- Routine Cancer Screening Saves Lives
- Evidence Guides Cancer Screening Recommendations
- Eligibility Criteria for Cancer Screening
- Recommendations for Cancer Screening
- Tests for Cancer Screening
- Research Improves the Science of Screening
- Effective Strategies Increase Screening Uptake
- Technological Innovations Advance Cancer Screening for Early Detection
Cancer Screening for Early Detection
In this section, you will learn:
- Cancer screening for early detection means checking for cancer in people who are at an average or increased risk of developing cancer but do not have symptoms of the disease.
- Detecting cancer early through routine screening saves and improves lives and reduces health care costs.
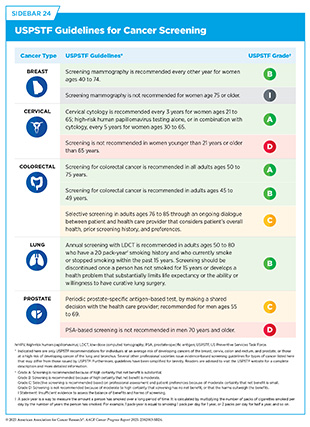
- The US Preventive Services Task Force, a panel of experts in evidence-based medicine, recommends routine screening for cancers of the breast, cervix, colon and rectum, lung and bronchus, and prostate.
- Improving access and adherence to routine cancer screening and follow-up care for all eligible individuals through evidence-based interventions can significantly reduce the burden of cancer.
- Technological advances in artificial intelligence, minimally invasive screening tests, and imaging are fueling optimism for improved approaches to detecting cancers early.
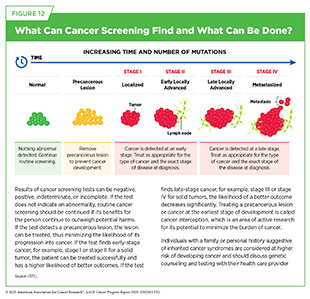
Cancer screening means checking for cancer or abnormal cells that may become cancerous in people who have no signs or symptoms of cancer. Routine screening plays an essential role in reducing the burden of cancer at the population level by detecting precancerous lesions and cancers at the earliest possible stage when they can be treated successfully before developing into advanced-stage cancer that spread to other parts of the body (see Figure 12).
Routine Cancer Screening Saves Lives
Routine cancer screening helps reduce the overall cancer burden by identifying and treating precancerous changes or early-stage cancers when treatment is more effective and the chance of better outcomes is higher. A study analyzing deaths averted from breast, cervical, colorectal, lung, and prostate cancers between 1975 and 2020 found that screening and prevention collectively prevented 4.75 million of the 5.94 million estimated cancer deaths during that period (67)Islami F, et al. (2025) CA Cancer J Clin, 75: 216.. The impact of each approach varied by cancer type. All of the reduction in cervical cancer mortality was due to screening and treatment of precancerous lesions. In colorectal cancer, 79 percent of lives were saved through screening and removal of precancerous polyps. Screening accounted for 56 percent of deaths averted from prostate cancer and 25 percent from breast cancer, with treatment advances responsible for the remainder. In contrast, nearly all of the lives saved from lung cancer during this time were due to prevention, particularly tobacco control efforts (67)Islami F, et al. (2025) CA Cancer J Clin, 75: 216..
Another large study showed that a negative result from colonoscopy—one with no signs of cancer or polyps—is associated with lower likelihood of colorectal cancer. A 20-year follow-up of nearly 200,000 people revealed that those with a negative screening colonoscopy had a 49 percent lower risk of developing colorectal cancer and a 44 percent lower risk of dying from it, compared to those who were never screened (373)Knudsen MD, et al. (2025) JAMA Oncol, 11: 46..
Routine cancer screening also reduces the likelihood of disease being diagnosed at a more advanced stage and may help avoid more intensive treatments. For example, a recent study of over 13,000 women ages 70 to 79, who were diagnosed with breast cancer between 2010 and 2017, found that women who had at least one screening mammogram in the 5 years before their diagnosis were 54 percent less likely to be diagnosed at a late stage and 36 percent less likely to die from breast cancer than those who had not been screened. The benefits were even greater for women who had three to four screenings; these women were 37 percent less likely to die from breast cancer compared to those who had one screening (374)Huang S, et al. (2025) JAMA Netw Open, 8: e255322..
In addition to reducing the burden of cancer at the population level, research is showing that cancer screening also costs less compared to cancer treatment. In 2021, the United States spent $43 billion on initial cancer screening. In comparison, the cost of the first 12 months of cancer treatment in 2021 was estimated to be $52.56 billion (375)Halpern MT, et al. (2024) Ann Intern Med, 177: 1170..
Routine cancer screening has proven to be one of the most effective tools in reducing cancer mortality, improving survival, and containing health care costs. Continued investment in equitable, evidence-based screening programs not only saves lives but also alleviates the broader burden of cancer on patients, families, and health systems.
Evidence Guides Cancer Screening Recommendations
Panels of subject matter experts—convened by government agencies and professional public health organizations—develop cancer screening recommendations through a structured, evidence-based review process. This report focuses on the recommendations issued by the US Preventive Services Task Force (USPSTF), a congressionally mandated independent panel convened by the Agency for Healthcare Research and Quality, part of the US Department of Health and Human Services (see Improving Screening Guidelines and Risk-based Approaches). USPSTF provides guidance designed for use in primary care to help prevent disease, including cancer. Its multistep process includes a comprehensive review of the available evidence, as well as engagement with both the scientific community and the public before issuing final recommendations. Other cancer-focused organizations also issue screening recommendations. While specific processes may vary across organizations, all aim to apply the same level of rigor to maximize benefit and minimize harm to public health.
