue New Commissioner’s National Priority Vouchers to Companies Supporting US National Interests. Accessed: June 30, 2025..
Regulatory Collaboration and Global Harmonization
Regulatory collaboration and global harmonization have become essential pillars in accelerating the development and delivery of safe, effective cancer therapies worldwide. These efforts reflect a shared understanding that health challenges, especially cancer, do not recognize borders. By aligning standards, processes, and scientific requirements across countries, regulatory harmonization facilitates faster access to new treatments, reduces inefficiencies, and helps ensure patients around the world benefit equally from biomedical innovation.
FDA has played a central role in these efforts, engaging with international stakeholders through several global organizations, including the International Council for Harmonization (ICH), the International Pharmaceutical Regulators Program (IPRP), and the International Coalition of Medicines Regulatory Authorities (ICMRA) (1143)US Food and Drug Administration. International Regulatory Harmonization. Accessed: June 30, 2025.. A key example is Project Orbis, launched in 2019 by OCE. Designed to enable concurrent submission and review of oncology drugs across multiple countries, Project Orbis has established a structured pathway for collaborative, cross-border evaluation of cancer therapies, reducing delays and enabling more patients to benefit from lifesaving treatments sooner. FDA, in collaboration with the Australian Therapeutic Goods Administration (TGA) and Health Canada (HC), conducted the first Project Orbis review (1144)Arora S, et al. (2020) Clin Cancer Res, 26: 5062.. Since then, Brazil, Israel, Singapore, Switzerland, and the United Kingdom have joined as partners. In 2024 alone, 23 oncology products were approved through Project Orbis, bringing the total to 112 approvals to date (1145)US Food and Drug Administration. Project Orbis. Accessed: June 30, 2025..
Regulatory collaboration has become a foundational element of the global oncology ecosystem, reflecting a sustained commitment to aligning regulatory science for faster and more equitable access to cancer therapies. As FDA and its international partners continue to expand these efforts, the future of cancer care will be shaped by enhanced representation, shared innovation, and faster delivery of life-saving cancer treatments.
Advancing Policies to Strengthen Cancer Prevention and Screening Programs
Nearly 40 percent of cancer cases in the United States can be attributed to preventable risk factors, such as tobacco use, dietary factors, and ultraviolet (UV) exposure (see Reducing the Risk of Cancer Development). Research has shown that routine screening for common cancers and early warning signs using evidence-based approaches is essential for improving treatment options and survival (see Cancer Screening for Early Detection). In fact, 80 percent of the lives saved from five of the most common cancers over the past 45 years have been the result of advances in prevention and screening (67)Islami F, et al. (2025) CA Cancer J Clin, 75: 216.. However, inequities in access to screenings and follow-up care contribute to health disparities and lower chances of survival in some patient populations (see Effective Strategies Increase Screening Uptake). Innovative and inclusive screening practices, from both health care providers and policymakers, are necessary to improve overall cancer prevention and screening rates. Digital health tools can improve cancer screening access and outcomes for populations with limited health care access, particularly those residing in rural areas (1146)Maita KC, et al. (2024) Perm J, 28: 130.. For states like Alabama, where 55 of their 67 counties are rural, equitable access to these lifesaving measures remains a focus for Senator Katie Britt (R-AL). In addition, targeted outreach with supportive patient navigation services can also boost cancer screening participation in rural populations (1147)Coronado GD, et al. (2025) JAMA Netw Open, 8: e250928.. Increased investment in Federally Qualified Health Centers will further improve capacities to address the unmet cancer screening needs in the US population (1148)Amboree TL, et al. (2024) JAMA Intern Med, 184: 671..
