Combatting Cancer through Science-based, Patient-centered Policies
In this section you will learn:
- Federal funding for medical research, most specifically through NIH and NCI, has a significant impact on our nation’s health and the United States economy.
- Regulatory science initiatives at the FDA are vital to accelerating progress against cancer.
- Policies and federally funded public health programs, many of which are supported by the CDC, ensure that individuals have access to preventive services, screening, and coverage for cancer treatment.
- Tobacco control policies improve public health and reduce cancer risk.
- Newly passed legislation aims to improve outcomes for children and adolescents who are diagnosed with cancer.
There has never been a time of greater promise in cancer research. As highlighted throughout this report, the rapid pace of progress and the broadening scope of the advances made in recent years have been extraordinary. Moreover, there has been an exceptionally strong and sustained commitment from our nation’s policy makers, in particular, Senator Roy Blunt (R-MO), Senator Patty Murray (D-Wa), Congressman Tom Cole (R-OK), and Congresswoman Rosa DeLauro (D-CT), for supporting medical research including cancer research. In their respective roles on the Labor-Health and Human Services (HHS)-Education Appropriations Subcommittees in the Senate and House, these four champions of medical research have been instrumental in securing four consecutive years of robust annual funding increases for the NIH. As a result of these increases, the NIH budget has increased by 30 percent from FY 2015 to FY 2019.
This positive momentum for robust annual funding increases for the NIH signals an awareness among members of Congress of the critical role that NIH-funded research plays in preventing, detecting, diagnosing, and treating cancer and other diseases. For research investments to yield dividends in the form of new medical products and community-based programs that improve public health, strong federal investments are also needed in the FDA and CDC, and in evidence-based policy making across and throughout the medical research ecosystem (see sidebar on Overcoming Cancer through Science Policy).
Sustaining the Momentum with Robust Annual Funding Increases for Medical Research
Federal funding through the NIH is the engine that drives medical research in the United States. It forms the foundation upon which many scientific discoveries are made and many life-changing treatments are developed. In fact, many of the major medical breakthroughs made in the last five decades, including much of the extraordinary progress detailed in this report, can be traced in part back to NIH research grants (28).
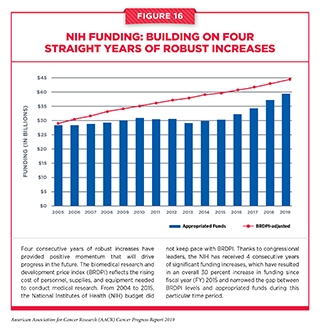
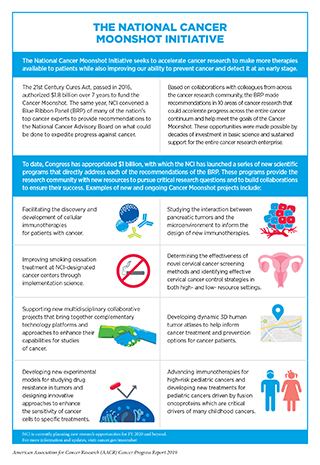
The accelerated pace and scope of the progress being made in cancer science and medicine would not have been possible without strong bipartisan leadership in the U.S. House and Senate, which has made medical research a national priority in recent years and has set the budgets for the NIH and NCI on a path of annual growth above inflation (see Figure 16). Since FY 2015, Congress has renewed its commitment to the promise of medical research and increased the NIH budget by a total of $9 billion over the past 4 years. With this growth, the troubling trend of stagnant budgets that persisted for more than a decade prior to 2015 has been halted. In addition, the NIH Innovation Fund, a multiyear, targeted funding stream created by the 21st Century Cures Act, is providing dedicated resources for the National Cancer Moonshot Initiative. The National Cancer Moonshot Initiative is designed to further accelerate the pace of progress in specific priority areas at the NCI where researchers are poised to make great strides in the coming years (see sidebar on the National Cancer Moonshot Initiative).
While this strong commitment to funding medical research has resulted in enormous accomplishments throughout the multidisciplinary field of cancer research, particularly in the area of precision medicine, a concerning trend is taking place. Specifically, there is a falling payline, and the success rate for the funding of investigator-initiated research project grants (RPGs) at the NCI has been declining. This may be attributable to the fact that the remarkable advances in and enthusiasm for cancer research have stimulated an unprecedented 50 percent increase in the number of investigator-initiated RPG applications to the NCI since FY 2013.