Eligibility Criteria for Cancer Screening
A number of factors determine a person’s lifetime risk of developing cancer and eligibility for cancer screening. People without a personal or family history of cancer or a known inherited genetic condition are considered at average risk. For these individuals, screening recommendations are generally based on age and sex assigned at birth.
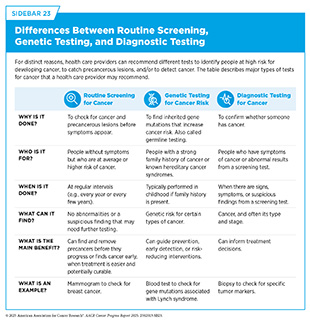
Certain people have specific cancer screening recommendations because they are at a higher risk of developing cancer than the general population. For example, exposure to cancer risk factors increases the likelihood of developing cancer (see Reducing the Risk of Cancer Development) and guides screening recommendations, such as those for lung cancer in people who smoke. Individuals who have been diagnosed with cancer (see Supporting Cancer Patients and Survivors) or those who have multiple immediate family members diagnosed with cancer are also at a higher risk. Similarly, those with hereditary cancer syndromes are at higher risk of developing cancer because of the genetic mutations they carry (see Figure 6). Experts recommend genetic testing and counseling for people with hereditary cancer syndromes. There are key differences between routine screening to detect cancer at the earliest possible stage, genetic testing to assess the risk of cancer, and diagnostic testing to confirm whether a person has cancer (see Sidebar 23).
The composition of specific tissues can also influence the risk of developing cancer. For example, research has shown that women with dense breast tissue have a higher risk of developing breast cancer. An analysis of mammograms from more than 33,000 women screened for breast cancer from 2000 to 2018 showed that women with dense breasts were 1.7 times more likely to develop breast cancer than those with less dense breast tissue (376)Lange JM, et al. (2025) Am J Epidemiol, 194: 441.. Having dense breasts is not abnormal but dense breast tissue and tumors both appear white on a mammogram, which can obscure cancer detection. This masking effect prompted the US Food and Drug Administration (FDA) to mandate all mammography facilities to provide patients with information about their breast density. The regulation, finalized in March 2023, went into effect in September 2024 (377)US Food and Drug Administration. FDA Updates Mammography Regulations to Require Reporting of Breast Density Information and Enhance Facility Oversight | FDA. Accessed: June 5, 2025.. Researchers are actively working to identify approaches that can supplement routine mammography for detecting breast cancer early in women with dense breasts. Women with dense breasts should consider a continued dialogue with their health care providers to stay informed about their potential risk for developing breast cancer.
Some factors that inform cancer screening decisions, such as exposure to cancer risk factors, differ across individuals and can change over time. Furthermore, screening guidelines are revised and updated as new evidence for the benefits or harm of screening comes to light. People should stay informed through regular dialogue with their health care provider and develop a personalized screening plan that reflects their changing risk of developing cancer and tolerance for the potential harms of screening over the course of their lifetime.
Recommendations for Cancer Screening
USPSTF makes screening recommendations for different types of cancer based on whether the benefits outweigh the potential harms. In some cases, USPSTF recommends against screening if the harm is likely to be greater than the benefits or issues an Insufficient Statement indicating there is not enough evidence to make a clear recommendation.
Each USPSTF recommendation is given a letter grade that reflects how strong the evidence is (see Sidebar 24). This grade also affects whether a screening test is covered without out-of-pocket costs under the Patient Protection and Affordable Care Act (ACA). Sometimes, USPSTF gives different grades for different groups of people for the same type of cancer. All final recommendations and the evidence supporting them are published in scientific journals and on the USPSTF website.
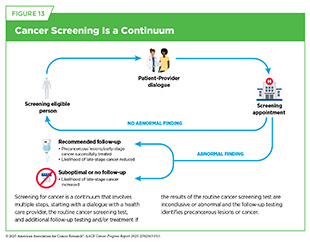
Cancer screening involves several steps, starting with a conversation between individuals and their health care provider about the benefits and risks of screening based on personal factors, such as family history. If the screening test shows no concerns, no further action is needed until the next scheduled screening. However, if the test finds possible signs of cancer or unclear results, follow-up care may be needed, including additional testing to confirm a diagnosis and, if necessary, starting treatment (see Figure 13).
Tests for Cancer Screening
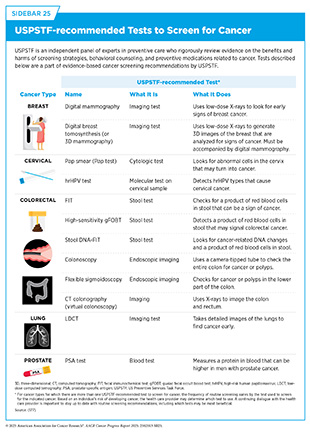
Cancer screening tests include laboratory procedures that detect cellular or molecular signs of cancer in blood or other biospecimens, as well as imaging or endoscopic procedures that identify tissue changes suggestive of cancer. USPSTF carefully weighs the potential benefits of tests against any risks associated with a given test, ensuring that recommendations will improve public health at the population level (see Sidebar 25).
Like all medical procedures, screening tests carry some limitations. For example, while complications (e.g., bleeding) from procedures such as colonoscopies are exceedingly rare (379)Lv XH, et al. (2025) Am J Gastroenterol., they are possible. Screening may also yield false-positive results, which can lead to unnecessary follow-up testing, concern and anxiety, or false-negative results, which may delay diagnosis. In some cases, screening may detect slow-growing cancers or medical conditions unrelated to cancer that would not have progressed or caused harm during a person’s lifetime. These findings can lead to further testing and interventions that may not always be necessary. Researchers continue to develop new screening tests and improve existing ones with the goal of minimizing potential harm from these tests without compromising their effectiveness in detecting cancer early.