A significant barrier to cancer prevention and screening services is the associated cost and lack of insurance coverage for many individuals. The Affordable Care Act (ACA) of 2010 required private insurers and Medicaid expansion programs to cover recommended cancer screenings (e.g., mammograms, Pap tests, colonoscopies, and lung cancer screening) without patient cost-sharing. Future legislation such as H.R. 842, the Nancy Gardner Sewell Medicare Multi-Cancer Early Detection Screening Coverage Act introduced by Representative Terri Sewell (AL-07) and Representative Jodey Arrington (TX-19), would allow for multi-cancer early detection (MCED) tests to be covered by Medicare in a timely manner upon approval by FDA, enhancing cancer screening rates across a broader population (1149)Congress.gov. H.R.842 – 119th Congress (2025-2026): Nancy Gardner Sewell Medicare Multi-Cancer Early Detection Screening Coverage Act. Accessed: June 30, 2025.. Expansion of health insurance through Medicare/Medicaid and growth of ACA marketplace plans will also improve access to preventive services, cancer screening, and early detection. Importantly, for some cancers, screening rates remain below pre-COVID-19 pandemic levels and will require additional interventions (1150)Star J, et al. (2025) JAMA, 333: 1543.. Continued progress in the fight against cancer will rely heavily on eliminating the inequities that exist in the prevention, screening, and early detection of cancer.
Mitigating Preventable Risk Factors
Research has shown that modifying certain health behaviors and other lifestyle choices can reduce the risk of cancer. Human papillomavirus (HPV) can cause several cancers, including nearly all cases of cervical cancer (see Prevent and Eliminate Infection From Cancer-causing Pathogens). Effective strategies are available to prevent HPV infection and its associated cancers, including vaccination, targeted screening, and timely follow-up care. HPV vaccination rates have increased among adolescents, though more still needs to be done to reach the Healthy People 2030 goal of an 80 percent vaccination rate among adolescents (1151)Healthy People 2030. Increase the proportion of adolescents who get recommended doses of the HPV vaccine — IID-08. Accessed: June 30, 2025.. Models have shown that HPV vaccination levels significantly vary across states and tailored approaches are needed to identify and address local barriers to coverage (1152)Alarid-Escudero F, et al. (2025) J Natl Cancer Inst, 117: 737..
Although progress has been made in HPV prevention, other cancer prevention efforts still fall short in addressing other major risk factors. For example, the current strategy notably lacks a focus on tobacco control—still the leading preventable cause of cancer. Comprehensive cancer prevention must also address other critical determinants of health, including alcohol consumption, environmental exposures, and structural inequities that shape access to healthy choices.
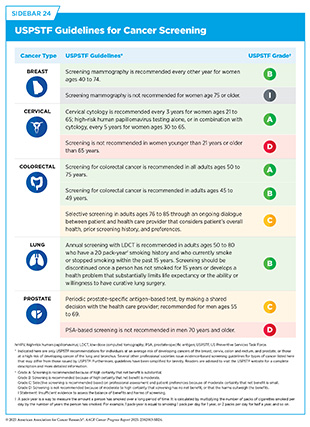
Improving Screening Guidelines and Risk-based Approaches
The US Preventive Services Task Force (USPSTF) routinely evaluates and updates cancer screening guidelines (see Sidebar 24). To address the rising cancer incidence in young adults, the USPSTF updated its guidelines in 2021 to recommend colorectal cancer screening for adults with average risk to 45 to 75 years instead of 50 to 75 years and in 2024 to recommend breast cancer screening using biennial mammography for women ages 40 to 74 years instead of 50 to 74 years. In December 2024, the USPSTF released draft changes to cervical cancer screening recommendations that include the addition of patient-collected high-risk HPV primary screening in women ages 30 to 65 years (1153)United States Preventive Services Taskforce. Draft Recommendation: Cervical Cancer: Screening. Accessed: June 30, 2025..
