- The State of Blood Cancers in 2025
- Blood Cancers: An Ongoing Challenge
- Unique Biology of Blood Cancers
- Precision Medicine Driving Advances Against Blood Cancers
- Diagnosing Blood Cancers With Increasing Precision
- A Revolution in Molecularly Targeted Therapies
- Transforming Blood Cancer Treatment With Immunotherapy
- Supporting Survivorship
- New Horizons in Blood Cancer Science and Medicine
A Decade of Progress: Transformative Advances in Blood Cancer Research and Treatment
In this section, you will learn:
- Blood cancers, or hematologic malignancies, make up a large group of cancers that originate from blood-forming tissues.
- Tremendous progress against blood cancers in the past decade has significantly decreased blood cancer–related deaths, improved survival rates, and helped survivors live fuller lives.
- Advances in understanding blood cancers have led the revolution in precision cancer medicine. Over the past decade, the US Food and Administration (FDA) approved 29 new molecularly targeted therapies and 21 new immunotherapeutics to treat various types of hematologic malignancies.
- Progress against blood cancers has often accelerated progress against solid tumors as well as against diseases beyond cancer.
- Despite the progress, certain populations within the United States, as well as certain regions around the globe, experience a disproportionate burden of blood cancers, primarily due to lack of access to breakthrough treatments and lack of infrastructure.
- Knowledge gleaned from decades of research is helping inform strategies to intercept and eliminate cells that may progress into blood cancers.
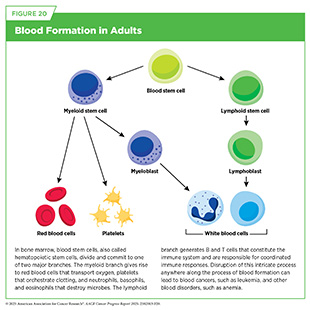
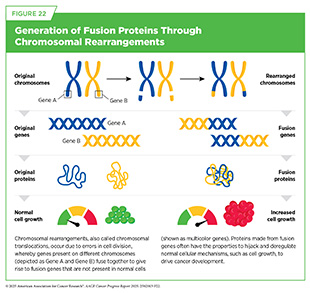
In adults, normal blood formation takes place in the bone marrow, which is found inside certain bones, such as the hips, ribs, and spine (see Figure 20). Blood cancers, also called hematologic malignancies, are a large group of cancers that arise when normal blood formation goes awry and results in abnormal, immature cells called blasts.
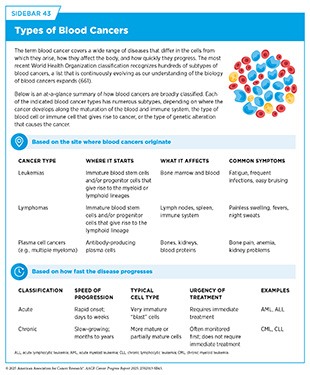
Blood cancer cells are often incapable of performing normal functions, such as defending the body against pathogens, dividing in a controlled manner, or eliminating abnormal or dead cells from the body (see Sidebar 39). Blood cancer cells also acquire properties, such as the ability to infiltrate vital organs or to evade the immune system, that make them aggressive. Blood cancers are broadly classified based on the tissue or cells from which they originate and how fast they grow (see Sidebar 43).
The State of Blood Cancers in 2025
Over the past few decades, research discoveries and technological advances have fueled tremendous progress against blood cancers. As an example, mortality rates for non-Hodgkin lymphoma (NHL)—the most common type of blood cancer in the United States (see Table 7)—have declined by 47 percent between 1997 and 2023 (4)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: June 31, 2025., largely because of advances in precision therapies driven by research. As of January 1, 2025, 879,290 individuals with an NHL diagnosis were living in the United States (9)Wagle NS, et al. (2025) CA Cancer J Clin..
Despite major advances, blood cancers pose a significant public health challenge, both in the United States and around the globe. In 2025 in the United States, blood cancers will account for about 9 percent of all cancer cases and deaths. Globally, blood cancers accounted for 6.6 percent of total cancer cases and 7.2 percent of total cancer-related deaths in 2022, the most recent year for which such data are available (9)Wagle NS, et al. (2025) CA Cancer J Clin..
Leukemias
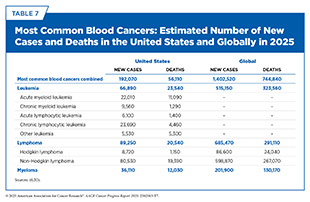
Leukemias are blood cancers that start in blood-forming tissue, such as the bone marrow, and cause large numbers of abnormal blood cells to be produced and enter the bloodstream. According to 2025 estimates, 66,890 new cases of leukemia and 23,540 deaths from the disease will occur in the United States (see Table 7). Leukemia is the most common cancer in children (ages 0 to 14) and adolescents (ages 15 to 19) (6)American Cancer Society. Cancer Facts and Figures. Accessed: June 13, 2025.. Depending on the cell of origin and how fast cancer grows (see Sidebar 43), there are four major categories of leukemias.
Acute myeloid leukemia (AML) and chronic myeloid leukemia (CML) affect the myeloid lineage of blood cells (see Figure 20). AML is a fast-growing cancer in which myeloid progenitor cells—cells capable of giving rise to all cell types in the myeloid lineage—lose their ability to mature and overcrowd the bone marrow, sometimes spilling into organs, such as gums or skin. AML is the second most common leukemia among adults age 20 years and older, accounting for 31 percent of all leukemia cases in this age group. AML mortality rates across age groups have declined every year from 1.9 percent in 2013 to 2.8 percent in 2022 (6)American Cancer Society. Cancer Facts and Figures. Accessed: June 13, 2025.; however, the 5-year survival remains at 33 percent (4)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: June 31, 2025..
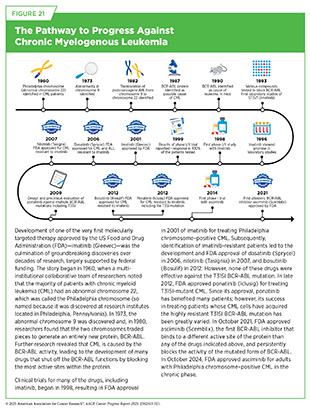
CML is a slow-growing cancer, which usually begins when a stem cell in the bone marrow acquires a genetic alteration known as the Philadelphia chromosome. The Philadelphia chromosome, in which pieces of chromosomes 9 and 22 break off and trade places with each other, produces the abnormal BCR-ABL1 protein that drives uncontrolled growth of cancer cells. CML usually occurs in older adults and rarely occurs in children. In 2025, nearly 10,000 new cases of CML are estimated to be diagnosed in the United States (see Table 7). While the overall CML incidence has increased slowly since the early 2000s, the mortality from the disease has halved (4)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: June 31, 2025., with 70 percent of CML patients age 20 and older living 5 years or more following their diagnosis (6)American Cancer Society. Cancer Facts and Figures. Accessed: June 13, 2025.. The incredible progress against the disease has been made possible by decades of research that led to understanding the biological underpinning of CML as well as FDA approval of several molecularly targeted therapies, significantly expanding treatment options for patients (see Figure 21).
Acute lymphocytic leukemia (ALL) and chronic lymphocytic leukemia (CLL) affect the lymphoid lineage of blood cells (see Figure 20). ALL is characterized by the rapid accumulation—mostly in the bone marrow but sometimes in the chest or brain—of immature lymphocytes, white blood cells that give rise to mature B and T immune cells. ALL is the most common type of leukemia in children and adolescents (ages 0 to 19) (6)American Cancer Society. Cancer Facts and Figures. Accessed: June 13, 2025., and thanks to research-driven advances in understanding the disease as well as the development of effective treatments, the 5-year relative survival rate is 90 percent in this age group (6)American Cancer Society. Cancer Facts and Figures. Accessed: June 13, 2025..
CLL is a slow-growing cancer that affects more mature antibody-producing B cells that accumulate in blood, bone marrow, and lymph nodes. In the modern classification of leukemias, CLL and small lymphocytic lymphoma (SLL), a type of NHL, are essentially the same disease but differ in where the cancer cells are primarily found. CLL cells are mostly in the blood and bone marrow while SLL cells are mainly in the lymph nodes and lymphatic tissues. CLL/SLL is the most common leukemia among adults ages 20 and older in the United States, accounting for about 38 percent of leukemia cases in this age group (see Table 7). The treatment landscape for patients with CLL/SLL has dramatically changed since the approval of the first molecularly targeted therapeutic, ibrutinib (Imbruvica), against the disease in 2014 (579)Arteaga CL, et al. (2014) Clin Cancer Res, 20: S1.. The 5-year survival rate for CLL/SLL is 92 percent for those diagnosed in 2017, the most recent data year (4)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: June 31, 2025..
Lymphomas
Lymphomas are blood cancers that begin in lymphocytes, which are a type of white blood cell that helps fight infection (see Figure 20). Lymphomas are the most common type of blood cancers in the United States, with an estimated 89,070 new cases and 20,540 deaths from the disease in 2025 (see Table 7). Lymphomas are broadly grouped into Hodgkin lymphoma (HL) and NHL, based on how the cancer cells look under a microscope and what mutations drive them. From 2012 to 2021, the overall incidence of both types of lymphoma has steadily declined (6)American Cancer Society. Cancer Facts and Figures. Accessed: June 13, 2025..
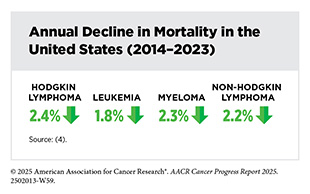
HL is marked by the presence of cells called Reed-Sternberg or Hodgkin cells, which are large, abnormal lymphocytes that may contain more than one nucleus. The number of these cells increases as the disease progresses. HL more commonly affects young adults and will account for about 10 percent of all lymphoma cases diagnosed in 2025 (see Table 7). Between 2013 and 2022, the HL death rate declined by 2.5 percent every year. For those diagnosed in 2017, the overall 5-year survival rate is more than 98 percent for children (ages 0 to 14) and adolescents (ages 15 to 19) (4)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: June 31, 2025.. As of January 1, 2025, 235,110 individuals with an HL diagnosis were living in the United States (9)Wagle NS, et al. (2025) CA Cancer J Clin..