Research Improves the Science of Screening
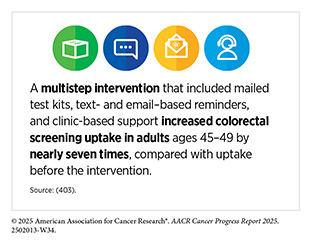
Cancer screening for early detection is a rapidly evolving field. Many studies focus on evaluating the real-world impact of recommended screening tests and identifying ways to reduce potential harm from routine screening. As one example, a recent study of over 10,000 patients found that the USPSTF-recommended fecal immunochemical test (FIT) for colorectal cancer screening reduced the risk of dying from colorectal cancer by 33 percent among study participants (380)Doubeni CA, et al. (2024) JAMA Netw Open, 7: e2423671., indicating the impact of easy-to-use screening tests on reducing the burden of cancer. A second study found that adjusting the test to be more sensitive helped find 8 percent more cases of colorectal cancer without increasing false-positive test results, making it affordable to improve noninvasive colorectal cancer screening (48). A study from the Netherlands found that at-home stool tests could safely replace follow-up colonoscopies after polyp removal without missing serious cases (381)Carvalho B, et al. (2025) Gastroenterology, 168: 121., thus reducing the use of an invasive screening test. Using annual FIT reduced the number of follow-up colonoscopies by up to 41 percent and still effectively detected advanced colorectal cancer (381)Carvalho B, et al. (2025) Gastroenterology, 168: 121.. Another recent study found that, compared to receiving multitarget DNA tests (see Sidebar 25) or blood-based tests every 3 years, annual FIT was most effective in low-resource community clinics, yielding 121 life-years gained per 1,000 screened individuals and a net monetary benefit of $5,883 per person (382)Nascimento de Lima P, et al. (2025) JAMA Netw Open, 8: e2454938.. Together, these findings support using at-home stool tests for colorectal cancer screening in reducing the burden of disease at the population level, and suggest that the test may be more cost-effective for screening and follow-up care than alternatives, especially in low-resource settings and for large-scale screening programs.
Researchers continue to evaluate the public health impact of existing screening recommendations and to identify aspects that can be improved further. A large national study of more than two million women evaluated the impact of the 2009 change to USPSTF breast cancer screening guidelines recommending biennial instead of annual mammograms for women under 50. Although less frequent mammograms led to a decline in early-stage breast cancer diagnoses from 2004 to 2019, this decline did not result in an increase in more advanced cancers, as experts had feared (384)Zhang-Petersen C, et al. (2024) JAMA Netw Open, 7: e2452688.(385)Hendrick RE, et al. (2011) AJR Am J Roentgenol, 196: W112.. Of note, overtreatment, i.e., surgeries to remove an entire breast, decreased following the revised recommendation (384)Zhang-Petersen C, et al. (2024) JAMA Netw Open, 7: e2452688..
USPSTF recommends a screening colonoscopy every 10 years after a colonoscopy with negative results. One recent study analyzed colonoscopy data from the past 40 years while taking into account personal risks and found that some individuals with a low risk of developing colorectal cancer can safely wait longer than 10 years for their next colonoscopy (373)Knudsen MD, et al. (2025) JAMA Oncol, 11: 46.. The findings of this study highlights the positive impact of evidence-based screening recommendations and identifies areas in which further improvements are possible.
Assessing newer and improved screening methods is another area of active research. For example, new findings are adding to growing evidence that digital breast tomosynthesis (DBT), an advanced form of three-dimensional mammography, may help detect breast cancer earlier and reduce the number of more serious, late-stage cases (387)Kim SY, et al. (2024) Radiology, 312: e242008.. A study of more than 270,000 screenings found that DBT identified more cancers than standard digital mammography (DM) and did so with fewer false-positive results (388)Philpotts LE, et al. (2024) Radiology, 312: e232841.. More importantly, the study found that among invasive cancers—those that have spread to nearby tissues—the proportion classified as advanced cancers—those that have spread to other organs extensively—was lower with DBT compared to DM (32.6 percent vs. 43.6 percent). This difference was even more striking during repeat screening (29.1 percent with DBT compared to 44 percent with DM), indicating that regular screening with DBT could lead to less likelihood of being diagnosed with more advanced cancers (388)Philpotts LE, et al. (2024) Radiology, 312: e232841.. In another study of over 208,000 women with a family history of breast cancer, DBT significantly outperformed DM by reducing false positives and unnecessary follow-ups. It also found fewer advanced cancers in women with extremely dense breasts, indicating that more aggressive tumors are diagnosed at an earlier stage (389)Li T, et al. (2025) JAMA Oncol.. Although potential concerns about overdiagnosis and equitable access to Black and Hispanic women remain, these findings indicate that DBT is superior in catching cancers earlier, especially in high-risk women, potentially reducing the need for more aggressive care.
Collectively, studies discussed here exemplify how new research improves early detection of cancer through development of smarter tools and more personalized strategies. These advances are helping make cancer screening more effective, efficient, and patient-centered.