Currently, eligibility for population-based cancer screening programs is primarily based on age and sex. Personalized risk-based screening (PRBS) for cancer is an emerging approach that considers individual risk factors such as genetics, environmental exposures, and health behaviors to tailor screening schedules. PRBS has shown promise for improving accessibility, increasing efficiency, and reducing health care costs of traditional screening programs (1154)Dunlop KLA, et al. (2024) Prev Med, 181: 107897.. PRBS is gaining traction as the potential next step for cancer prevention and control among both the general public and health care community (1155)Tan NQP, et al. (2025) Lancet Public Health, 10: e85.. The development of artificial intelligence (AI)–based tools has enhanced the ability to utilize personalized preventive medicine techniques such as PRBS. Application of AI algorithms can effectively stratify cancer risk within the general population and shift patients from general to targeted cancer screening (see Advances in Artificial Intelligence (AI)) (1156)Gentile F, et al. (2024) ESMO Real World Data and Digital Oncology, 5: 100046.. Furthermore, the use of AI-based predictive models could define cancer prevention strategies by giving patients a greater understanding of their personal risk factors and the importance of appropriate screening, even for cancers that currently have no screening guidelines (1157)Lee KH, et al. (2025) Qual Manag Health Care, 34: 186.. These findings have fueled a shift towards PRBS to enhance the value of cancer screening; however, implementation strategies and policies are needed to address the barriers at the individual, provider, and health care system levels as well as challenges with algorithmic bias and validation of predictive models.
Cancer Prevention and Screening Programs
CDC plays a key role in promoting cancer screening and addressing cancer screening disparities as well as educating the public about cancer prevention. CDC’s DCPC runs critical programs to increase overall cancer screening rates including the NBCCEDP, National Comprehensive Cancer Control Program (NCCCP), and CRCCP. DCPC has public health campaigns that help educate people on cancer risks, including targeted outreach to those with higher risks and medically underserved populations (see Sidebar 59). CDC also compiles and provides Cancer Screening Change Packages as a resource for clinical teams to support the delivery of cancer screening services. CDC released its first set of Cancer Screening Change Packages in the summer of 2023 and released a breast cancer screening change package on July 16, 2024 (1158)Centers for Disease Control and Prevention. Breast Cancer Screening Change Package. Accessed: June 30, 2025..
Within CDC, the National Institute for Occupational Safety and Health (NIOSH) plays an important role in identifying carcinogenic hazards, tracking occupational cancer risks, and developing mitigation strategies. NIOSH maintains the List of Hazardous Drugs in Healthcare Settings, last updated in December 2024, that includes carcinogens (1159)Centers for Disease Control and Prevention. NIOSH List of Hazardous Drugs in Healthcare Settings, 2024. Accessed: June 30, 2025.. This function is essential to cancer prevention efforts in the broader population.
Sustained progress towards increasing overall cancer screening rates requires continued investment in CDC and support for legislation like H.R.2381, SCREENS for Cancer Act of 2025, introduced by Representative Joseph Morelle (D-NY-25), which would reauthorize and improve the NBCCEDP for FY 2026 through 2030 (1160)Congress.gov. H.R.2381 – 119th Congress (2025-2026): SCREENS for Cancer Act of 2025. Accessed: June 30, 2025.. Furthermore, renewed support for CDC programs and initiatives would help meet future cancer screening goals including the NBCCEDP aim to increase the percentage of women who receive overdue cervical cancer screening from 20 percent to 35 percent by 2026 (1161)Centers for Disease Control and Prevention. What CDC Is Doing to Achieve Equity in Cancer Control. Accessed: June 30, 2025..
Leveraging Policy to Reduce Tobacco-related Illness
Tobacco use remains the leading cause of preventable cancers and is linked to 17 different types of cancers, in addition to lung cancer, which is the number one cause of cancer deaths in the United States (see Eliminate Tobacco Use). Nearly half a million Americans die from cigarette smoking annually, with over 7,000 Americans dying from lung cancer related to secondhand smoke. This does not include the approximate 34,000 annual deaths from heart disease from secondhand smoke (1163)Centers for Disease Control and Prevention. The Health Consequences of Smoking – 50 Years of Progress: A Report of the Surgeon General. Accessed: June 6, 2025.(1164)Centers for Disease Control and Prevention. Tobacco-Related Mortality. Accessed: June 30, 2025.. Currently, approximately 28.8 million adults smoke cigarettes (1165)American Lung Association. Tobacco Trends Brief: Overall Tobacco Trends Brief. Accessed: June 30, 2025.. Additionally, due to predatory marketing campaigns from the tobacco industry, tobacco use remains higher in certain communities, including Black individuals and Native Americans (1166)Truth Initiative. Tracing the racist tactics of the tobacco industry. Accessed: June 30, 2025.. Members of the LGBTQ+ community also smoke tobacco at a higher rate than heterosexual/straight adults (15.3 percent compared to 11.4 percent) (1167)Centers for Disease Control and Prevention. LGBTQ+ People | For Specific Groups | Tips From Former Smokers. Accessed: June 30, 2025..