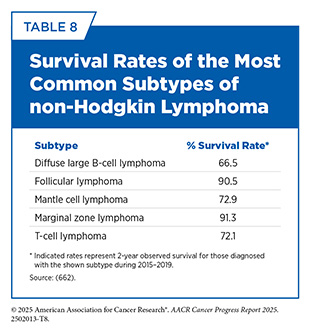
NHL is a more diverse group of over 80 distinct cancers, some slow-growing and others very aggressive. NHL is the most common type of blood cancer in the United States and globally (see Table 7). NHL can occur at any age and can be formed from either B cells, such as diffuse large B-cell lymphoma (DLBCL), or T cells, such as anaplastic large cell lymphoma (ALCL). Unlike HL, for which the overall risk peaks during adolescence and early adulthood, the overall risk of developing NHL increases with age. Five-year survival rates for those diagnosed with NHL between 2015 and 2021 are 74 percent (4)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: June 31, 2025.; however, these rates vary widely across the subtypes (see Table 8).
Multiple Myeloma
Multiple myeloma is a cancer of plasma cells, which are responsible for producing antibodies. In this disease, cancerous plasma cells crowd the bone marrow and release abnormal antibodies (M proteins) that can damage the kidneys, weaken bones, and cause anemia and high calcium levels. Multiple myeloma often starts quietly, first appearing as a precancerous state like monoclonal gammopathy of undetermined significance (MGUS) or smoldering multiple myeloma, which carries risk but does not cause symptoms (see Intercepting Precancerous Conditions in Blood Cancers).
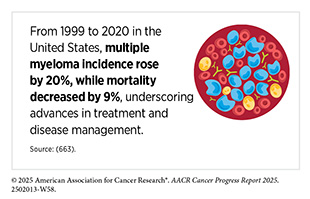
According to the most recent estimates, multiple myeloma will account for 18 percent of all blood cancer cases and 20 percent of all deaths from blood cancers in the United States in 2025 (see Table 7). The 5-year relative survival rate is 64 percent for all stages but varies significantly based on the stage at which the cancer is detected. For example, the 5-year survival rate is just 62 percent for those diagnosed when the cancer has metastasized—about 96 percent of all multiple myeloma cases—compared to 81 percent for those diagnosed when the cancer is still confined to the primary site—about 3 percent of all myeloma cases (4)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: June 31, 2025.. As of 2022, the most recent year for which such data are available, an estimated 192,000 people in the United States were living with multiple myeloma (4)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: June 31, 2025..
There are also several hematologic malignancies that can progress into one of the main subtypes discussed above. As one example, myelodysplastic syndromes (MDS) are a group of bone marrow disorders in which immature blood cells fail to develop properly, leading to low counts of one or more types of blood cells. These disorders are often caused by mutations acquired during a person’s lifetime. MDS can remain stable or progress to leukemia, and intervention varies from surveillance to intensive treatment.
Blood Cancers: An Ongoing Challenge
Incidence and mortality rates for all blood cancers combined have been steadily declining in recent years, but trends vary by age, sex, race, and blood cancer subtype. For example, the overall incidence of HL decreased by 1.4 percent every year between 2013 and 2022, but the incidence of CML rose by 1 percent annually during the same time frame.
Another example of varying trends is the multiple myeloma incidence rates by sex. Between 2013 and 2022, the multiple myeloma incidence rose at more than twice the rate among females (3.2 percent every year) compared to males (1.4 percent every year) (4)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: June 31, 2025.. Similarly, while 5-year relative survival has increased across subtypes of blood cancer since 2000 (4)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: June 31, 2025., survival gains greatly differ among different subtypes of blood cancers. As one example, 5-year relative survival spans from 89 percent in HL to just 33 percent in AML (4)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: June 31, 2025..
Global Burden of Blood Cancers
Over the past three decades, the global burden of hematologic malignancies has evolved considerably, with both encouraging progress and persistent challenges. Leukemia continues to account for the leading cause of mortality from hematologic malignancies; in 2022, leukemia was the 13th most frequently diagnosed and the 10th leading cause of cancer deaths globally. However, there are encouraging long-term trends. From 1990 to 2019, leukemia mortality rates declined significantly, with the steepest decreases seen in regions with high sociodemographic index (SDI)—a composite measure of a country’s development, based on income per capita, educational attainment, and fertility rate (664)Zhang N, et al. (2023) Blood Cancer J, 13: 82.. Among leukemia subtypes, CML has seen the largest mortality declines, especially in high-SDI countries (664)Zhang N, et al. (2023) Blood Cancer J, 13: 82.. In contrast, low-SDI regions bear a disproportionate mortality burden despite lower incidence (664)Zhang N, et al. (2023) Blood Cancer J, 13: 82..
In 2022, NHL was the most common hematologic malignancy and the 10th most diagnosed and 11th leading cause of cancer deaths globally (56)Bray F, et al. (2024) CA Cancer J Clin, 74: 229.. Rates of mortality and disability-adjusted life-years (DALY)—a measure of overall disease burden that combines years of life lost due to premature death and years lived with disability—for NHL declined from 1999 to 2021, particularly in high-SDI regions (such as North America) (666)Wang S, et al. (2025) BMC Public Health, 25: 1223.. HL has also shown global declines in both incidence and mortality, with the most progress observed in high-SDI regions (666)Wang S, et al. (2025) BMC Public Health, 25: 1223.. These encouraging global trends are also observed in childhood NHL; from 1990 to 2021, rates of mortality and DALY for childhood NHL have each declined by 40 percent (667)Xie J, et al. (2025) Front Pediatr, 13: 1618810..
Multiple myeloma contributed to about 1 percent of all cancer diagnoses and cancer-related deaths globally in 2022 (56). During the period from 1990 to 2019, the incidence of multiple myeloma increased across all five SDI regions, with the largest estimated annual change of 0.8 percent in middle-SDI regions (664)Zhang N, et al. (2023) Blood Cancer J, 13: 82..
Despite major advances in the treatment of blood cancers, access to breakthrough therapies like molecularly targeted therapies and immunotherapeutics, such as CAR T-cell therapy, and hematopoietic stem cell transplants remains highly uneven across the globe. Many low- and middle-income countries face critical shortages in infrastructure that make it difficult to support the necessary specialists trained in precision diagnostics, as well as to provide supportive care needed by patients and their caregivers, further exacerbating disparities in outcomes (668)Flowers CR, et al. (2025) Blood Cancer Discov, 6: 79.(669)Miranda-Galvis M, et al. (2023) Blood Adv, 7: 6466.. Addressing these global inequities will require sustained investment in workforce training, technology transfer, and regionally adapted care delivery models.
Disparities in the Burden of Blood Cancers in the United States
Despite tremendous progress in recent years, disparities in the burden of blood cancers continue to span the entire cancer research and care continuum. As an example, in 2023, the most recent year for which data are available, the HL incidence rate was the same among Black and White individuals under the age of 50. However, from 2019 to 2023, HL mortality in this age group declined at nearly four times the rate among White individuals (9.8 percent every year) compared to Black individuals (2.6 percent every year) (4)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: June 31, 2025.. Examples of such disparities exist at many levels (see Sidebar 44) and are influenced by biology, lived experiences, access to health care systems, and broader social drivers of health (668)Flowers CR, et al. (2025) Blood Cancer Discov, 6: 79..
Certain genetic variants linked with ancestry—such as those in GATA3, IKZF1, and SETD2 genes—are more common in Black and Hispanic populations and influence not only the likelihood of developing blood cancers but also how well patients respond to treatment (679)de Smith AJ, et al. (2024) Cell Genom: 100526.(680)Churchman ML, et al. (2018) Cancer Cell, 33: 937.(681)de Smith AJ, et al. (2023) Front Oncol, 13: 1299355.(682)Lee MJ, et al. (2020) Cancer, 126: 3493.(683)Peres LC, et al. (2022) Blood Adv, 6: 3767.. These biological differences are often compounded by structural barriers. People from medically underserved communities are more likely to live in areas with poor health care access, face higher rates of comorbidities, and be diagnosed at more advanced stages. In many cases, they mistrust the medical system, and also face lower enrollment in clinical trials, as well as longer delays to treatment (12)American Association for Cancer Research®. AACR Cancer Disparities Progress Report 2024. Accessed: June 14, 2025.. New policies and national initiatives must be coupled with sustained investment in community trust, culturally tailored care, and workforce diversity if we are to have a meaningful impact on reducing disparities (668)Flowers CR, et al. (2025) Blood Cancer Discov, 6: 79..
Growing evidence indicates that access to health care plays an important role in reducing the burden of the disease across population groups. As one example, when Black and White patients with multiple myeloma received comparable treatment and had similar socioeconomic status, overall health, and the stage at which the disease was diagnosed, disparities in survival were effectively eliminated (684)Dong J, et al. (2022) Blood Cancer J, 12: 34.. In fact, Black patients had a 4.6 percent higher 5-year survival rate than White patients within the matched cohort (684)Dong J, et al. (2022) Blood Cancer J, 12: 34.. Findings of this study reaffirm an increasingly evident view that differences in health outcomes for patients with cancer are largely attributable to differences in access and delivery of care (12)American Association for Cancer Research®. AACR Cancer Disparities Progress Report 2024. Accessed: June 14, 2025.. Addressing disparities requires a reimagining of how care is delivered, who identifies and shapes research priorities, and how social drivers of health—such as income inequality and educational access—are integrated into cancer control and care strategies.
Unique Biology of Blood Cancers
Unlike solid tumors that grow as distinct masses, blood cancers arise from a dynamic system of blood and immune cells. The fluid nature of blood and its continuous renewal via hematopoietic stem cells—capable of giving rise to the entire blood and immune system—and progenitor cells—capable of giving rise to mature myeloid or lymphoid cells—creates a unique biological landscape (685)Fernandez-Maestre I, et al. (2024) Blood Cancer Discov, 5: 377.. As a result, leukemias, lymphomas, and multiple myeloma often manifest not in one organ but throughout the circulatory or lymphatic system (see Sidebar 45 and Circulatory and Immune Systems).
Genetic and epigenetic disruptions in blood cancers frequently alter the functions of genes involved in the regulation of cell growth, identity, and chromosome structure. In myeloid leukemias, mutations in genes, such as DNMT3A, TET2, RUNX1, NPM1, and IDH1/2, are often early events contributing to cancer development, as well as to treatment resistance (686)Hogg G, et al. (2023) Cancer Genet, 278-279: 38.(687)Lachowiez CA, et al. (2025) Blood Cancer Discov.. For example, mutations in the DNMT3A gene alone occur in about 20 percent to 30 percent of AML cases and are associated with an altered epigenome (688)Huang G, et al. (2025) Ann Hematol, 104: 1399.. These mutations often co-occur and cooperate with mutations in cellular processes that regulate cell proliferation, such as mutations in the FLT3 gene, to drive leukemia (689)Meyer SE, et al. (2016) Cancer Discov, 6: 501..