This dramatic increase in investigator-initiated RPG applications is unique to the NCI compared with other NIH institutes, where the number of applications has remained relatively constant during this same period. Given that NCI’s budget has not increased at the same rate as its investigator-initiated RPG applications, the payline for these awards in 2018 was only 8 percent of approved grants, and the success rate was 11.3 percent. This means that a disproportionate and increasing number of exceptional applications (that is, those in the 11 – 15 percent scoring range) are not being funded at the NCI. If current trends persist or worsen, they will create a significant disincentive for investigators at all stages of their careers to submit grant applications to the NCI and even to remain in cancer research. This will have a stifling effect on cancer research at a time when impactful new discoveries are occurring at an ever-increasing pace and are having a beneficial impact on reducing cancer incidence and mortality rates.

As noted by Senator Richard Shelby, medical research is one of the most important investments that our government can make, and the impact is felt in every part of the country. In fact, of the funds appropriated by Congress to the NIH, the vast majority (nearly 85 percent) is awarded to scientists in all 50 states and the District of Columbia through a competitive review process. The impact of federal support for the NIH and NCI also reaches well beyond the laboratory and the clinic. As the largest single public funder of medical research in the world, NIH-funded research throughout the country generated more than $74 billion in U.S. economic activity last year alone and supported more than 430,000 jobs (342).
The significant NIH funding increases of the last 4 years have been critical in returning medical research funding to a trajectory of steady growth. However, with so many opportunities for further advances against cancer and other diseases, it is vital that our elected officials take the steps necessary to ensure that this momentum for robust and sustained annual funding increases continues in the future (see The AACR Call to Action).
A Strong, Diverse Research Workforce Depends on Predictable Funding
Many innovative research questions and fresh ideas come from scientists early in their careers. Ensuring the continued, rapid pace of progress against cancer requires that the next generation of cancer researchers be recruited, supported, and encouraged. The cancer workforce of tomorrow also must reflect the increasing diversity in our country, including disciplinary, gender, racial, ethnic, and geographic diversity. Robust, sustained, and predictable annual funding increases for the NIH, coupled with federal, state, and private sector-funded programs to assist early-career scientists, play a critical role in cultivating tomorrow’s scientific leaders.
Members of Congress and NIH officials have recognized the importance of supporting scientists early in their careers, and they have taken steps to assist these investigators through legislative provisions in the 21st Century Cures Act and the new policies enacted through the NIH Office of Extramural Research, including the Next Generation Research Initiative. This special program is focused on ensuring the long-term stability and strength of the U.S. medical research enterprise by supporting early-stage investigators and mid-career investigators through specific funding efforts.
The NCI has also implemented innovative programs to support early-career investigators. For example, in January 2018, the NCI announced the establishment of the Method to Extend Research in Time (MERIT) (R37) Award, which is a grant that provides longer term support to early-career investigators. Eligible investigators may obtain up to 7 years of support in two segments: an initial 5-year award and an opportunity for an extension of up to two additional years, based on an expedited NCI review of the accomplishments during the initial funding segment. By providing an opportunity for longer term support, the NCI hopes to provide these individuals with more opportunities to take creative risks in their research projects and allow them to have additional time to successfully establish their careers before having to submit renewal applications.
Advancing Regulatory Science and Policy
As the pace of innovation accelerates and our armamentarium against cancer expands, the FDA must be equipped to review an ever-increasing number of advances. Therefore, it is vital that the agency also receive consistent, robust support from Congress through the annual appropriations process. Appropriated dollars support critical regulatory science initiatives that foster the development of evidence-based regulatory policies that promote cutting-edge scientific advancement and expedited approval of medical products.
Authorized by the 21st Century Cures Act, the Oncology Center of Excellence (OCE) was established in 2017 to streamline the review of anticancer therapeutics and increase regulatory efficiency by bringing together staff with oncology expertise from the medical product centers of the FDA’the Center for Drug Evaluation and Research (CDER), the Center for Biologics Evaluation and Research (CBER), and the Center for Devices and Radiological Health (CDRH). In 2018, the OCE approved 19 new anticancer therapeutics.
In addition to coordinating the reviews of anticancer therapeutics, the OCE is focused on regulatory innovation. For example, the OCE began two pilot programs known as Real-Time Oncology Review (RTOR) and Assessment Aid to accelerate review timelines for anticancer therapeutics (see sidebar on Pilot Programs Showcase Regulatory Innovation). RTOR and Assessment Aid have already paid dividends, initially for expanding the uses of previously approved anticancer therapeutics, but in May 2019, the FDA used these programs when it approved the new molecularly targeted therapeutic alpelisib for treating certain patients with breast cancer (see Providing New Options for Patients with Breast Cancer).