However, despite these grim statistics, notable progress has been made in both tobacco prevention and cessation, particularly among youth. While 2.25 million youth still use tobacco products, the rate of youth cigarette smoking is at a 25-year low (189)Jamal A, et al. (2024) MMWR Morb Mortal Wkly Rep, 73: 917.. These landmark numbers are a sign of progress and show the success of national anti-smoking and nicotine programs coordinated by both federal and non-federal entities, such as the recently launched Outsmart Nicotine campaign (1168)Truth Initiative. Outsmart Nicotine. Accessed: June 30, 2025. and the long-standing 1-800-QUIT-NOW helpline (1169)Centers for Disease Control and Prevention. Five Reasons Why Calling a Quitline Can Be Key to Your Success | Quit Smoking | Tips From Former Smokers. Accessed: June 30, 2025.. Therefore, while there is still progress to be made, overall cigarette smoking rates have continued to fall (1165)American Lung Association. Tobacco Trends Brief: Overall Tobacco Trends Brief. Accessed: June 30, 2025..
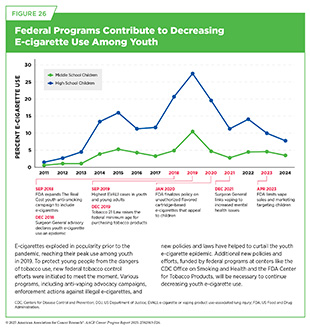
At the peak of the electronic cigarettes (e-cigarettes) epidemic in 2019, 31 percent of high school students and 12.5 percent of middle school students reported current use of a tobacco product, with the vast majority of use consisting of e-cigarettes (1170)Wang TW, et al. (2019) MMWR Surveill Summ, 68: 1.. In 2024, these rates fell to 10.1 percent and 5.4 percent, respectively, driven by major decreases in e-cigarette use (1171)US Food and Drug Administration. Results from the Annual National Youth Tobacco Survey (NYTS). Accessed: June 30, 2025.. In fact, youth e-cigarette use reached its lowest level in a decade, with 5.9 percent of youth reporting current use. These successes resulted in large part from swift policy action and federal funding for e-cigarette prevention programs and should be celebrated (see Figure 26).
Unfortunately, no tobacco product is safe and tobacco product use remains the leading cause of preventable disease and death in the United States. While tobacco use is generally decreasing, nearly 20 percent of adults still report use of any tobacco product and 14.6 percent report use of combustible tobacco products, the most harmful type of product (1172)Centers for Disease Control and Prevention. Current Cigarette Smoking Among Adults in the United States | Smoking and Tobacco Use. Accessed: June 30, 2025.. In the face of massive cuts and reorganization to federal tobacco control and regulation organizations including CDC’s Office of Smoking and Health (OSH) and FDA’s CTP, it is imperative to highlight the successes of these organizations and continue pushing for and implementing innovative tobacco control policies and research to further drive the decline in tobacco use (1173)American Lung Association. Lifesaving Programs to Prevent and Reduce Tobacco Use Go Up in Smoke. Accessed: June 30, 2025..