Effective Strategies Increase Screening Uptake
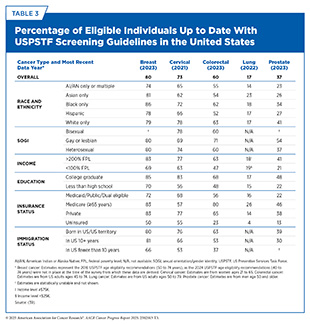
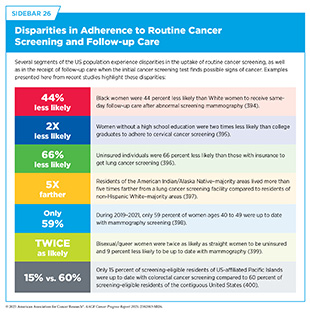
Getting screened for cancer according to recommendations is one of the most effective ways to reduce cancer deaths, yet many people in the United States are not up to date on their screenings (see Table 3). Screening rates are especially low among certain groups, including racial and ethnic minorities, residents of sovereign Native Nations, and people with limited access to health care (12)American Association for Cancer Research®. AACR Cancer Disparities Progress Report 2024. Accessed: June 14, 2025.. Suboptimal uptake of routine screening also means getting screened when it is not necessary. A recent study found that it can take up to 13 years or even longer for unnecessary and potentially harmful cancer screenings to decline in use after new guidelines are introduced (390)LeLaurin JH, et al. (2025) BMJ Qual Saf.. This is concerning because unnecessary screenings can potentially lead to overdiagnosis and other issues, such as anxiety associated with medical procedures.
If the initial screening test suggests cancer, it is important to confirm the result with a follow-up test (see Figure 13). Unfortunately, certain population groups in the United States continue to experience low adherence to routine cancer screening and follow-up care because of multilevel factors, including systemic and structural racism, lack of access to quality health care, minimal or no awareness of the benefits of routine cancer screening, and mistrust in the health care system (see Sidebar 26). For example, Black adults are 15 percent less likely to receive the follow-up colonoscopy after an abnormal stool-based test (392)Mohl JT, et al. (2023) JAMA Netw Open, 6: e2251384.. New research shows that if the rates of follow-up colonoscopies as well as polyp detection by endoscopists—the health care professional who performs colonoscopies—were the same in Black adults as in White adults, colorectal cancer deaths in the Black population could drop by 19 percent (393)Alagoz O, et al. (2024) J Natl Cancer Inst, 116: 1807.. These findings underscore the importance of follow-up care and its quality in reducing the population-level burden of cancer.
It is important to understand whether low rates of cancer screening and follow-up care are part of the reason why some population groups experience higher cancer burden. One way to improve screening is to design care that meets the unique needs of different communities and helps overcome barriers, such as cost, access, or mistrust of the health system. It is also crucial that screening guidelines and programs are based on data that reflect the specific populations they are meant to serve.
Reducing Structural Barriers
Structural barriers to health care access, such as lack of nearby health care facilities, health insurance, and public transit, play a significant role in low adherence to cancer screening and follow-up care. Research shows that reducing structural barriers to health care can increase participation in routine cancer screening and follow-up care. For example, multiple barriers to colonoscopy participation have been identified, including access to nearby health care facilities, lack of provider recommendation, and lack of knowledge and awareness (401)Paudel YR, et al. (2025) Journal of Public Health.. A 20-year colorectal cancer screening initiative in California addressed structural barriers through a systematic outreach that included reminding individuals who were overdue for colorectal cancer screening. People had the option to be sent FIT kits for at-home tests. The initiative more than doubled screening rates from 37 percent to 80 percent across 1.1 million adults, leading to a 30 percent drop in cancer incidence and a 50 percent decline in deaths. Furthermore, the program also sharply reduced racial disparities in outcomes, cutting colorectal cancer mortality among Black patients by over half (402)The ASCO Post. 20-Year Screening Program Drives Down Colorectal Cancer Cases, Deaths. Accessed: June 30, 2025..
Lung cancer screening is another example in which lack of access to specialized facilities and trained staff poses major structural barriers to participation in routine screening and follow-up care. Only 17 percent of eligible US adults were up to date with lung cancer screening in 2022 (see Table 3). Researchers are working to identify ways that can increase participation of eligible individuals in lung cancer screening. A review of 12 global studies found that mobile lung cancer screening units using low-dose CT scans are a powerful way to detect lung cancer early, especially in rural, low-income, and uninsured communities (404)Karanth SD, et al. (2024) J Thorac Dis, 16: 7143.. Across programs in the United States, the United Kingdom, Japan, China, and Brazil, mobile screening units detected 80 percent of lung cancers at early, more treatable stages. These findings support expanding mobile screening to save lives, increase adherence to screening recommendations, and reduce health disparities (404)Karanth SD, et al. (2024) J Thorac Dis, 16: 7143..
Researchers are finding that similar approaches are effective for breast cancer screening, which also requires specialized facilities and trained staff to perform mammography. A national study of over 2.6 million women on Medicare fee-for-service plans found that mobile mammography helped reach underserved women—especially those living in rural areas, lower-income communities, and among American Indian/Alaska Native (AI/AN) populations. While only 0.4 percent of women used mobile mammography, those who did had significantly fewer years without a mammogram compared to those who used only facility-based services. Mobile mammography was over five times more likely to be used by AI/AN women and three times more likely among rural residents (405)Pelzl CE, et al. (2025) Clin Breast Cancer, 25: e288.. These findings show that mobile units are helpful ways to expand access for population groups who might otherwise go unscreened for lung and breast cancers.
Examples highlighted here underscore the effectiveness of innovative approaches—such as mailed test kits and mobile screening units—in expanding access, increasing early detection, and narrowing disparities across cancer types. These interventions can save lives and reduce or eliminate disparities in cancer prevention.