Unlike solid tumors, blood cancers carry fewer overall somatic mutations (see Genetic Alterations), but a higher frequency of mutations in proteins, such as EZH2 and cohesin, that are involved in epigenetic regulation of cell functions (690)Wozniak M, et al. (2025) Ther Adv Med Oncol, 17: 17588359241306026.(691)Khouri MR, et al. (2025) Cancer, 131: e35846.. Similarly, mutations in genes—such as GATA2 and CEBPA that are critical for normal development and maturation of immune cells—lead to the development of B-cell lymphomas and multiple myeloma, as well as confer treatment resistance in these cancer types (692)Zoller J, et al. (2023) Front Oncol, 13: 1205855.. Inherited or germline mutations (see Figure 6) play a substantial role in blood cancer development, with approximately 5 percent to 10 percent of myeloid malignancy cases harboring pathogenic variants in genes, such as RUNX1, GATA2, and CEBPA (693)Gong Y, et al. (2021) Oncol Rep, 45: 49.. These variants often increase a person’s lifetime risk for conditions such as MDS and AML. As our understanding of inherited risk genes deepens, genetic testing, especially at diagnosis, is becoming essential for tailoring treatment and guiding family risk management strategies (694)Godley LA, et al. (2024) Am Soc Clin Oncol Educ Book, 44: e432218..
Chromosomal rearrangements are structural genetic alterations in which two chromosomes exchange genetic material to make a new gene that can lead to the development of cancer by a variety of mechanisms (see Sidebar 8 and Figure 22) (695)Mitelman F, et al. (2007) Nat Rev Cancer, 7: 233.. Blood cancers are characterized by a higher frequency of chromosomal rearrangements; about 30 percent of leukemias, lymphomas, and multiple myeloma carry chromosomal rearrangements, compared to roughly 5 percent to 10 percent of solid tumors (696)Rabbitts TH (1994) Nature, 372: 143.. In fact, the first chromosomal rearrangement between chromosomes 9 and 22 and the resulting BCR-ABL1 fusion gene was described in CML (see Figure 21). The higher frequency of chromosomal translocations in blood cancers partly reflects the biology of hematopoietic cells, which are naturally programmed to rearrange their DNA to produce diverse antibodies and other proteins against pathogens.
Many subtypes of blood cancers are very rare—those with fewer than 15 cases out of 100,000 people each year. Among some of the blood cancer subtypes with less than 1 case out of 100,000 people every year are rare leukemias (e.g., hairy cell leukemia, acute promyelocytic leukemia), rare lymphomas (e.g., mantle cell lymphoma, anaplastic large cell lymphoma), and other rare hematologic malignancies (e.g., Waldenström macroglobulinemia). As is the case with other rare cancers, rare blood cancers suffer from lack of expertise across the research and care continuum (see Sidebar 38), underscoring the urgent need for sustained investment in foundational and translational research.
Precision Medicine Driving Advances Against Blood Cancers
Decades of research leading to major advances in blood cancer treatments have laid the groundwork for a new era of oncology that transcends traditional boundaries between hematologic and solid tumors. Blood cancers, particularly myeloma, have also served as models for developing therapeutic strategies that not only target the cancer cells directly but also invoke the immune system to destroy cancer cells (697)Gulla A, et al. (2021) Blood Cancer Discov, 2: 468..
Pioneering successes in the development of molecularly targeted therapies for blood cancers introduced the concept that cancer can be effectively treated by selectively targeting its genomic drivers rather than focusing on its site of origin. As one example, imatinib—the first molecularly targeted therapy—transformed once-fatal CML)into a manageable condition by blocking the BCR-ABL1 protein that drives the disease (698)Senapati J, et al. (2023) Blood Cancer J, 13: 58.(699)Jabbour E, et al. (2024) Am J Hematol, 99: 2191.. On the basis of recent findings, some CML patients with deep, sustained responses can stop taking imatinib entirely and remain in remission while enjoying a better quality of life (700)Atallah E, et al. (2021) JAMA Oncol, 7: 42..
A decade after the approval for CML, FDA expanded the use of imatinib for a rare digestive tract cancer known as gastrointestinal stromal tumor (GIST). This approval was based on research showing that many GISTs are driven by activating mutations in the KIT protein, another target of imatinib in addition to BCR-ABL1 (692)Zoller J, et al. (2023) Front Oncol, 13: 1205855.(701)Catalano F, et al. (2023) Cancers (Basel), 15.(702)Venkataraman V, et al. (2023) Oncologist, 28: 671.(703)Barnett CM, et al. (2013) Hematol Oncol Clin North Am, 27: 871.. Long-term follow-up confirmed that GIST patients who stayed on imatinib had far better disease control and survival than those who stopped, with benefits growing over time (704)Blay JY, et al. (2024) Lancet Oncol, 25: 1163.. By underscoring that a therapeutic to treat blood cancers could change the outlook for patients with a solid tumor, imatinib has ushered in a new paradigm of precision cancer medicine (see Figure 21).
The development and success of imatinib inspired a new generation of small molecule inhibitors targeting genomic drivers in blood cancers, and now similar strategies are being used to target common alterations in solid tumors, such as those in BRAF, NTRK, IDH1/2, RET, and FGFR genes (705)Cohen P, et al. (2021) Nat Rev Drug Discov, 20: 551.. More specifically, it established the family of protein kinases as viable therapeutic targets, paving the way for the approval of more than 80 kinase inhibitors by FDA (706)Roskoski R, Jr. (2024) Pharmacol Res, 200: 107059.. While most current FDA approvals for these molecularly targeted therapeutics remain limited to solid tumors, many of these same genomic alterations also exist—and are targetable—in blood cancers.
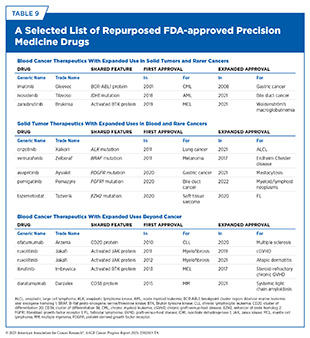
Several therapeutics that were initially FDA-approved to treat blood cancers have been subsequently approved to treat solid tumors, and vice versa (see Table 9). An integrated approach that applies lessons learned from one field to the other promises not only more efficient drug development but also broader therapeutic access and innovation for more patients across the cancer spectrum (707)Adashek JJ, et al. (2025) Med, 6: 100550..
Repurposing existing drugs for new uses beyond their approved indications is particularly important to speed progress against rarer blood cancers that share treatable molecular targets with solid tumors or more common blood cancers (708)Brown A, et al. (2024) Cancers (Basel), 16.(709)Christyani G, et al. (2024) Int J Mol Sci, 25.. As an example, a basket trial, the clinical study that groups patients by genetic features (see Sidebar 28), enabled the 2017 FDA approval of vemurafenib (Zelboraf), first approved in 2011 for melanoma, to treat Erdheim-Chester disease, an ultra-rare hematologic malignancy. Between 50 percent and 70 percent of patients with Erdheim-Chester disease harbor BRAF gene mutations, the same alteration that is common in melanoma and targeted by the BRAF inhibitor vemurafenib. Studies have shown that patients with Erdheim-Chester disease responded well to vemurafenib treatment (710)Hyman DM, et al. (2015) N Engl J Med, 373: 726.(711)Subbiah V, et al. (2020) Cancer Discov, 10: 657.(712)Doke R, et al. (2025) Naunyn Schmiedebergs Arch Pharmacol, 398: 6407..
Although long-term follow-up studies have also shown serious side effects (713)Goyal G, et al. (2025) Blood, 145: 2100., the generally favorable response to vemurafenib in patients with Erdheim-Chester disease illustrates how knowledge from one cancer type can inform another (712)Doke R, et al. (2025) Naunyn Schmiedebergs Arch Pharmacol, 398: 6407.. This example also underscores the importance of integrating genomics, molecular diagnostics, and cross-disciplinary collaboration to identify actionable mutations and develop treatments for understudied malignancies.
In the following sections, we present a broad overview of some of the transformative advances in precision medicine from the past decade that are being used in the clinic for patients with different types of blood cancers.
Diagnosing Blood Cancers With Increasing Precision
Over the past decade, breakthroughs in sequencing technologies, biospecimen processing, and computational biology have fueled innovations in molecular diagnostics for hematologic malignancies (714)Kwon R, et al. (2024) Haematologica, 109: 379.. In addition to foundational technologies, such as imaging for detection of chromosomal alterations and polymerase chain reaction—which enables amplifying DNA amount from a sample—for detection of minimal residual disease, next-generation sequencing of cancer DNA and RNA has now become a standard for diagnosis, prognosis, and therapeutic decision-making (see Modernizing Clinical Trial Design for Blood Cancers) (715)Cho YU (2024) Blood Res, 59: 11.. AML—the deadliest form of blood cancer (see Table 7), with relapse rates still around 40 percent to 50 percent—is just one example for which rapid and tailored molecular profiling is becoming more crucial (716)Haferlach T (2020) Hematol Rep, 12: 8957..
Innovations like loop-mediated isothermal amplification sequencing, which combines rapid increase in the amount of DNA and RNA as well as their sequencing from very small amounts of blood, are helping to identify the genetic alterations in hematologic malignancies within 2 days, sometimes within hours (717)Boonbanjong P, et al. (2022) Biosensors (Basel), 12.(718)Ludwig KU, et al. (2021) Nat Biotechnol, 39: 1556.. Because chromosomal alterations play a pivotal role in many blood cancers, detecting these structural variations with speed and accuracy is key to informing clinical decisions. In this regard, optical genome mapping, a high-resolution imaging technique that allows long stretches of DNA to be examined under the microscope for detecting even the most complex structural variations in chromosomes, is proving vital in gathering clinically relevant information about a patient’s cancer (719)Dremsek P, et al. (2021) Genes (Basel), 12.. Similarly, tests that identify methylation patterns in genomes of patients with AML are unraveling epigenetic underpinnings of the disease and are at the cusp of broader clinical use for diagnosing AML (see Epigenetic Changes) (720)Yang X, et al. (2019) Int J Mol Sci, 20.(721)Kuhn MWM, et al. (2025) Leukemia..