Flavored Tobacco Products
Evidence-based policies that address the evolution of tobacco products to include new additives and flavors are needed to reduce tobacco-related illness and health disparities. While cigarettes with characterizing flavors other than menthol were nationally prohibited in 2009, the addition of flavors to tobacco products remains prevalent. Menthol cigarettes and flavor additives in other combustible and non-combustible products remain common. This increases their appeal, is associated with greater rates of cigarette initiation, facilitates sustained use, and decreases rates of successful cessation, particularly among youth (1174)Bold KW, et al. (2024) Annu Rev Clin Psychol, 20: 381.. As a result of the substantial scientific evidence supporting prohibition of menthol and other flavors in tobacco products, FDA proposed draft rules in 2022 that would ban menthol as a characterizing flavor in cigarettes and all characterizing flavors, including menthol, in cigars. However, on January 21, 2025, the proposed rule was withdrawn (1175)Reuters. Trump administration withdraws FDA plan to ban menthol cigarettes. Accessed: June 30, 2025.. Currently, multiple states and localities have implemented restrictions on flavored tobacco products (1176)Campaign for Tobacco-free Kids. States and Localities That Have Restricted the Sale of Flavored Tobacco Products. Accessed: June 30, 2025.. Notably, while there has been state and local adoption of limitations on flavored tobacco products, these policies have primarily targeted electronic nicotine delivery systems (ENDS) and non-combustible tobacco products (1177)Whitacre TR, et al. (2025) Tob Control.. Novel regulatory approaches to banning flavors in tobacco products are needed to realize the tremendous potential public health benefits. Comprehensive policies that prohibit flavors as well as ingredients or additives that affect sensory properties would close possible loopholes and better achieve the intended health outcomes (1178)Kyriakos CN, et al. (2025) Tob Control, 34: 239.. In addition, research is needed to identify effective public health messaging for flavored tobacco products to prevent use initiation, promote cessation, and advance harm reduction. Robust policies that restrict both flavored tobacco product sales and marketing contribute to lower levels of adolescent exposure to flavored tobacco product advertising and may reduce product use (1179)Clark D, et al. (2024) Public Health Rep: 333549241300095.. Reducing the addictiveness of tobacco products by prohibiting flavors and sensory modifiers would help prevent tobacco-related cancers and save millions of lives in the coming decades.
Funding Federal Regulatory, Enforcement, and Control Activities
FDA’s tobacco regulatory and enforcement activities, helmed by CTP, are entirely funded by user fees charged to manufacturers and importers of certain tobacco products. However, unlike all other tobacco product manufacturers, ENDS (e.g., e-cigarettes) manufacturers currently do not pay user fees to FDA. The bipartisan-supported Resources to Prevent Youth Vaping Act (S. 3653) was introduced in the 118th Congress to authorize FDA to begin collecting user fees from e-cigarette manufacturers and would provide FDA’s CTP an additional $100 million in funding per year, but no further legislative action has occurred (1180)Congress.gov. US Food and Drug Administration (FDA) Regulation of Electronic Nicotine Delivery Systems (ENDS): Background and Selected Policy Issues. Accessed: June 30, 2025.. Requiring e-cigarette manufacturers to pay user fees to FDA will be critical to supporting efforts to control tobacco use, including enforcement actions against illegal products, as CTP currently experiences an outsized workload related to these products. Despite this gap in funding, FDA’s CTP has recently made strides across four key areas it manages: regulation and guidance, compliance and enforcement, science and research, and public health education.
DA CTP’s regulatory efforts are multifaceted, including product approvals and rulemaking, among other roles. FDA CTP has received an unprecedented number of premarket tobacco applications (PMTAs), submitted by tobacco companies seeking authorization to sell their products, having processed over 26 million out of the total 27 million applications submitted (1181)Energy & Commerce Committee Democrats. Hearing on “Evaluating FDA Human Foods and Tobacco Programs”. Accessed: June 30, 2025.. While the vast majority have led to marketing denial orders, some products have been authorized. In particular, there are now 34 FDA-authorized e-cigarette products, and marketing authorization was recently granted to a novel nicotine pouch product (1182)US Food and Drug Administration. FDA Authorizes Marketing of 20 ZYN Nicotine Pouch Products after Extensive Scientific Review. Accessed: June 30, 2025.(1183)FDA Center for Tobacco Products. FDA Tobacco Education & Prevention Resources: Posters, Flyers, & More | FDA Authorized E-Cigarette Products. Accessed: June 30, 2025.. Although these products pose health risks, as do all nicotine products, FDA determined that the benefits of these products outweigh the risks to the population as a whole. When authorizing new tobacco products, FDA scrutinizes the potential benefit of completely switching to the new product for adults who use cigarettes and/or other smokeless tobacco products, as these products are expected to confer lower risk of cancer and other tobacco-related illnesses. This is balanced against the risk of new users, including youth, who may begin to use the new products. FDA continues to monitor these decisions in the post-market setting to ensure that they meet the necessary public health standards. Additionally, FDA finalized guidance outlined by legislation in 2019 to prohibit the sale of tobacco products to those younger than 21 years of age. While this law has been in effect since 2019, this recent finalization brings FDA regulation into line with legislation and signifies FDA’s commitment to preventing youth tobacco use (1184)US Food and Drug Administration. FDA Issues Final Rule Increasing the Minimum Age for Certain Restrictions on Tobacco Sales. Accessed: June 30, 2025.. FDA has also proposed a rule that would reduce nicotine levels in many combustible products by 95 percent. If finalized, this rule is projected to cause nearly 13 million people to quit smoking within one year, prevent 1.8 million tobacco-related deaths by 2060, and save more than $1.1 trillion (1185)Federal Registrar. Tobacco Product Standard for Nicotine Yield of Cigarettes and Certain Other Combusted Tobacco Products. Accessed: June 30, 2025..