Integrating Patient Navigation
Patient navigation refers to personalized, one-on-one support that helps individuals overcome logistical, financial, and informational barriers—such as scheduling appointments, understanding test results, and securing transportation or insurance coverage (see Sidebar 52). Patient navigation has been effective across the cancer care continuum, including increasing participation in routine cancer screening and follow-up care and reducing disparities (406)Chan RJ, et al. (2023) CA Cancer J Clin, 73: 565..
A recent study involving 260 individuals experiencing homelessness showed that patient navigation significantly increases lung cancer screening participation. Patient navigation increased screening completion by nearly five-fold, with 43 percent of participants completing lung cancer screening within 6 months compared to only 9 percent without navigation (407)Baggett TP, et al. (2024) JAMA Intern Med, 184: 892.. A review of 18 studies found that patient navigation—using bilingual community health workers to guide individuals through the screening process—significantly improved screening rates for breast, cervical, and colorectal cancers (408)Jang J, et al. (2025) J Gen Intern Med.. In some settings, colorectal screening increased from 11 percent to 90 percent, and cervical screening rose from about 2 percent to 60 percent (408)Jang J, et al. (2025) J Gen Intern Med.. Together, these studies underscore patient navigation as a highly effective strategy to enhance screening uptake as well as address disparities in early cancer detection.
Another recent study examined the impact of implementing patient navigation in a safety-net setting to improve cervical cancer screening. Approximately 2,500 women ages 30 to 65 who were overdue for cervical cancer screening were enrolled in a randomized controlled trial to evaluate the impact of patient navigation on participation of underserved women in cervical cancer screening (410)Montealegre JR, et al. (2025) JAMA Intern Med.. Participants, 67 percent of whom were from underserved communities, received either telephone reminders for screening at the clinic, telephone reminders along with a mailed self-collection HPV test, or telephone reminders, mailed self-collection HPV test, and patient navigation. Researchers found that the introduction of patient navigation increased participation from 17 percent among those who only received a telephone reminder to 47 percent (410)Montealegre JR, et al. (2025) JAMA Intern Med.. These results highlight patient navigation as essential for reducing cervical cancer disparities in underserved communities.
Collectively, the examples discussed above show that patient navigation is a powerful tool for improving access to cancer screening, particularly among underserved and high-risk populations. As cancer screening guidelines evolve, embedding patient navigation into care delivery models will be key to improving equity in early detection (see Advancing Policies to Strengthen Cancer Prevention and Screening Programs). This point is reinforced by the President’s Cancer Panel’s report, Enhancing Patient Navigation with Technology to Improve Equity in Cancer Care. The report, released in November 2024, underscores the importance of patient navigation in cancer care and recommends implementing technologies to connect patient navigators with cancer patients (411)The White House. Enhancing Patient Navigation with Technology to Improve Equity in Cancer Care: A Report to the President of the United States from the President’s Cancer Panel. Accessed: June 30, 2025..
Engaging Communities
Evidence shows that community engagement and tailored approaches are highly effective in increasing participation in cancer screening and follow-up care, especially among medically underserved populations (412)Okasako-Schmucker DL, et al. (2023) Am J Prev Med, 64: 579.. A national study of nine breast and cervical cancer screening programs found that partnerships between clinics and community-based organizations, such as churches, shelters, radio stations, and cultural centers, played a crucial role in reaching medically underserved women (413)Subramanian S, et al. (2024) Health Promot Pract: 15248399241303891.. Researchers have also found that members of closely connected communities are more likely to be up to date on routine screenings, even in the absence of consistent access to health care. This observation presents a powerful opportunity: When one person in a tightly knit community has a positive screening experience, they often share it with others. Because trust runs high within these networks, such word-of-mouth can spur broader uptake, making tailored, community-based interventions especially impactful. For instance, a recent study found that Mexican immigrant women living in rural areas had higher mammography rates (54.4 percent) than those in urban areas (45.6 percent) (414)Bekteshi V, et al. (2024) J Prim Care Community Health, 15: 21501319241295916.. These differences were linked to stronger family ties and traditional beliefs that supported health-seeking behaviors. Similarly, another study found that Black immigrant women with stronger social ties were nearly three times more likely to be up to date on cervical cancer screening (415)Cofie LE, et al. (2024) J Racial Ethn Health Disparities.. These findings underscore why community-based strategies can be so powerful in improving screening uptake.
Real-world interventions reinforce this evidence. In Central Texas, a lung cancer screening program combined bilingual outreach with patient navigation to screen more than 600 high-risk adults (416)Pignone M, et al. (2025) Am J Prev Med, 68: 227.. From 2020 to 2023, 83 percent of patients who agreed to screening completed it—regardless of race, age, or insurance status. Nearly half of current smokers in the program received help for smoking cessation, and one in three reported success. The program demonstrates how culturally tailored, community-based interventions can eliminate barriers to routine screening, which can save lives. Community-based strategies have also proven effective for informed decision-making. A review of clinical trials found that tailored educational interventions, delivered through health care settings and churches, helped Black men make more informed decisions about prostate cancer screening (417)Lopez A, et al. (2024) J Racial Ethn Health Disparities.. These programs used group discussions and decision-making tools to improve knowledge and readiness to act.
Together, these studies show that engaging trusted community leaders, organizations, and peers fosters trust and strengthens ties to the health care system. Tailored strategies, such as culturally relevant education, language-specific materials, and flexible service delivery, can bridge gaps in cancer screening and lead to better outcomes for all population groups.
Technological Innovations Advance Cancer Screening for Early Detection
Driven by innovative research, advances in molecular diagnostics, artificial intelligence (AI), and minimally invasive screening approaches are enabling earlier and more precise cancer detection. Here, we highlight four areas in which rapid progress is poised to further transform cancer early detection.