The emergence of single-cell sequencing has dramatically reshaped the understanding of blood cancers, revealing great heterogeneity in their transcriptomes, epigenomes, and cellular characteristics, even within the same subtype (722)Garcia-Sanz R, et al. (2021) Cancers (Basel), 13.. These technologies have identified unique features not only of cancer cells overall but also of those cancer cell populations that confer resistance to treatment (723)Zeng AGX, et al. (2025) Blood Cancer Discov: OF1., knowledge that can help design more effective therapies. Parallel strides in computational biology are reshaping how data derived from these technologies are interpreted.
However, many of these state-of-the-art approaches require significant investments in specialized expertise and infrastructure, making them out of reach for many researchers and patients alike (714)Kwon R, et al. (2024) Haematologica, 109: 379.. While remarkable progress has been made for diagnosing blood cancers, additional work is needed to overcome major hurdles in translating these technological gains into universal, precise, and accessible care for patients with blood cancers.
A Revolution in Molecularly Targeted Therapies
Over the past decade, molecularly targeted therapies have transformed the treatment landscape for blood cancers. Advances in genomic profiling have identified the genetic alterations in blood cancers, which has enabled the development of drugs that precisely inhibit mutated forms of proteins, such as BTK, BCL-2, FLT3, and IDH, that drive uncontrollable proliferation and growth of cancer cells. These therapies offer deeper responses, prolonged survival, and improved quality of life. This section provides a broad overview of the key advances that have revolutionized treatment options for patients with blood cancers.
Breakthroughs in Treating Leukemias
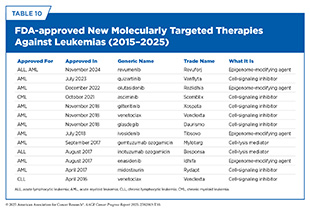
Over the past two decades, the treatment of leukemia has undergone a profound transformation, moving away from a reliance on chemotherapy toward therapies designed to target the specific molecular drivers of disease (see Table 10). Uncovering the molecular underpinnings of CML pioneered this revolution, whereby the discovery of the Philadelphia chromosome and the resulting BCR-ABL1 fusion gene led to the development of small molecule inhibitors, such as imatinib, specifically targeting functions of the abnormal BCR-ABL1 protein (see Figure 21). Imatinib, which was approved by FDA for CML treatment in 2001, remains the gold standard today (698)Senapati J, et al. (2023) Blood Cancer J, 13: 58..
Newer and improved BCR-ABL1 inhibitors, such as dasatinib, nilotinib, bosutinib, and ponatinib, have since emerged, offering options in the face of resistance mutations like T315I that render imatinib ineffective (724)Jayavel S, et al. (2025) J Egypt Natl Canc Inst, 37: 12.. Most recently, in 2021, FDA approved asciminib, a first of its kind BCR–ABL1-targeted treatment that binds to a different active site of the protein than any of the drugs indicated above and persistently blocks the activity of the mutated form of BCR-ABL1. Thanks to these treatments, CML has been transformed from a disease with a typical life expectancy of 3 to 5 years into one in which most patients can expect to live a normal lifespan.
The approach of targeting key proteins involved in cancer cell proliferation, growth, and survival has since been extended to AML (see Figure 23). As one example, mutations in the FLT3 gene—which is involved in proliferation and survival of hematopoietic stem and progenitor cells—are present in roughly one-third of patients with AML. In 2017, FDA approved the first molecularly targeted therapeutic, midostaurin (Rydapt), to treat AML patients whose cancer cells harbor FLT3 mutations (725)Tessmer MS, et al. (2017) Clin Cancer Res, 23: 5325.. Since then, two additional molecularly targeted therapeutics—gilteritinib (Xospata) and quizartinib (Vanflyta)—have been approved by FDA to treat patients with AML. Each of the three therapeutics has helped patients with AML live longer and without detectable disease (726)Erba HP, et al. (2023) Lancet, 401: 1571.(727)Dohner H, et al. (2022) Blood Adv, 6: 5345.(728)Abbas HA, et al. (2019) Cancer Manag Res, 11: 8817.(729)Onate G, et al. (2023) Blood Cancer J, 13: 69.(730)Gupta SV, et al. (2024) Princ Pract Clin Res, 10: 47..
Another major advance in the personalized treatment of AML has been the development of therapeutics targeting IDH1 and IDH2—important proteins that mediate a key step in cellular energy production and epigenome regulation (731)Waitkus MS, et al. (2018) Cancer Cell, 34: 186.. Mutated forms of IDH1/2, found in approximately 20 percent of AML cases, promote the development of leukemia (732)Nassereddine S, et al. (2017) Ann Hematol, 96: 1983.. As of June 30, 2025, FDA has approved three IDH inhibitors for AML—ivosidenib (Tibsovo) in 2017, enasidenib (Idhifa) in 2018, and olutasidenib (Rezlidhia) in 2022—all of which have shown a complete remission in 20 percent to 40 percent of patients with AML (733)Norman M, et al. (2024) Expert Rev Hematol, 17: 755.. Targeting IDH1/2 mutations in solid tumors have also shown great promise (see Bringing the Promise of Precision Medicine to Children, Adolescents, and Young Adults With Cancer) (734)Carosi F, et al. (2024) Cancers (Basel), 16., further underscoring how knowledge gleaned from the underpinnings of hematologic malignancies is driving progress against certain solid tumors.
During the 12 months covered by this report (July 1, 2024 to June 30, 2025), FDA further expanded its armamentarium of AML therapies by approving revumenib (Revuforj), a new molecularly targeted treatment for patients, including children, who carry a common structural variation in the KMT2A gene. Structural variations, also known as rearrangements, in the KMT2A gene are observed in 80 percent of infants with ALL and in 5 percent to 15 percent of children and adults with acute leukemia, including cases that originate in myeloid or lymphoid cells, or a mix of both (see Sidebar 43) (735)Issa GC, et al. (2023) Nature, 615: 920..
The KMT2A gene encodes a protein called MLL1, which plays a critical role in normal blood cell development by regulating gene expression through epigenetic mechanisms. When the KMT2A gene is rearranged, it disrupts normal cell development by causing blood cells to revert to an immature, stem cell–like state, preventing them from maturing into functional blood cells. This process is driven by the interaction of MLL1 with another protein called menin. Together, menin and MLL1 form a complex that binds to DNA in the cell nucleus and triggers harmful genetic programs that lead to leukemia. Acute leukemia with KMT2A rearrangements is associated with treatment resistance and poor prognosis (736)Issa GC, et al. (2025) J Clin Oncol, 43: 75.. In addition to KMT2A rearrangements, mutations in the NPM1 gene—detected in up to 30 percent of acute AML cases in adults—also depend on menin to promote leukemia development (737)Issa GC, et al. (2021) Leukemia, 35: 2482..
These discoveries led to the development of menin-targeted therapies (738)Garber K (2024) Nat Rev Drug Discov, 23: 567., culminating in the November 2024 FDA approval of revumenib (Revuforj), the first menin inhibitor, for adult and pediatric patients (1 year and older) with acute leukemia harboring KMT2A rearrangements who never responded to or experienced relapse after initial treatments. Revumenib works by blocking the interaction between menin and MLL1. By binding to menin, it prevents the menin–MLL1 complex from attaching to DNA, thereby halting the abnormal genetic programs that fuel leukemia. As a result, leukemia cells are either driven to mature into blood cells with some normal properties or are eliminated.
FDA approval was based on a phase I/II clinical trial in which more than 21 percent of patients experienced complete remission (cancer no longer detectable in the bone marrow, number of healthy blood cells returned to normal levels) or complete remission with partial recovery of their blood counts (cancer no longer detectable in the bone marrow, partial recovery of the number of healthy blood cells). The benefits lasted a median of over 6 months. Revumenib has provided patients such as John C. (Jack) Moorman with a personalized treatment option that is much less aggressive than traditional chemotherapeutics.
Ongoing studies are looking to identify mechanisms of resistance to revumenib treatment and evaluating revumenib as the initial treatment as well as in combination with other molecularly targeted therapeutics or chemotherapeutics to improve outcomes for patients.
Molecularly targeted therapies have significantly advanced the treatment of ALL, especially in certain subtypes and relapsed/ refractory disease. Approximately 20 percent to 30 percent of adult ALL cases are Philadelphia chromosome–positive (Ph+ ALL), while among children, the prevalence is much lower at about 2 percent to 5 percent (739)Kantarjian H, et al. (2025) Am J Hematol, 100: 1205.. Ph+ ALL is driven by the Philadelphia chromosome product, the BCR-ABL1 fusion protein, molecularly targeted therapeutics against which have revolutionized treatment of Ph+ ALL, in addition to CML.
Another class of molecularly targeted therapeutics that is gaining momentum in the treatment of blood cancers comprises antibody–drug conjugates (ADCs) (see Delivering Cytotoxic Agents Precisely to Cancer Cells). Unlike the therapeutics discussed thus far in this section—all are small molecules inhibiting functions of one or more proteins in the cells—ADCs kill cancer cells in an entirely different manner (740)Wang R, et al. (2025) J Hematol Oncol, 18: 51..
Inotuzumab ozogamicin (Besponsa), an ADC, was approved by FDA in 2017, for treating patients with Ph+ ALL. ADCs represent an exciting new class of molecularly targeted therapeutics that function by finding certain proteins present on the surface of cancer cells and then delivering toxic compounds directly to them, thus minimizing side effects from the treatment (740)Wang R, et al. (2025) J Hematol Oncol, 18: 51.. Combining these molecularly targeted therapies with chemotherapeutics or immunotherapeutics has transformed outcomes for patients with ALL, with about 73 percent of patients living 5 years or more following diagnosis (4)NCI Surveillance, Epidemiology, and End Results Program. NCI SEER*Explorer. Accessed: June 31, 2025.(739)Kantarjian H, et al. (2025) Am J Hematol, 100: 1205..
Despite the expanding number of precision medicine drugs against leukemia, resistance to molecularly targeted therapy remains a universal challenge. Thanks to research, combining different classes of precision therapeutics with traditional forms of treatment, often tailored to a patient’s molecular profile, is helping to increase remissions, delay relapses, and in some cases, eliminate the need for chemotherapy altogether. For example, treating patients who have CLL with a combination of two molecularly targeted therapies (venetoclax [Venclexta], which triggers leukemia cell death, and acalabrutinib [Calquence], which blocks leukemia cell proliferation), along with an immunotherapeutic (obinutuzumab [Gazyva], which invokes the immune system to kill leukemia cells) kept the disease from progressing in more people at 3 years than was found with standard chemotherapy (741)Brown JR, et al. (2025) N Engl J Med, 392: 748.. The treatment was also generally safe and well tolerated.