FDA has also recently increased its enforcement efforts to prevent unauthorized illegal tobacco products from being sold. Last year, an FDA-Department of Justice task force was created to curtail the massive illegal e-cigarette marketplace, which constitutes 86 percent of all e-cigarette products and includes nearly all flavored products (1186)US Food asnd Drug Administration. Justice Department and FDA Announce Federal Multi-Agency Task Force to Curb the Distribution and Sale of Illegal E-Cigarettes. Accessed: June 30, 2025.(1187)Truth Initiative. US retail sales data show 86% of e-cigarette sales are for illegal products. Accessed: June 30, 2-25.. Enforcement actions have ramped up since the creation of this task force, seizing millions of dollars of products, issuing warning letters, and seeking fines (1188)US Food and Drug Administration. A Year in Review: FDA’s Progress on Tobacco Product Regulation in 2024. Accessed: June 30, 2025.. However, most of these actions have been limited to smaller or foreign organizations and have not yet substantially impacted this multibillion-dollar market.
Federal educational campaigns to prevent tobacco initiation have also come to fruition. Exposure to FDA’s The Real Cost e-cigarette prevention campaign was directly correlated with decreased likelihood of reporting e-cigarette initiation and prevented an estimated 450,000 youth from initiating cigarettes between 2023 and 2024 (1189)MacMonegle A, et al. (2025) Am J Prev Med.. CDC OSH’s Tips from Former Smokers (Tips) campaign was also significantly associated with calls to federal quit lines, generating an estimated 2.1 million calls between 2012 and 2023, or nearly 25 percent of all calls (1190)Mann NH, et al. (2025) Nicotine Tob Res, 27: 326..
In addition to the Tips campaign, CDC OSH’s other programs have significantly contributed to the protection of the American public from the harms of tobacco use. OSH tracks tobacco product use and analyzes changes in tobacco-related disease incidence, including contributing to the yearly National Youth Tobacco Survey (1171)US Food and Drug Administration. Results from the Annual National Youth Tobacco Survey (NYTS). Accessed: June 30, 2025. alongside FDA CTP, providing essential longitudinal insight into how youth are interacting with tobacco products and how to curb future use. CDC OSH also supports all state tobacco control programs through their National and State Tobacco Control Program (1191)Centers for Disease Control and Prevention. CDC-RFA-DP20-2001: National and State Tobacco Control Program | Smoking and Tobacco Use. Accessed: June 30, 2025., which provides funding, increases reach and accessibility, and supplies expertise and technological support. CDC OSH is set to fund state tobacco control programs for nearly $70 million in the 2024–2025 year (1191)Centers for Disease Control and Prevention. CDC-RFA-DP20-2001: National and State Tobacco Control Program | Smoking and Tobacco Use. Accessed: June 30, 2025.. Historically, every $1 spent on these programs leads to a $55 return on investment in averted health care costs (1192)Centers for Disease Control and Prevention. About the Office on Smoking and Health | National Center for Chronic Disease Prevention and Health Promotion (NCCDPHP). Accessed: June 30, 2025., highlighting the significant costs of tobacco-related chronic disease and the incredible benefits of a strong CDC OSH.