Advances in Artificial Intelligence (AI)
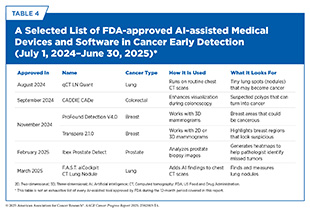
AI is the ability of a computer to perform tasks commonly associated with human intelligence, such as how to act, reason, and learn. The use of AI in cancer screening for early detection is rapidly advancing, with research fueling innovations that are reshaping how cancers are identified and diagnosed. These advances are already reflected in the growing number of FDA-approved AI-assisted tools designed to enhance cancer screening and diagnosis (see Table 4).
Recent studies demonstrate the transformative potential of AI in improving how breast cancer is detected, while also reducing the workload of radiologists. A large Swedish screening trial involving over 100,000 women showed that AI-assisted mammography detected 29 percent more cancers without increasing false positives, which are cases in which a test incorrectly indicates that a person has cancer when they do not. In addition, it led to a 44 percent reduction in the number of screenings requiring radiologist review (418)Hernstrom V, et al. (2025) Lancet Digit Health, 7: e175.. In a randomized controlled trial, AI triage reduced time from mammogram to biopsy by 30 percent—nearly 17 fewer days—with no missed cancers (419)Friedewald SM, et al. (2025) Breast Cancer Res Treat, 211: 1.. Another study, involving nearly half a million women, found that using AI to support mammogram interpretation improved breast cancer detection by nearly 18 percent (420)Eisemann N, et al. (2025) Nat Med, 31: 917..
A US-based study using magnetic resonance imaging (MRI) found that an AI model could predict breast cancer up to 1 year in advance, even when radiologists saw no abnormalities (421)Hirsch L, et al. (2025) Acad Radiol, 32: 1218.. The AI system correctly identified 30 percent of cancers before diagnosis and flagged the correct location in over half of those cases. Another large study of racially and ethnically diverse women that used current as well as up to 4 years of prior mammograms to train an AI model for predicting a woman’s risk of developing breast cancer found that this approach markedly improved accuracy of the model (422)Jiang S, et al. (2025) JAMA Netw Open, 8: e2512681.. These findings offer a powerful tool for high-risk populations such as women with dense breasts, for whom many organizations recommend receiving MRI to supplement mammography (423)Sardanelli F, et al. (2024) Insights Imaging, 15: 96.. Together, these studies support a future in which AI helps detect breast cancer earlier and more precisely without overwhelming health care systems.
AI-assisted tools are also revolutionizing colorectal cancer detection. Two recent studies offer strong evidence that AI significantly improves polyp detection during colonoscopy, a key factor in preventing colorectal cancer. An analysis of 28 screening trials with over 23,000 participants found that AI-assisted colonoscopy improved polyp detection rates by 20 percent and reduced the rate of missed polyps by 55 percent (425)Makar J, et al. (2025) Gastrointest Endosc, 101: 68.. However, the improvements were primarily for small, low-risk lesions, suggesting the need for further refinement.
In another study from the United Kingdom involving 2,000 patients, the FDA-approved GI Genius AI module improved detection of small, high-risk polyps that often go unnoticed. These benefits were observed in patients both with and without symptoms, indicating real-world utility across screening populations (426)Seager A, et al. (2024) Lancet Gastroenterol Hepatol, 9: 911..
Although AI holds immense promise for transforming early cancer detection, experts caution against rapid adoption without addressing potential risks (see New Advances in Artificial Intelligence). A recent review of 11 studies involving nearly 20,000 patients found that AI-based tools improved lung cancer detection by up to 20 percent, but also increased false positives—potentially leading to unnecessary follow-up scans and anxiety (427)Geppert J, et al. (2024) Thorax, 79: 1040.. Many current AI models are trained on nondiverse or low-quality datasets, which may limit their performance in underrepresented groups, such as patients with rare cancer subtypes or dense breast tissue (428)Diaz O, et al. (2024) Eur J Radiol, 175: 111457..
Ensuring that AI lessens, not worsens, disparities in cancer care requires continued investment in research as well as better data diversity and regulatory oversight (see New Advances in Artificial Intelligence). While AI technology is advancing quickly, careful implementation is essential to maximize benefits and minimize unintended harms.
Advances in Minimally Invasive Screening Approaches
Catching cancer early saves lives but most traditional screening tools, such as the Pap test, mammogram, or colonoscopy, either apply to only a few cancer types or require invasive procedures (see Sidebar 25). In the 12 months covered in this report, several new minimally invasive tests and devices have been approved by FDA, reflecting the rapid pace of innovation in this field (see Table 5).
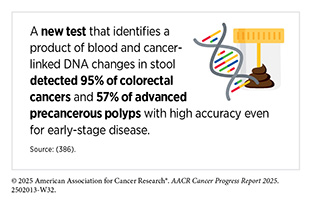
Several recent studies underscore the promise of minimally invasive tests in detecting colorectal cancer early. One study using samples from patients with colorectal cancer found that a next-generation stool DNA test correctly identified 95 percent of colorectal cancers and over 57 percent of advanced precancerous lesions (386)Gagrat ZD, et al. (2024) Cancer Prev Res (Phila), 17: 119.. Another study evaluated a new blood test that detected more than 80 percent of early-stage colorectal cancers, while producing fewer false positives than standard methods (47)Chung DC, et al. (2024) N Engl J Med, 390: 973.. Both tests received FDA approval in 2024 (see Table 5).