In 2019, the OCE launched two exciting regulatory science and policy initiatives, Project Renewal and Project Facilitate. Project Renewal is a critical public health initiative designed to update safety and efficacy information on outdated product labels of long-standing, generic anticancer therapeutics. Currently, physicians may not be able to depend on the prescribing information described in the out-of-date labels of generic drugs such as 5-fluorouracil, and this causes health care providers to seek information from other sources and increases risk to patients.
Working together with the cancer research community, the OCE is seeking to transform outdated generic product labels for 40 generic drugs into living documents with clearly established processes for updating the information. Under Project Facilitate, the OCE established a – one-stop shop – call center at the FDA to serve and support physicians seeking to use the agency’s Expanded Access Programs for oncology patients [see sidebar on Facilitating Patient Access to Promising Investigational Drugs (Project Facilitate)].
The FDA is building on advances in technology to incorporate real-world data (RWD) and real-world evidence (RWE) into its processes for approving treatments, expanding drug labels, and monitoring therapies currently on the market. RWD can come from a variety of sources, including medical claims data, electronic health records, patient-reported outcomes, and product or disease registry data. RWE is clinical evidence generated from these data. Leveraging RWE for regulatory purposes continues to increase as policy mandates, established by the 21st Century Cures Act and other legislation, accelerate efforts to characterize regulatory-grade real-world evidence and develop methodologies to support its use. The “Framework for FDA’s Real-World Evidence Program,” which the agency released at the end of 2018, described their multifaceted approach to identify and use RWE to support regulatory decisions regarding the effectiveness of drugs, devices, and biologics. Demonstration projects, stakeholder engagement, and establishing internal processes for evidence evaluation will be vital to the program’s success.
Advancing Effective Cancer Prevention, Treatment, and Control Efforts
To maximize societal benefit from investments in medical research and the development of innovative medical products and technologies, the advances made must reach individuals, families, and communities through clinical care and public health programs. Public policies play a role in supporting equitable access to care and the delivery of effective public health programs such as screening, treatment, and vaccination programs (see sidebar on CDC Cancer Prevention and Control Programs).
Through advances in research for the prevention, diagnosis, and treatment of cancers caused by the human papillomavirus (HPV) (see Prevent and Eliminate Infection with Cancer-causing Pathogens), the elimination of cervical cancer and other HPV-related cancers is now an ambitious but feasible goal. According to experts, expanding the use of existing medical products and technologies could lead to the global elimination of HPV-related cancers as a public health problem within the century (127).
Unfortunately, the most effective prevention tool, the HPV vaccine, is underutilized in the United States. HPV vaccination rates in U.S. adolescents have risen in recent years, but thus far are only about half of the national 80 percent goal set by the U.S. Department of Health and Human Services in Healthy People 2020 (52). In November 2018, the President’s Cancer Panel recommended several actions by policy makers and health care stakeholders to increase HPV vaccination rates to at least 80 percent of eligible recipients, including ensuring that providers are recommending HPV vaccine to all eligible adolescents and young adults during provider visits, evidence-based communication campaigns to increase parents – acceptance of HPV vaccination, and continued health insurance coverage of HPV vaccine for eligible populations (120). In addition to HPV vaccination, cervical cancer screening and treatment for women who screen positive are needed to reduce and eventually eliminate cervical cancer. As of 2016, only 80 percent of eligible women were screened as recommended for cervical cancer, far below the Healthy People 2020 goal of 93 percent of eligible women screened. Continued funding for screening programs such as CDC’s National Breast and Cervical Cancer Early Detection Program is essential for reaching the national screening goals. The elimination of HPV-related cancers in the U.S. will only be possible through concerted efforts by policy makers and stakeholders to increase vaccination rates and to improve screening and treatment of HPV-related cancer cases.
Globally, 630,000 people develop HPV-related cancers each year, and the vast majority – 530,000 – are cases of cervical cancer (343). In January 2019, at the World Health Organization (WHO) Executive Board meeting, the U.S. government was one of several countries that requested the development of a WHO global strategy to accelerate cervical cancer elimination, centering on improved HPV vaccine coverage, screening and treatment of cervical precancer, and treatment of invasive cancers. The global public health strategy is being created through a consultative process and will be presented for approval by member states at the 2020 World Health Assembly. The global targets under consideration for 2030 include: achieving 90 percent coverage of HPV vaccination for eligible populations, achieving 70 percent screening of eligible women for cervical cancer, and achieving 90 percent treatment of women identified with precancers and invasive cancers. In low- and middle-income countries, achieving these targets will include financing of HPV vaccination by organizations, such as the Global Vaccine Alliance, the development and market availability of HPV tests and other screening tests, and optimizing service delivery to ensure that women who screen positive are provided prompt treatment.