The efforts outlined here, spanning regulation, education, and research, highlight the importance and impact of funding for federal tobacco-related programs. While these programs have generated tangible health benefits for the American public, their futures remain uncertain. Additional policies supported by these programs that could reduce tobacco-related illness include improved insurance coverage for evidence-based smoking cessation therapies; further restrictions on tobacco product advertising and promotions; and increased funding for FDA, NCI, and CDC smoking awareness, research, and cessation programs. As highlighted by the 2024 US Surgeon General’s Report, Eliminating Tobacco-Related Disease and Death, though there has been much progress in tobacco control, tobacco remains a major cause of death and poses an outsized danger to vulnerable populations. Efforts proposed in the Surgeon General’s Report to eliminate the burden of death and disease resulting from tobacco use include further restrictions to flavored products and consumer marketing of tobacco products as well as the implementation of the proposed rule for nicotine reduction (1193)Centers for Disease Control and Prevention. Surgeon General’s Report: Eliminating Tobacco-Related Disease and Death: Addressing Disparities. Accessed: June 30, 2025..
Addressing Cancer Disparities and Improving Patient Outcomes
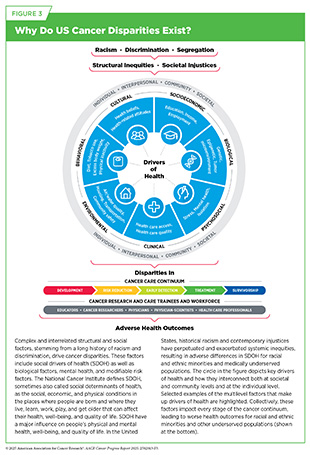
As described in the AACR Cancer Disparities Progress Report 2024 (12)American Association for Cancer Research®. AACR Cancer Disparities Progress Report 2024. Accessed: June 14, 2025., cancer and other health disparities are driven by a complex set of interrelated causes, including social, economic, and environmental factors—collectively known as social determinants or social drivers of health (SDOH) (see Figure 3) (1194)Healthy People 2030. Social Determinants of Health. Accessed: June 30, 2025.. Addressing the negative impact of SDOH is essential for achieving health equity and will continue to require multifaceted solutions, including new policies at the federal, state, and local levels, as well as collaboration between policymakers, health care providers, and patients.
Environmental Justice and Cancer
Within SDOH, environmental factors remain an ongoing subject of discussion on how marginalized communities can face increased cancer risks. Environmental justice as a movement arose in response to a disproportionate exposure of harmful pollution, toxic sites and facilities, and other carcinogenic factors. The “Cancer Alley,” a regional nickname for a stretch of land between Baton Rouge and New Orleans, Louisiana, is home to over 150 oil refineries, plastic plants, and chemical facilities (1195)United Nations News Center. Environmental racism in Louisiana’s ‘Cancer Alley’, must end, say UN human rights experts. Accessed: June 30, 2025., and has a 40 percent Black population, as compared with the national 12 percent average. Given the petrochemical environment, which has ramifications for both quality of life and for health outcomes, and other factors, the risk of cancer in “Cancer Alley” is 95 percent higher than in most of the country (see Limit Exposure to Environmental Risk Factors) (1196)James W, et al. (2012) Int J Environ Res Public Health, 9: 4365..
Environmental justice is not limited to geographic factors, with matters like economic status also impacting health outcomes. In older housing environments, exposure to lead in paint, dust, and pipes has resulted in one in five children in these homes having higher blood lead levels (1197)Gochfeld M, et al. (2011) Am J Public Health, 101 Suppl 1: S53., as compared to one in eight in other settings. Communities impacted by environmental factors deserve additional resources in prevention, screening, and treatment. Addressing these externalities must remain a priority for policymakers, to ensure no one faces a higher risk due to environmental factors.