Beyond tests for early detection of individual cancers, researchers are also developing multi-cancer early detection (MCED) tools that can detect multiple cancer types by analyzing molecular markers, such as circulating tumor DNA (ctDNA) shed by cancer cells. These mostly blood-based tests offer the potential to simplify cancer screening by identifying a wide range of cancers, including those without current screening methods.
Minimally invasive screening aims to detect multiple cancers before symptoms arise. A large international study tested a blood-based MCED method that analyzed DNA methylation patterns in cell-free DNA (cfDNA), which are fragments of DNA shed by normal and unhealthy cells into the bloodstream. The test analyzed 13 different types of cancers and detected 79 percent of early-stage malignancies, with very few false positives. It also performed well in finding 58 percent of early-stage pancreatic cancers and 100 percent of early-stage liver cancers, both highly aggressive cancers that are rarely caught early (431)Bao H, et al. (2025) Nat Med..
Minimally invasive cancer screening, especially with blood-based tests, has the potential to transform how and when cancer is found. A growing body of evidence, including several of the studies highlighted here, shows that these tools can detect cancer signals with remarkable accuracy, even in people with no symptoms (433)Alix-Panabieres C, et al. (2025) Cancer Cell, 43: 161.. But important challenges remain, such as unequal access, limited insurance coverage, lack of transparency about what markers the tests are detecting and what the findings might mean, and concerns about missing cancers, especially precancerous lesions or early-stage cancers, or unclear results (434)Carethers JM (2024) N Engl J Med, 390: 1045.(435)Perachino M, et al. (2025) Commun Med (Lond), 5: 167.(436)Semenkovich NP, et al. (2024) JAMA Oncol, 10: 1023.. Continued research is essential to improving test performance, ensuring equity, and validating how well these tools work in everyday clinical settings so that their benefits reach all communities, with the ultimate goal to reduce cancer-related mortality.
Advances in Imaging
Imaging plays a critical role in the early detection of cancer by revealing tumors and abnormal tissue changes before symptoms appear. These technologies often guide diagnosis and treatment decisions. Advances in low-dose CT, MRI, and molecular imaging are now making it possible to detect cancer earlier, with greater precision and fewer harms. Although not recommended by USPSTF, whole body MRI—an established screening tool for children with cancer predisposition syndrome (e.g., Li-Fraumeni syndrome)—is also being used in adults to look for cancer (437)Parillo M, et al. (2025) Cancers (Basel), 17.. Increasingly, researchers are also integrating traditional imaging methods with AI and novel ways to visualize cancer cells to improve the accuracy of cancer detection and reduce the chances of missed diagnoses.
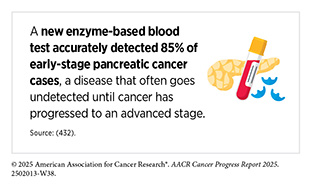
In a promising application, researchers are exploring how advanced MRI techniques might enable earlier detection of pancreatic cancer, one of the deadliest cancer types due to its typically late diagnosis. A recent study tested two specialized MRI methods in mice as well as in human pancreatic tissue (438)Bilreiro C, et al. (2025) Invest Radiol, 60: 397.. These imaging techniques were able to detect early warning signs of pancreatic cancer, called PanIN lesions, that are invisible on standard scans. This approach may offer health care teams a new way to identify pancreatic cancer before it progresses, offering hope for earlier intervention in a disease with extremely low survival rates.
Imaging is also improving how prostate cancer is diagnosed after elevated prostate-specific antigen (PSA) levels are detected. Traditionally, a biopsy is performed after a suspicious PSA result, but this invasive procedure often detects slow-growing cancers that would not have advanced or caused harm (439)Rebello RJ, et al. (2021) Nat Rev Dis Primers, 7: 9.. New evidence supports using MRI after a positive PSA test to decide whether a biopsy is necessary. MRI improves visualization of suspicious prostate lesions, including cancers that require clinical intervention, and helps distinguish them from benign cancers that are less likely to pose a threat to a person’s health (440)Launer BM, et al. (2025) Urol Oncol, 43: 15.. In a study of more than 13,000 men ages 50 to 60, MRI-guided screening led to fewer unnecessary biopsies and reduced low-risk cancer diagnoses by 57 percent, without missing the number of aggressive or incurable cancers detected (441)Hugosson J, et al. (2024) N Engl J Med, 391: 1083.. Another study found that 86 percent of men who had a negative MRI result completely avoided the need for a biopsy at a 3-year follow-up. MRI still detected cancers that required treatment (442)Hamm CA, et al. (2025) JAMA Oncol, 11: 145.. These findings support a shift in prostate cancer screening toward a more precise, MRI-guided approach that limits harm and avoids overdiagnosis without missing prostate cancer cases that require treatment.
The advances discussed here highlight the expanding role of imaging in transforming early cancer detection. From identifying aggressive tumors to reducing invasive procedures, imaging innovations are helping to make cancer screening more accurate, more personalized, and less burdensome. With continued research and clinical validation, these tools could dramatically improve how we detect and ultimately treat cancer across many different types.
Genetic Insights Advancing Cancer Risk Reduction and Interception
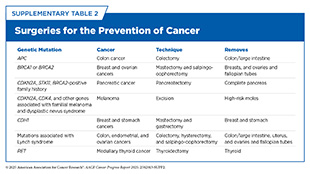
Genetic testing examines DNA to identify changes that may influence cancer risk, diagnosis, or treatment. Germline testing, which looks for inherited genetic mutations (see Genetic Alterations), is not a tool for detecting cancer itself but plays a critical role in identifying individuals at elevated lifetime risk—particularly those with a strong family history of cancer (see Sidebar 23). When a harmful mutation is found, patients can receive personalized care that may include genetic counseling, targeted screening, or preventive interventions like risk-reducing surgeries (see Supplementary Table 2).