Milestones in Managing Lymphomas
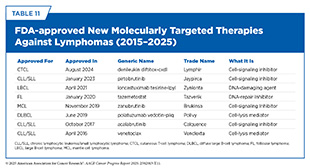
In recent years, the therapeutic landscape for NHL has undergone a profound shift, offering new hope for patients who historically had limited options beyond chemotherapy. This shift reflects a deeper understanding of lymphoma biology and the identification of proteins that can be selectively inhibited with targeted therapies (see Table 11). As a result, molecularly targeted therapies are reshaping treatment across major NHL subtypes by improving survival, enhancing quality of life, and reducing treatment-related toxicity (742)Wang L, et al. (2020) Signal Transduct Target Ther, 5: 15..
NHL is the most common type of blood cancer, with over 80 subtypes (see Lymphomas). Some common forms include DLBCL, which is the most aggressive and diagnosed subtype (about 30 percent to 35 percent of NHL cases); follicular lymphoma (FL), which is slow-growing and makes up about 20 percent to 25 percent of NHL cases; and mantle cell lymphoma (MCL), which is relatively less common (about 5 percent to 7 percent of NHL cases) but highly aggressive (662)Cerhan JR, et al. (2024) Am J Hematol, 99: 408.. Over the past decade, an expanding number of molecularly targeted therapies have become available for patients with DLBCL, FL, and MCL, leading to notable gains in response rates and durability of remission (743)Crisci S, et al. (2019) Front Oncol, 9: 443.(744)Thomas CJ, et al. (2024) Cancers (Basel), 16.(745)D’Alo F, et al. (2024) Cancers (Basel), 16..
One protein that has become an attractive target for developing targeted therapies against NHL is Bruton’s tyrosine kinase (BTK). Research has shown that BTK plays a crucial role in B-cell development, survival, and proliferation. In certain lymphomas, BTK becomes overactive, making it a central contributor to the disease and an ideal target for therapy (746)Pal Singh S, et al. (2018) Mol Cancer, 17: 57.. Small molecules inhibiting BTK function have been especially transformative in treating various subtypes of NHL.
As of June 30, 2025, FDA has approved four BTK inhibitors: ibrutinib, approved in 2013; acalabrutinib, approved in 2017; pirtobrutinib (Jaypirca), approved in 2023; and zanubrutinib (Brukinsa), approved in 2024. These molecularly targeted therapies have been approved to treat several NHL subtypes, as well as certain types of hematologic malignancies that can progress into lymphomas (see Table 11) (747)Mehra S, et al. (2024) Int J Mol Sci, 25.. Although ibrutinib is the first FDA-approved BTK inhibitor, the newer three inhibitors have improved selectivity, reduced off-target effects, and address treatment resistance. Pirtobrutinib, for instance, has a unique mechanism of action that makes it effective even against mutated forms of BTK that are resistant to other BTK-targeted therapeutics. Collectively, these therapeutics have enabled durable responses with less toxicity in many patients with NHL (747)Mehra S, et al. (2024) Int J Mol Sci, 25..
Researchers have also leveraged another cellular process, called apoptosis or programmed cell death, to develop molecularly targeted therapeutics that induce cell death in lymphomas (748)Adams CM, et al. (2018) Front Oncol, 8: 636.. In normal cells, apoptosis is a tightly controlled process, in which members of the BCL family of proteins play important roles in preventing cell death in healthy cells and triggering apoptosis in damaged or unhealthy cells. Cancer cells, including some lymphoma subtypes, often have higher levels of proteins that prevent cell death, thus helping them escape apoptosis (748)Adams CM, et al. (2018) Front Oncol, 8: 636..
In 2016, FDA approved venetoclax for the treatment of CLL in which part of a chromosome is deleted, and extended this approval in 2018 for CLL or SLL with the same genetic alteration. Venetoclax selectively inhibits the activity of BCL-2, a member of the BCL protein family, that prevents lymphoma cells from dying. In 2020, FDA approved selinexor (Xpovio), which also triggers lymphoma cell death, albeit by a different mechanism. Selinexor was approved for the treatment of DLBCL, the most common and most aggressive form of NHL.
ADCs are also proving effective in the treatment of NHL. As of June 30, 2025, FDA has approved three ADCs to treat different NHL subtypes (see Table 11). Polatuzumab vedotin (Polivy), approved in 2019, targets a protein called CD79b, which is abundantly present on the surface of lymphoma cells. Since its approval, polatuzumab vedotin has emerged as a major addition to the treatment of relapsed/refractory DLBCL, particularly in combination with bendamustine, a chemotherapeutic, and rituximab, another molecularly targeted therapeutic (749)Ghione P, et al. (2024) Haematologica, 109: 2802.. Because of its selective mechanism—delivering cytotoxic agents directly into lymphoma cells—the therapeutic also has reduced toxicity.
The other two ADCs approved for treatment of lymphoma subtypes, loncastuximab tesirine-lpyl for large B-cell lymphoma and brentuximab vedotin for ALCL, bind to two different proteins, CD19 and CD30, on the lymphoma cell surface (750)Al Sbihi A, et al. (2024) Cancers (Basel), 16.. In MCL, a highly aggressive type of NHL, ADCs are increasingly being studied in combination with other molecularly targeted therapeutics to overcome resistance to chemotherapy (751)Jain N, et al. (2023) J Hematol Oncol, 16: 99..
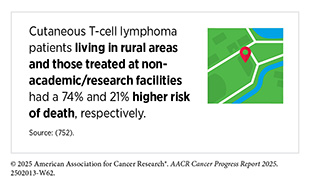
While much of the therapeutic progress has focused on the more common B-cell subtypes of NHL, researchers are also advancing novel treatments for rarer and clinically distinct forms of the disease, such as cutaneous T-cell lymphoma (CTCL), which mainly affects the skin. Early-stage CTCL tends to grow slowly and may not impact life expectancy, but about 30 percent of patients progress to advanced stages within 10 years with significant reduction in survival rates. Treatments for early-stage disease focus on managing skin symptoms and slowing disease progression, starting with topical agents or radiation, while those with advanced disease are treated with additional therapies including systemic treatments. However, current treatments for advanced CTCL often stop working within months and can cause serious side effects.
Researchers are taking innovative approaches to target CTCL. One such approach involves designing synthetic proteins that can target cancer cells and directly deliver toxic molecules to kill them. In August 2024, FDA approved denileukin diftitox-cxdl (Lymphir) for treating adults with CTCL who have received at least one prior systemic therapy and whose disease has returned or stopped responding to treatments. FDA approval was based on results from a phase III clinical trial in which 36 percent of patients responded to denileukin diftitox-cxdl and nearly 9 percent had a complete disappearance of their cancer (753)Foss FM, et al. (2025) J Clin Oncol, 43: 1198..
Denileukin diftitox-cxdl consists of part of the diphtheria toxin linked to interleukin-2 (IL-2), a molecule produced by the immune system that activates immune cells to fight infections and attack cancer cells. Denileukin diftitox-cxdl targets IL-2 receptors found on both cancer cells and certain immune cells, delivering the diphtheria toxin directly to these cells. Once the drug attaches to the IL-2 receptor, it is taken into the cell, where it is broken apart to release the toxin. The toxin blocks the cell’s ability to make proteins, ultimately causing cancer cells to die. In addition to attacking cancer cells, denileukin diftitox-cxdl also eliminates specific immune cells that help protect the cancer, potentially making the treatment more effective.
As research continues to illuminate the molecular underpinnings of lymphoma, the pace of therapeutic innovation has never been stronger. From small molecule inhibitors that block lymphoma survival to ADCs that directly deliver toxins to cancer cells, current treatments reflect decades of progress in basic and translational science. Sustained investment in research will be critical to maintaining this momentum—transforming even the most aggressive or treatment-resistant subtypes into manageable and, in some cases, curable diseases.
Progress in Targeting Multiple Myeloma
In recent years, the development of molecularly targeted therapies and immunotherapies has reshaped the treatment landscape of multiple myeloma (see Table 12), offering new hope for patients whose disease had historically relied on conventional chemotherapy and corticosteroids (see Sidebar 41, Redirecting T Cells to Eradicate Blood Cancers, and Flagging Blood Cancer Cells for Destruction by the Immune System). These novel approaches are driven by an improved understanding of multiple myeloma biology, processes that control plasma cell proliferation and growth, and mechanisms of drug resistance (754). As a result, a new generation of precision medicines is emerging, aimed at selectively interfering with survival pathways, stress responses, and metabolism in multiple myeloma cells (755)Lin CH, et al. (2024) Int J Mol Sci, 25..
A growing list of small molecule inhibitors has expanded treatment options and improved outcomes for relapsed and refractory disease. The first FDA approval of a molecularly targeted therapy to treat multiple myeloma was of bortezomib (Velcade) in 2003; bortezomib inhibits the proteasome, the cell’s machinery designed to eliminate unneeded or damaged proteins. Proteasome inhibitors have been particularly effective against multiple myeloma because multiple myeloma cells rely heavily on their ability to properly manage protein production and disposal (756)Sedlacek J (2025) Clin Exp Med, 25: 176.(757)Fricker LD (2020) Annu Rev Pharmacol Toxicol, 60: 457.. Studies have shown that in patients with newly diagnosed myeloma, the addition of bortezomib to immunomodulatory agents such as lenalidomide (Revlimid) and dexamethasone resulted in significantly improved progression-free and overall survival with manageable side effects (758)Durie BGM, et al. (2017) Lancet, 389: 519..
In 2012, FDA approved a second proteosome inhibitor, carfilzomib (Kyprolis), and in 2015, a third proteosome inhibitor, ixazomib (Ninlaro), to treat patients with multiple myeloma. All three therapeutics induce multiple myeloma cell death by disrupting protein degradation pathways, and have improved outcomes with less serious side effects (754)Jurczyszyn A, et al. (2025) Pol Arch Intern Med, 135.. In addition, unlike bortezomib, which is administered either subcutaneously or intravenously, or carfilzomib, which is administered intravenously, ixazomib is given orally and is more convenient for some patients.