Ongoing efforts from scientific communities working together with members of Congress, NIH, CDC, and other federal agencies are needed to support and accelerate the elimination of HPV-related cancers in the U.S. and globally through public policy.
Despite advances in cancer research and care, there are persistent disparities in health outcomes for certain segments of the U.S. population, including racial and ethnic minorities, individuals of low socioeconomic status, and residents of rural areas (see sidebars Which U.S. Population Groups Experience Cancer Health Disparities? and U.S. Cancer Health Disparities). Many drivers of cancer health disparities have been identified, and policy solutions are needed to help achieve health equity. One major issue is that many populations are underrepresented in clinical trials for developing new anticancer therapeutics. Barriers to patient participation in clinical trials that need to be addressed include financial barriers, restrictive eligibility criteria, and lack of information about and access to clinical trials.
Public policies are also needed to support continued innovation and greater access to treatment and diagnostic options for all cancer patients. The Centers for Medicare and Medicaid Services (CMS) recently issued a National Coverage Determination (NCD) for CAR T-cell therapy, a novel, revolutionary therapy for certain patients with blood cancer (see Treatment with Immunotherapy). CMS will cover CAR T-cell therapies that are administered for FDA-approved indications and off-label use that is approved by CMS. While the coverage policy does not change how much CMS reimburses for CAR-T treatments, it is a helpful step toward ensuring that patients have access to innovative treatments as they are developed and approved.
In March 2018, CMS announced their decision on national Medicare coverage for next-generation sequencing (NGS) diagnostic tests for advanced cancers. At the time, CMS stated they would provide coverage for FDA approved or cleared companion in vitro diagnostic tests that specify treatment options for patients with recurrent, relapsed, refractory, metastatic, or advanced stage III or IV cancer that has not been tested previously with the same NGS test or for repeat testing using the same test when a new primary cancer diagnosis is made by the treating physician. Though the scope of the coverage decision was broader than initially proposed in earlier drafts, repeat NGS testing for same primary cancers and germline hereditary testing did not receive coverage from CMS. In April 2019, CMS reopened the NCD for such NGS tests for advanced cancers to specifically seek comment from the public on coverage for germline hereditary tests. CMS should carefully consider stakeholder feedback on these and future cancer technologies with the explicit goal to set coverage policy that supports continued innovation and ensures patient access to safe, effective, and clinically useful cancer treatments.
Supporting Public Health Policies to Reduce the Use of Tobacco Products
E-cigarettes, which first hit the U.S. market more than a decade ago, have grown in popularity during the past few years, despite little research on their long-term health effects. Most concerning is the fact that e-cigarettes are now the most commonly used tobacco product among U.S. youth and young adults. According to the 2018 National Youth Tobacco Survey (NYTS), a nationally representative survey funded by FDA and CDC, current e-cigarette use or “vaping” – among middle and high school students increased alarmingly between 2017 and 2018, with over 3.6 million kids using e-cigarettes in 2018. Among high-school students, e-cigarette use jumped 78 percent from 2017 to 2018. This is especially concerning because we know that youth who use e-cigarettes are more likely to try combustible cigarettes later (66).
The NYTS study authors hypothesized that last year’s increase in e-cigarette use among youth could be attributable to use of USB-flash-drive-like e-cigarettes, including JUUL, which have garnered popularity among youth. These products have high nicotine content; appealing flavors; and the ability to be easily concealed and used discreetly.
In response to these alarming data, many public health experts believe that youth e-cigarette use has reached an epidemic proportion. In September 2018, the FDA launched massive enforcement actions against retailers for selling e-cigarettes to minors. In addition, the FDA announced plans to limit most e-cigarette sales to age-restricted, in-person locations and to heighten age-verification measures for online sales to try to ensure that minors are not able to buy popular flavored e-cigarette pods. Additionally, on December 18, 2018, the U.S. Surgeon General issued an advisory on youth e-cigarette use, urging parents, teachers, health professionals, and government officials to take “aggressive steps” to keep children from using e-cigarettes (65). He also called for new local restrictions, including increased taxes on electronic nicotine products and indoor e-cigarette use bans.