Germline testing differs from somatic testing, which analyzes tumors for mutations acquired during a person’s lifetime. Somatic mutations are not inherited and help guide cancer treatment, prognosis, and monitoring. Both approaches are crucial but germline testing is uniquely positioned to shift cancer care from reactive to proactive and can also help family members plan their care accordingly if a close relative tests positive for a germline mutation that may place them at higher risk of developing cancer.
An increasing body of evidence shows that it is not always one mutation, but the combined small effects of many inherited genetic changes, that leads to cancer (443)Yang X, et al. (2023) Nat Rev Cancer, 23: 619.. A rapidly emerging tool within germline testing is the polygenic risk score (PRS), which quantifies the cumulative effect of dozens to hundreds of common inherited variants. PRS is now used in clinical settings to enhance breast cancer risk prediction and tailor screening strategies in women with mutations that almost always cause cancer (444)Roberts E, et al. (2023) Breast, 67: 71.. Furthermore, researchers are evaluating the effectiveness of PRS in determining the risk of other cancer types. Findings from a recent study showed that PRS improved selection of patients with prostate cancer who needed clinical intervention, as well as reducing unnecessary biopsies and overdiagnosis (445)McHugh JK, et al. (2025) N Engl J Med, 392: 1406.. Ongoing research is also investigating the role of environmental factors in increasing cancer risk and exploring whether incorporating environmental factors along with PRS will further refine the effectiveness of such scores (446)Kachuri L, et al. (2020) Nat Commun, 11: 6084.(447)Mallabar-Rimmer B, et al. (2024) Eur J Hum Genet, 32: 1456..
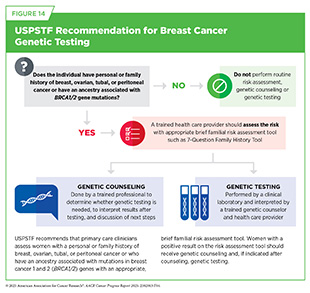
Individuals with a family or personal history suggestive of inherited cancer syndromes should discuss genetic counseling and testing with their health care provider. Expert panels issue clinical guidelines to support this process. For example, USPSTF recommends that primary care clinicians assess women with a personal or family history of breast, ovarian, tubal, or peritoneal cancer—or with ancestry linked to BRCA1/2 mutations, such as those of Ashkenazi Jewish ancestry—using a validated risk tool. Those with positive results should be referred for genetic counseling and, if appropriate, genetic testing (see Figure 14). These recommendations are currently being updated.
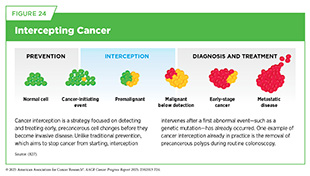
The understanding of genetic mutations associated with cancer-predisposing syndromes (see Figure 6) is also enabling strategies, such as cancer interception (see Figure 24), that can mitigate or altogether eliminate a person’s chances of developing cancer. Researchers are developing preventive vaccines that aim to stop cancer from developing and that are particularly promising for individuals at high risk of developing cancer, such as those with inherited mutations or predisposition syndromes. One example of such an approach is an ongoing clinical study using a vaccine to prevent cancer development in individuals with Lynch syndrome, who have germline mutations in genes involved in DNA repair (448)Leoni G, et al. (2020) Cancer Res, 80: 3972.. Another example is an mRNA vaccine to prevent cancer in individuals who carry germline mutations in BRCA1/2 genes and are at higher risk of developing cancers of the breast, ovary, pancreas, and prostate (449)Domchek SM, et al. (2025) Journal of Clinical Oncology, 43: 10505.. Although in the early stages of clinical testing, these preventive cancer vaccines have the potential to revolutionize cancer surveillance, prevention, and care in people with germline mutations.
Germline mutations account for approximately 10 to 15 percent of all childhood cancers (450)Zhang J, et al. (2015) N Engl J Med, 373: 2336.(451)Parsons DW, et al. (2016) JAMA Oncol, 2: 616.(452)Grobner SN, et al. (2018) Nature, 555: 321. and often confer a high lifetime cancer risk with early onset of disease. Identifying these mutations enables early surveillance and timely intervention. While historically few standardized surveillance protocols existed for these syndromes, the American Association for Cancer Research convened a 2016 workshop to develop consensus recommendations for early cancer detection in affected children (453)Brodeur GM, et al. (2017) Clin Cancer Res, 23: e1.. A follow-up workshop in 2023 expanded and updated these guidelines to reflect new evidence and include newly identified syndromes, while also exploring emerging surveillance technologies and potential prevention strategies for high-risk pediatric populations (454)Brodeur GM, et al. (2025) Clin Cancer Res.. Surveillance now begins at birth or during early childhood, depending on the syndrome, with imaging and blood tests prioritized over CT to reduce radiation exposure. These protocols aim to catch tumors early when cure rates exceed 90 percent, such as for Wilms tumor or hereditary retinoblastoma.
These advances underscore the expanding role of genetic testing in cancer prevention, not by detecting cancer itself but by identifying those most likely to develop it. As clinical guidelines evolve and tools like PRS mature, germline testing will continue to shift cancer care toward earlier, more personalized intervention—often before a single symptom appears.
Next Section: Unifying Cancer Science and Medicine—A Continuum of Innovation for Impact Previous Section: Reducing the Risk of Cancer Development