In 2019, FDA approved selinexor (Xpovio), which also triggers apoptosis but by trapping certain proteins in the multiple myeloma cell nucleus, where they can restore control over cell growth and trigger cancer cell death. Since its approval, selinexor has proven to be an effective treatment for multiple myeloma. A recent study showed that a combination of selinexor with bortezomib, lenalidomide, and dexamethasone achieved a 100 percent overall response rate (see Sidebar 32) in newly diagnosed patients with a particularly aggressive form of multiple myeloma. The combination was well tolerated with manageable side effects, highlighting its potential as an effective and feasible first treatment given to this high-risk population (759)Yin J, et al. (2024) Sci Rep, 14: 28557..
Molecularly targeted therapeutics represent a promising frontier in management of multiple myeloma—particularly for patients ineligible for immunotherapies or those with resistant disease. As resistance mechanisms evolve, combinations of these agents, informed by the biology of multiple myeloma calls, may pave the way for durable, chemotherapy-free control of multiple myeloma.
In addition to molecularly targeted therapeutics approved by FDA to treat various subtypes of leukemia, lymphoma, and multiple myeloma, FDA has also approved a number of therapeutics to treat hematologic malignancies that can give rise to one of the three major types of blood cancers (see Table 13).
Transforming Blood Cancer Treatment With Immunotherapy
Groundbreaking basic research in the field of immunology laid the foundation of modern immunotherapy, one of the most exciting new areas of cancer treatment along with molecularly targeted therapeutics (see Advances in Treatment With Immunotherapeutics). Cancer immunotherapeutics leverage the natural ability of the immune system to fight cancer and have been transformative for patients with different types of blood cancer.
FDA-approved immunotherapeutics against blood cancers work in various ways including amplifying the cancer-killing ability of the immune cells, releasing the brakes on the immune system, and flagging cancer cells for destruction by the immune system.
Harnessing CAR T Cells to Treat Blood Cancers
Chimeric antigen receptor (CAR) T-cell therapy is a form of cellular immunotherapy (see Sidebar 41) that has revolutionized the treatment landscape for blood cancers. As of June 30, 2025, FDA has approved seven CAR T-cell therapies, all for the treatment of blood cancers, including leukemia, lymphoma, and multiple myeloma (see Sidebar 46). Collectively, CAR T-cell therapies represent a major breakthrough in the treatment of adults and children with blood cancers (760)Dana H, et al. (2021) Acta Pharm Sin B, 11: 1129..
The first of these treatments, tisagenlecleucel (Kymriah), was approved by FDA in 2017 for the treatment of children and young adults with B-cell ALL that had not responded to standard treatments or had relapsed at least twice. Tisagenlecleucel allowed some children whose leukemia had returned or stopped responding to other treatments to experience complete remission. A long-term follow-up of patients treated with tisagenlecleucel showed that more than 60 percent were living 3 years or longer after their first infusion of CAR T cells (761)Laetsch TW, et al. (2023) Journal of Clinical Oncology, 41: 1664.. Additionally, more than 50 percent of patients were living without their disease coming back at the end of 3 years after treatment completion, suggesting that CAR T cells can lead to durable cancer control.
The second FDA-approved CAR T-cell therapy, axicabtagene ciloleucel (Yescarta), which also became available in 2017, was shown to be the first treatment in nearly three decades to improve overall survival for patients with relapsed or refractory large B-cell lymphoma (762)Westin JR, et al. (2023) N Engl J Med, 389: 148..
Most recently, researchers have provided the first-of-its-kind proof that the CAR T-cell therapy ciltacabtagene autoleucel (Carvykti) can induce long-term remission and is potentially curative for patients with relapsed or refractory multiple myeloma. Based on a recent analysis, after only a single infusion of the CAR T cells, 33 percent of patients remained alive and progression-free after 5 years (763)Jagannath S, et al. (2025) J Clin Oncol: JCO2500760.. This represents a major milestone in the treatment of multiple myeloma.
Several types of leukemia and lymphoma arise in B cells, including most cases of ALL (see The State of Blood Cancers in 2025). While treatments like chemotherapy, stem cell transplants, molecularly targeted therapeutics, and immunotherapeutics including CAR T-cell therapy have improved outcomes, survival remains low for older adults and patients whose cancer comes back or does not respond to treatment. This low survival underscores the need for more effective therapies, a need that may be addressed by the most recently approved CAR T-cell therapy, obecabtagene autoleucel (Aucatzyl), approved in November 2024 for treating adults with B-cell ALL that has relapsed or become refractory to other treatments.
Like other CAR T-cell therapies approved for leukemia and lymphoma that arise in B cells, obecabtagene autoleucel is made by collecting a patient’s T cells, multiplying them, and genetically modifying them to express a CAR that targets CD19, a protein on the surface of B cells. The modified cells are then infused back into the patient to seek out and destroy cancer cells. However, unlike existing CAR T-cell therapies, obecabtagene autoleucel is uniquely engineered with a lower binding strength to CD19, which may reduce its side effects and help the CAR T cells persist longer in the body, potentially addressing two major challenges of current CAR T treatments for B-cell ALL—high toxicity and short-lived responses (765)Roddie C, et al. (2021) J Clin Oncol, 39: 3352..
FDA approval of obecabtagene autoleucel was based on results from a phase I/II clinical trial in which 42 percent of patients treated with the CAR T-cell therapy experienced a complete disappearance of their cancer within 3 months of receiving the treatment. Those who responded within 3 months stayed cancer-free for a median of about 14 months, with some patients still doing well beyond that time.
Compared to brexucabtagene autoleucel, another CAR T-cell therapy that was approved for adults with B-cell ALL in 2021, obecabtagene autoleucel caused fewer severe side effects, including lower rates of serious immune reactions and brain-related side effects (766)Roddie C, et al. (2024) N Engl J Med, 391: 2219.. Notably, the severe side effects with obecabtagene autoleucel were mostly seen in patients with a high number of leukemia cells in their bone marrow, suggesting that in patients with lower disease levels the procedure may be safe as outpatient treatment.
While CAR T-cell therapies are clearly showing immense benefit for patients with blood cancer, they have many limitations including toxicities, treatment resistance, as well as barriers to manufacturing and treatment delivery.
In November 2023, FDA announced that it was investigating a link between receiving CAR T-cell therapy and developing secondary cancers later on after initial clinical reports suggested a potential link. This prompted extensive investigation and a surge of research across the field (767)Hamilton MP, et al. (2024) N Engl J Med, 390: 2047.(768)Elsallab M, et al. (2024) Blood, 143: 2099.(769)Tix T, et al. (2024) Clin Cancer Res, 30: 4690.(770)Ghilardi G, et al. (2024) Nature Medicine, 30: 984.. Several studies have now concluded that the risk of secondary cancer is very rare and is no greater among patients treated with CAR T-cell therapy than among those treated with other treatments.
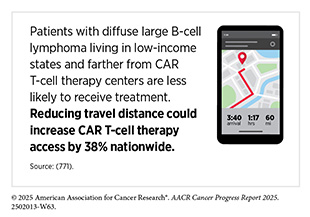
There are disparities in the access to CAR T-cell therapies, especially among medically underserved populations (676)Valtis YK, et al. (2025) JAMA Oncol.(771)Chung A, et al. (2025) Blood Adv.. Because of serious or life-threatening immune-related adverse reactions, FDA required CAR T cells to be administered only at specially certified large academic hospitals by qualified health care professionals with appropriate medical support. However, most cancer patients receive their treatment at community settings, which limits their access to this rapidly growing class of lifesaving immunotherapies (772)Byrne MT, et al. (2025) JAMA Oncol, 11: 481..
Innovations in simplifying manufacturing and broadening delivery are needed so more patients can benefit from CAR T-cell therapies. In this regard, in June 2025, FDA removed the special safety requirements for approved BCMA- and CD19-directed CAR T-cell therapies, a decision expected to expand access by allowing more treatment centers—including those in community and rural areas—to administer these therapies without additional regulatory hurdles (773)US Food and Drug Administration. FDA Eliminates Risk Evaluation and Mitigation Strategies (REMS) for Autologous Chimeric Antigen Receptor (CAR) T cell Immunotherapies. Accessed: June 30, 2025..
Access to CAR T-cell therapies vary globally, with significant barriers to access in low- and middle-income countries (774)Medina-Olivares FJ, et al. (2024) Front Oncol, 14: 1397613.. Based on an online survey of clinicians outside the United States, less than 20 percent of survey respondents reported adequate access to CAR T-cell therapies for patients with multiple myeloma (775)Atallah R, et al. (2025) JCO Glob Oncol, 11: e2400441..
To overcome some of the current limitations of CAR T-cell treatments, researchers are exploring a new generation of cell-based therapies for blood cancer (764)Brudno JN, et al. (2024) JAMA, 332: 1924.. Innovations include CAR T cells that recognize novel targets, including those that work by binding to multiple targets on blood cancer cells, as well as their use in combination with other immunotherapies. Researchers are also exploring “universal” CAR T cells that can be used across multiple blood cancer types (776)Wellhausen N, et al. (2023) Sci Transl Med, 15: eadi1145.. Additionally, to address the complex manufacturing issues, researchers are attempting to engineer CAR T cells in the patient’s body instead of the laboratory, which could make them faster to produce and more affordable (777)Hunter TL, et al. (2025) Science, 388: 1311.(778)Willyard C (2025) Nature, 641: 1090..
Another promising strategy involves targeting specific proteins found on AML cells to expand the use of CAR T cells to a broader patient population (779)Casirati G, et al. (2023) Nature, 621: 404.. Furthermore, the potential of natural killer (NK) cells, which can be engineered to target cancer like CAR T cells but are easier and faster to manufacture and may have fewer adverse effects, is being investigated. Together, these advances could expand access, improve safety, and enhance the effectiveness of cellular immunotherapies for a wider range of blood cancers.
Driven by its success in cancer, CAR T-cell therapies are now being evaluated in the treatment of autoimmune diseases such as lupus, in which harmful B cells drive the illness. Unlike current treatments that often leave some disease-causing B cells hidden in tissues, CAR T cells can reach difficult-to-access areas and achieve more complete B-cell elimination (780)Rampotas A, et al. (2025) Bone Marrow Transplantation, 60: 6..
Unleashing the Immune System Against Blood Cancers
Immune checkpoint inhibitors (ICIs) release the brakes on immune cells, which unleashes T cells to find and destroy cancer cells. These immunotherapeutics have become the foundation of treatment for a wide range of solid tumors, including previously intractable cancers such as advanced kidney cancer, lung cancer, and melanoma, for which they have transformed patient outcomes (see Figure 19). However, ICIs have had limited success in the treatment of blood cancers thus far.