The rise of e-cigarette use puts the United States at risk of losing the progress that has been made against smoking in the last 30 years. Over the past three decades, the smoking rate among U.S. adults has fallen almost 12 percentage points (from 25.5 percent in 1990 to 14 percent in 2017) and, with tobacco use as the leading cause of preventable death, we cannot afford to lose the tremendous progress that has been made in curbing tobacco use (18), (344). Therefore, the strategies that worked to reduce the rates of combustible cigarette use, which included increasing the price, warning the public of the risks, and prohibiting flavors, may need to be applied to e-cigarettes, especially since these are proven evidence-based measures that have resulted in a reduction of tobacco use by youth in the past.
While additional research is needed to fully understand the short- and long-term harms of e-cigarette use, it is very clear that these products have no place in the hands of youth or young adults. However, a challenge that we are facing today involves the fact that youths and young adults often have a misconception that e-cigarettes are safe. This misconception must be corrected, especially when considering that there is now an expanded group of youth who are at risk of a lifetime addiction to nicotine products. This has prompted the FDA to begin a youth prevention campaign to help increase awareness among young people of the health risks of e-cigarettes
The AACR, along with other public health groups, strongly supports prohibiting the sale of tobacco products to individuals under the age of 21. Nearly all tobacco use begins in youth and young adulthood, and 95 percent of adult smokers begin smoking before they turn 21. However, we also recognize that this is one, among several, important federal policy changes needed to address the public health crisis of e-cigarette use among U.S. youth and young adults. Other potential actions include prohibiting the manufacture and sale of all flavored tobacco products (unless they are FDA-approved to aid in tobacco cessation); restricting online sale of all tobacco products, particularly to underage purchasers; strongly supporting the actions that the Center for Tobacco Products at the FDA is taking to regulate the manufacturing, distribution, and marketing of tobacco products; and increasing funding of the prevention and cessation activities of the CDC Office on Smoking and Health.
Promoting Policies to Further Our Progress against Pediatric Cancer
Cancer remains the second leading cause of death among U.S. children ages 1 – 14 (345). In the last few years, important strides have been made to further progress against pediatric cancers through the passage and implementation of two important pieces of legislation and programs at the NCI (see sidebar on the NCI Childhood Cancer Data Initiative).
On August 18, 2017, key provisions of the Research to Accelerate Cures and Equity (RACE) for Children Act were signed into law as part of the FDA Reauthorization Act of 2017. Under these provisions, the FDA may require companies developing targeted cancer drugs for adults to develop those drugs for children with cancer as well. This is an important policy change, because the relatively small population of children with cancer provides little market incentive for the biopharmaceutical industry to develop new pediatric oncology drugs.
The RACE Act provisions are an update to the Pediatric Research Equity Act (PREA) of 2003. Under PREA, drug companies were required to develop drugs to treat diseases for children as well as adults. This was not applied to cancer, however, because cancers develop in different organs in children and adults. Under the new law, companies developing a cancer treatment for adults would also undertake PREA studies in children when the molecular target of the drug is relevant to a pediatric cancer.
As required by the RACE Act provisions, the FDA has developed a Pediatric Molecular Target List to provide guidance to industry in planning for new drug and biologic submissions. The list includes molecular targets that have potential relevance to the growth or progression of at least one type of pediatric cancer and those molecular targets for which there is no evidence of association with the growth or progression of pediatric tumors and will be updated as new evidence becomes available. The requirement for companies to assess the potential use of new therapies for pediatric as well as adult cancer patients will go into effect on August 18, 2020.
In May 2018, Congress passed the Childhood Cancer Survivorship, Treatment, Access, and Research (STAR) Act of 2018. This is the most comprehensive childhood cancer legislation passed by Congress to date, and it aims to address some of the most challenging issues in childhood cancer research and care. Specifically, the STAR Act:
- Increases opportunities for childhood cancer research by authorizing NCI to expand existing efforts to collect biospecimens for childhood cancer patients enrolled in NCI-sponsored clinical trials.
- Improves childhood cancer surveillance by authorizing grants to state cancer registries to identify and track incidences of child, adolescent, and young adult cancer.
- Enhances research on the late effects of childhood cancers to improve the lives of childhood cancer survivors.
- Ensures pediatric expertise at the NIH by requiring the inclusion of at least one pediatric oncologist on the National Cancer Advisory Board.
In January 2019, the NCI announced a request for applications (RFA) titled Improving Outcomes for Pediatric, Adolescent and Young Adult Cancer Survivors. This announcement builds on the NCI’s ongoing commitment toward advancing cancer survivorship for these patients and directly aligns with STAR Act provisions for research that will help us better understand and meet the needs of childhood cancer survivors.