Patient-centered Care and Outcomes and Survivorship
As cancer research continues to improve overall detection and treatment, there are millions of cancer survivors. As of January 2022, an estimated 18.1 million cancer survivors live in the United States, including nearly 500,000 who were diagnosed before the age of 20 (9)Wagle NS, et al. (2025) CA Cancer J Clin., underscoring the impact of sustained investment in research. As more people survive cancer, the need to study the long-term health of these survivors continues to grow, particularly for pediatric survivors whose treatment options are more likely to have many more long-term consequences (see Addressing the Unique Challenges Faced by Vulnerable Populations). Policies like the Childhood Cancer STAR (Survivorship, Treatment, Access, Research) Act, which saw bipartisan sponsors, including Co-Chair of the House Cancer Caucus Representative Mike Kelly (R-PA), help provide researchers with the tools to better address these research gaps.
Quality cancer care includes survivorship, and continued research investments in this area are essential for overall health. Patient-centered care can improve overall survival quality of life, for both the survivor and their support system. Both the physical and mental health of cancer survivors should remain an integral part of holistic cancer research. CDC supports cancer survivors by providing resources with nuanced tools for all stages of survival—including factors like hair loss (1198)Centers for Disease Control and Prevention. Cancer Survivors. Accessed: June 30, 2025. and stress mitigation tools for the post-treatment period.
For so many pediatric cancer survivors and their support systems, returning to a sense of normalcy can be complex and often includes late effects of treatment (1199)National Cancer Institute. Childhood Cancer Survivor Study: An Overview. Accessed: June 30, 2025.. Tools like the NCI’s Childhood Cancer Data Initiative (CCDI) (1200)National Cancer Institute. Childhood Cancer Data Initiative (CCDI). Accessed: June 12, 2025. can help researchers access necessary information to improve survivorship and mitigate negative side effects. The Children’s Oncology Group (COG), which is a member of the NCI’s National Clinical Trials Network (NCTN), is the world’s largest organization devoted exclusively to childhood and adolescent cancer research. COG has more than 100 active clinical trials. There are approximately 12,000 patients registered on COG trials each year. These trials include front-line treatment for many types of childhood cancers, studies aimed at determining the underlying biology of these diseases, and trials involving new and emerging treatments, supportive care, and survivorship (1201)Children’s Oncology Group. Survivorship Guidelines. Accessed: June 30, 2025.(1202)St. Jude Children Research Hospital. CCSS. Childhood Cancer Survivor Study. Accessed: June 30, 2025..
Improving the Use of Digital Information in Cancer Treatment and Management
To improve the delivery of care to patients and improve outcomes for survivors, it is critically important to expand the use of digital health information, including making health registries more robust, timely, and inclusive of information beyond what they currently collect. There is an accompanying need to standardize the collection, security, and dissemination of this information for patients, caregivers, clinicians, and researchers. Although progress has been made in the expansion of electronic health records and telemedicine, opportunities to maximize the benefits of digital health care technologies in oncology have been growing (1203)Parikh RB, et al. (2022) J Natl Cancer Inst, 114: 1338.. For example, patient information is often stored across multiple databases, depending on the systems used by a patient’s health care providers, pharmacies, and insurers. Tracking patient data, including patient reported outcomes, is therefore incredibly difficult, hampering effective care (1204)Bradley CJ, et al. (2022) J Natl Cancer Inst, 114: 1065.. There are also significant shortcomings in the collection and systematic organization of different types of potentially relevant patient data, including the patient’s race and ethnicity, sexual orientation and gender identity, patient reported outcomes of cancer therapy, and diagnostic results (1203)Parikh RB, et al. (2022) J Natl Cancer Inst, 114: 1338.(1204)Bradley CJ, et al. (2022) J Natl Cancer Inst, 114: 1065..
Federal investments in programs like NCI’s CCDI (1200)National Cancer Institute. Childhood Cancer Data Initiative (CCDI). Accessed: June 12, 2025. underscore how increased data sharing can better improve overall treatment and survivorship. CCDI is tasked with gathering data on every child, adolescent, and young adult with a childhood cancer diagnosis and with developing a platform to share the research data involved in their treatment. Having shareable data on rare pediatric cancers, which are often hard to diagnose and lack standardized care, can help improve both outcomes and survivorship.
Next Section: The AACR Call to Action Previous Section: Envisioning the Future of Cancer Science and Medicine