Currently, the only FDA-approved ICIs for blood cancers include pembrolizumab, approved for both classic HL and a specific NHL subtype called primary mediastinal B-cell lymphoma, and nivolumab, approved for classic HL. Both are indicated for patients with relapsed or refractory disease. However, recent evidence shows that nivolumab, when combined with standard chemotherapy, can also significantly improve outcomes for patients with newly diagnosed advanced classic HL (781)Herrera AF, et al. (2024) N Engl J Med, 391: 1379.. This regimen was better tolerated, with fewer serious side effects, such as nerve damage, underscoring the potential of ICIs as the initial treatment for these patients.
While ICIs have shown success in treating classic HL and primary mediastinal B-cell lymphoma, they have had limited impact on other NHL subtypes, multiple myeloma, or myeloid malignancies. Researchers are exploring new immune checkpoint targets, such as LAG-3, TIM-3, TIGIT, and CD47, and developing next-generation ICIs, including bispecific antibodies that target two checkpoint proteins.
Researchers are also testing combination strategies, including pairing different ICIs or combining them with ADCs, to enhance effectiveness against blood cancers. While many of these approaches are still in early stages, ongoing and future efforts may identify patients who will benefit the most, determine the best timing for treatment, and improve long-term outcomes through optimal combinations.
Redirecting T Cells to Eradicate Blood Cancers
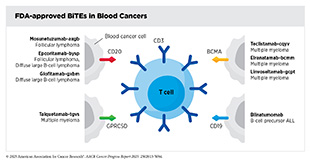
Certain immunotherapeutics, such as bispecific T-cell engagers (BiTEs), work by flagging cancer cells for destruction by the immune system. By attaching to cell surface proteins on T cells and cancer cells using its two arms, BiTEs bring the two cell types together, directing the T cells to home in on the cancer cells. As a result, T cells are activated, and they destroy the adjacent cancer cells.
The first of these agents, blinatumomab, was approved by FDA in 2014 for treating adult patients with relapsed or refractory Philadelphia chromosome–negative B-cell precursor ALL. Blinatumomab binds to the protein CD19 on the surface of ALL cells and the protein CD3 on the surface of immune T cells. Since its first approval in 2014, FDA has allowed blinatumomab to be used in more groups of people with B-cell ALL, including those who still have small amounts of cancer left after treatment and even for those who are in remission and have no trace of their disease.
One example is the expanded approval of blinatumomab in 2017 for treating children whose ALL has returned following at least one course of treatment. FDA’s decision was based on clinical studies showing that in children, AYAs, and adults with B-cell ALL that had relapsed or is not responding to therapy, treatment with blinatumomab led to better outcomes and fewer side effects compared to chemotherapy alone (782)Brown PA, et al. (2021) JAMA, 325: 833.(783)Locatelli F, et al. (2021) JAMA, 325: 843.(784)Kantarjian H, et al. (2017) N Engl J Med, 376: 836.. Moreover, research has shown that even patients who have responded extremely well to chemotherapy and have no trace of ALL, live longer when blinatumomab is added to their maintenance treatment (785)Litzow MR, et al. (2024) N Engl J Med, 391: 320..
Another key finding was that the addition of blinatumomab to chemotherapy was highly effective for infants with newly diagnosed ALL with certain genetic alterations known as KMT2A rearrangement, a disease historically associated with poor outcomes (786)van der Sluis IM, et al. (2023) N Engl J Med, 388: 1572..
Ongoing research is exploring the effectiveness of blinatumomab in combination with other therapeutics as well as in earlier stages of the disease, and even as the initial treatment for certain patients with B-cell ALL. In this regard, a large NCI-supported study of more than 1,400 children newly diagnosed with B-cell ALL, who were considered at lower risk for cancer coming back, showed that those who received blinatumomab along with standard chemotherapy had better outcomes. After about two and a half years, 96 percent of children who received the combination treatment remained cancer-free, compared to 88 percent who received only chemotherapy (787)Gupta S, et al. (2025) N Engl J Med, 392: 875..
Four of the FDA-approved BiTEs are for the treatment of patients with multiple myeloma, a disease that remains incurable and primarily affects older adults. In recent years, the development and FDA approval of new classes of therapeutics—including proteasome inhibitors like bortezomib and carfilzomib, immunomodulatory agents like lenalidomide and pomalidomide (Pomalyst), and immunotherapeutics like daratumumab (Darzalex) (see Flagging Blood Cancer Cells for Destruction by the Immune System)—have improved outcomes for patients. Despite these advances, many patients who initially respond to these new therapies eventually experience relapses due to the development of treatment resistance.
Research has shown that the proteins GPRC5D and BCMA are highly expressed on the surface of most multiple myeloma cells. The BiTEs approved for multiple myeloma—linvoseltamab-gcpt, teclistamab-cqyv and elranatamab-bcmm, which target BCMA, and talquetamab-tgvs, which targets GPRC5D—have all demonstrated the ability to induce deep and durable responses in patients who have exhausted several prior treatments and have limited remaining options (1)American Association for Cancer Research®. AACR Cancer Progress Report 2024. Accessed: June 11, 2025.(177)American Association for Cancer Research. AACR Cancer Progress Report 2023. Accessed: Feb 29, 2025.(788)Firestone R, et al. (2023) Blood Cancer Discov, 4: 433.. Notably, in addition to disease control, many patients also experienced improvements in symptoms and quality of life.
Ongoing investigations are aimed at using these agents more effectively to further improve outcomes for more patients with multiple myeloma. For instance, researchers are evaluating the use of BiTEs earlier in the treatment course as well as combinations of BiTEs with other therapies (789)Shouse G (2025) Blood Rev, 69: 101251.. As one example, teclistamab-cqyv is being evaluated in combination with daratumumab and lenalidomide, with early data showing that nearly 90 percent of patients respond to the treatment (790)D’Souza A, et al. (2024) Blood, 144: 495.. Researchers are also working to overcome resistance to the currently used BiTEs in multiple myeloma, which can occur when myeloma cells reduce the levels of or alter the structure of BCMA or GPRC5D—proteins that these therapies are designed to target (791)Lee H, et al. (2024) Blood, 143: 1211.. To address this issue, ongoing studies are evaluating dual-targeting approaches, such as combining teclistamab-cqyv and talquetamab-tgvs, to reduce the likelihood of treatment resistance (792)Cohen YC, et al. (2025) N Engl J Med, 392: 138..
The other three BiTEs approved by FDA for blood cancers are for the treatment of two different forms of NHL that arise in immune cells called B cells, FL and DLBCL. FL is a slow-growing cancer but can eventually progress to become a fatal disease, while DLBCL is an aggressive subtype that can be rapidly fatal without effective intervention. For patients who experience relapse after standard treatments such as chemotherapy and CAR T-cell therapy, therapeutic options have been limited. For FL and DLBCL, BiTEs have emerged as a transformative new treatment that redirects the immune system to eradicate cancer. By binding to the proteins CD3 on T cells and CD20 on malignant B cells, BiTEs enable targeted killing of cancer cells.
Several BiTEs have now received FDA approval for treating relapsed or refractory FL and DLBCL. Mosunetuzumab-axgb (Lunsumio) and epcoritamab-bysp (Epkinly) were approved for FL based on results from clinical studies in which 80 percent of patients saw their cancer shrink and 60 percent had no signs of cancer after treatment (789)Shouse G (2025) Blood Rev, 69: 101251.. Longer follow-up showed a median duration of response to mosunetuzumab-axgb of 36 months, indicating durable benefit for patients who responded to the BiTE (793)Sehn LH, et al. (2025) Blood, 145: 708..
For people with aggressive DLBCL, both epcoritamab-bysp and glofitamab-gxbm (Columvi) are now approved by FDA (789)Shouse G (2025) Blood Rev, 69: 101251.. Epcoritamab-bysp helped nearly two out of three patients who received the treatment, and some responses lasted more than 2 years. Follow-up studies have underscored the long-term benefit of epcoritamab-bysp across subgroups of patients, including those with difficult-to-treat disease and poor prognosis (794)Thieblemont C, et al. (2024) Leukemia, 38: 2653.. Glofitamab-gxbm has also shown strong benefits, helping over half of patients, with many seeing complete remission that lasted more than a year and a half.
BiTEs are one of the most rapidly growing therapeutic areas in cancer and are providing new hope for many patients who have few other choices remaining. Despite its effectiveness, BiTE therapy may cause serious adverse effects. Researchers are exploring ways to mitigate the range of side effects, including infections, low blood counts, cytokine release syndrome, neurologic symptoms, and other symptoms that may be associated with these treatments, especially among patients with weaker immune systems. Another key concern is that most patients eventually relapse due to the development of treatment resistance, indicating the need for better predictors of long-term benefit and ways to manage or prevent resistance.
Ongoing research is evaluating the effectiveness of BiTEs earlier in the course of treatment and in combination with other therapies. A critical area of investigation is to determine the best sequence for using BiTEs versus CAR T-cell therapies, especially when they are both approved for the same cancer type and target the same protein (e.g., tisagenlecleucel and blinatumomab are both approved for ALL and target CD19; three out of four BiTEs and both CAR T-cell therapies approved for multiple myeloma target BCMA), since giving one treatment first may affect how well the other works later on (795)Dima D, et al. (2025) Blood Cancer J, 15: 111..
Another area of investigation is to examine whether combining two BiTEs or using biological markers to select patients can make the treatments safer and more effective. Researchers are working to identify patient characteristics, such as T-cell functional state, that predict the best response to BiTEs. In addition, efforts are underway to engineer trispecific (ability to bind to three targets) or multispecific antibody designs. These efforts aim to expand the number of patients who can benefit from these revolutionary immunotherapeutics, further improve outcomes, reduce side effects, and ultimately help more patients live longer and better lives.
Flagging Blood Cancer Cells for Destruction by the Immune System
Before an immune cell can destroy a cancer cell, it must find it. Many therapeutic antibodies that have been approved by FDA over the past decade for treating patients with blood cancers work, at least in part, by helping immune cells find cancer cells.
Notable agents within this group of immunotherapeutics are daratumumab and elotuzumab (Empliciti), approved in 2015, and isatuximab-irfc (Sarclisa), approved in 2020, for treating patients with multiple myeloma that has progressed despite treatment with several other therapeutics.
Both daratumumab and isatuximab-irfc work by attaching to a protein called CD38, which is found at high levels on the surface of myeloma cells. This attachment has several effects on the myeloma cells, most notably flagging them for immune cells, which upon attaching to another part of daratumumab are triggered to destroy the myeloma cells.
Elotuzumab attaches to SLAM7F, another protein found at high levels on the surface of myeloma cells. This protein is also found at high levels on immune cells called natural killer (NK) cells. Elotuzumab has distinct effects on myeloma cells and NK cells after attaching to SLAM7F on their surfaces, thus providing a two-pronged attack on multiple myeloma. It directly activates NK cells, enhancing their ability to kill myeloma cells, and it flags myeloma cells for a number of immune cell types, which once directed to the myeloma cells, attack and destroy them.
Since its initial approval, daratumumab has been evaluated in many additional studies including in combination with a variety of molecularly targeted therapeutics and immunotherapeutics among patients with newly diagnosed as well as relapsed or refractory multiple myeloma. Data from these studies have confirmed the added benefits of daratumumab combinations in delaying disease progression and even increasing overall survival for patients (796)Dimopoulos MA, et al. (2023) J Clin Oncol, 41: 1590.(797)Sonneveld P, et al. (2023) J Clin Oncol, 41: 1600..
Based on its transformative impact on patient outcomes, FDA has expanded the use of daratumumab in many additional settings, including as the initial treatment for multiple myeloma. Additionally, researchers are evaluating whether daratumumab can prevent the progression of smoldering multiple myeloma, a precursor disease of multiple myeloma for which no treatments are currently approved (see Intercepting Precancerous Conditions in Blood Cancers) (798)Dimopoulos MA, et al. (2025) N Engl J Med, 392: 1777..
Most recently, in 2024, FDA approved daratumumab as well as isatuximab-irfc in combination with a standard initial treatment (bortezomib, lenalidomide, and dexamethasone) for patients with newly diagnosed multiple myeloma. These approvals reflect how scientifically informed combination therapies are improving patient outcomes at the clinic.
The 2024 approval of daratumumab was based on a clinical trial in which adding daratumumab to the standard regimen that includes bortezomib, lenalidomide, and dexamethasone (VRd regimen) led to deeper and more durable responses, with 84 percent of patients remaining progression-free at four years compared to 68 percent for those receiving the standard regimen (799)Sonneveld P, et al. (2024) N Engl J Med, 390: 301.. Additionally, patients in the daratumumab group were more likely to have no detectable cancer in their bone marrow, which is known as being minimal residual disease (MRD)–negative, than those who received standard therapy.
The approval of isatuximab was based on a study which showed that adding isatuximab to the standard VRd regimen led to significantly better outcomes, with 63 percent of patients progression-free at nearly five years, compared to 45 percent treated with VRd alone. More patients receiving isatuximab achieved complete responses and sustained MRD negativity, compared to the standard treatment group (799)Sonneveld P, et al. (2024) N Engl J Med, 390: 301.(800)Facon T, et al. (2024) N Engl J Med, 391: 1597.. Researchers have further demonstrated that patients who achieve MRD negativity after treatment with an isatuximab-containing regimen do not benefit from subsequent stem cell transplantation and can safely avoid the risks and side effects of a transplant (801)Perrot A, et al. (2025) Blood, 146: 52..
The rapid development of multiple effective immunotherapies for multiple myeloma has also led to new research questions for health care providers, such as determining the best combinations and optimal sequences of treatment for individual patients and understanding whether these therapies need to be given continuously or if patients can take planned breaks. While combination regimens that include daratumumab are clearly more effective than those without it, comparative data are lacking, and health care providers currently rely on clinical judgment to guide treatment decisions. Researchers emphasize the need for clinical trials that conduct head-to-head comparisons of treatment combinations and evaluate optimal sequencing and duration of therapy to better guide future clinical practice.
Two additional immunotherapeutics approved in the past decade that work by flagging cancer cells for destruction by the immune system are mogamulizumab-kpkc (Poteligeo) and tafasitamab-cxix (Monjuvi). Mogamulizumab-kpkc was approved in 2018 for treating CTCL. It is an antibody that targets CCR4, a protein present on certain cancerous T cells, helping the immune system destroy them. Tafasitamab-cxix was approved in 2020 for relapsed or refractory DLBCL. It targets CD19 on B cells and boosts the immune attack on cancer cells. In June 2025, FDA expanded the use of tafasitamab-cxix for patients with relapsed or refractory follicular lymphoma.
Taken together, the numerous approaches and therapeutics outlined in the previous sections highlight the transformative role of immunotherapy in reshaping the treatment landscape and improving outcomes for patients with blood cancers.
Supporting Survivorship
Thanks to the rapid pace of progress in precision early detection, prevention, diagnostics, and medicine, there are over 1.6 million survivors with a diagnosis of blood cancer living in the United States as of January 2025 (9)Wagle NS, et al. (2025) CA Cancer J Clin..
A person can be diagnosed with blood cancer at any age depending on the type of blood cancer, as well as a multitude of genetic, environmental, and behavioral factors that can increase the risk of developing blood cancer. Consequently, survivors of blood cancers face many of the same challenges faced by survivors of any cancer (see Supporting Cancer Patients and Survivors). In addition, survivors of blood cancers also experience a uniquely complex set of long-term health challenges, many of which stem from the immunologic and physiologic toll of their disease and treatments.
Unlike solid tumors, blood cancers directly involve the immune system and its central components—bone marrow, lymphoid organs, and circulating immune cells. As a result, therapies such as toxic chemotherapy, B-cell–depleting monoclonal antibodies, bispecific T-cell engagers, and CAR T-cell therapies can leave survivors with a persistently weakened immune system (802)Alshammari F, et al. (2025) Hematology, 30: 2448898.(803)Camacho-Arteaga L, et al. (2025) JAMA Netw Open, 8: e2461683.(804)Bouchkouj N, et al. (2025) JAMA, 333: 1354.(805)Nath K, et al. (2024) Blood Cancer J, 14: 88.. This immunosuppressed state not only increases susceptibility to future infections but also often necessitates revaccination to restore protection against common pathogens (806)Toret E, et al. (2021) Hum Vaccin Immunother, 17: 1132.(807)Kamboj M, et al. (2024) JCO Oncol Pract, 20: 889.(808)Malard F, et al. (2021) Blood Cancer J, 11: 142..
Stem cell transplantation is a common treatment for patients with blood cancers in which they receive their own blood-forming stem cells collected before the treatment (called autologous stem cell transplantation) or from a healthy donor (called allogeneic stem cell transplantation). Compounding the treatment-related challenges, survivors of blood cancers may face the burden of chronic graft-versus-host disease (GVHD), a potential complication of stem cell transplantation in which donor immune cells attack the recipient’s tissues. GVHD affects approximately 40 percent of allogeneic transplant survivors, and can manifest as skin thickening, inflamed airways, dry eyes, and overwhelming fatigue (809)Murray J, et al. Graft-Versus-Host Disease (GvHD). In: Kenyon M, Babic A, editors. The European Blood and Marrow Transplantation Textbook for Nurses: Under the Auspices of EBMT. Cham (CH)2018. p 221. Survivors also face an elevated lifetime risk of secondary malignancies (810)Westerveld ASR, et al. (2024) Blood Cancer J, 14: 150.(811)Rosenberg AS, et al. (2021) Blood Cancer J, 11: 5.(812)Kumar V, et al. (2019) Blood Cancer J, 9: 75., cardiotoxicity (813)Palfi S, et al. (2025) Blood Cancer J, 15: 63.(814)Jabbour E, et al. (2025) JAMA, 333: 1618.(815)El-Cheikh J, et al. (2023) Blood Cancer J, 13: 83., and cognitive impairments (816)Geraghty AC, et al. (2025) Cell, 188: 3238.(817)Ho MH, et al. (2025) Support Care Cancer, 33: 312.(818)Hshieh TT, et al. (2018) JAMA Oncol, 4: 686.(819)Stefanski KJ, et al. (2021) J Natl Cancer Inst, 113: 481.. Fatigue, among the most commonly reported and least visible effects, often persists for years after treatment ends and significantly erodes quality of life (820)Dai H, et al. (2025) Sci Rep, 15: 17763..

Moreover, the financial toll of survivorship is also substantial for patients with blood cancers and caregivers. Continuous surveillance, immunotherapies, and supportive care contribute to significant out-of-pocket burdens, while persistent physical and cognitive deficits can delay or prevent a return to work, further compounding economic strain (821)Baum J, et al. (2023) Sci Rep, 13: 22856.(822)Ouchveridze E, et al. (2022) Blood Cancer J, 12: 74.(823)Sparano F, et al. (2024) JCO Oncol Pract, 20: 438.(824)Owsley KM, et al. (2025) J Cancer Surviv..
To address these multifaceted challenges, multidisciplinary teams that bring together hematologists, infectious disease experts, cardiologists, pulmonologists, endocrinologists, rehabilitation specialists, mental health care providers, and patient navigators offer a coordinated approach to managing chronic complications and improve quality of life (825)Zhang E, et al. (2024) Blood, 144: 3650.. As an example, one study that implemented financial navigators for patients with hematologic cancer and their caregivers found that the program secured an average of $2,500 in financial benefits for each participant (826)Edward JS, et al. (2023) JCO Oncol Pract, 19: e696.. The navigators screened patients to determine if they were at an increased risk of financial toxicity and provided assistance and resources. In addition, research has shown that embedding personalized survivorship care plans in electronic health records—with automated prompts for vaccinations, screenings, and follow-ups—can help ensure continuity in care.
New Horizons in Blood Cancer Science and Medicine
Over the past decade, advances in science and technology have transformed how hematologic malignancies are detected and diagnosed. From identifying at-risk individuals before symptoms emerge to decoding the genetic makeup of blood cancers with unprecedented precision, the field has moved rapidly toward earlier and more personalized care. This section highlights breakthroughs in genomics-based approaches that are reshaping how blood cancers are classified, monitored, and treated. Together, these innovations are poised to redefine what it means to detect, diagnose, and treat blood cancers at their earliest stages.
Intercepting Precancerous Conditions in Blood Cancers
Rapid technological advances, more effective treatments, and better ways to identify people who are at higher risk of cancer have led to a better understanding of precancers and their path to become cancer and the emergence of cancer interception (see Figure 24) (827)Domchek SM, et al. (2024) Cancer Discov, 14: 600.. It is an exciting area of research because treating precancerous cells at such an early stage minimizes the chances of advanced cancer and improves health outcomes.
Cancer interception in hematologic malignancies focuses on identifying and targeting precancerous states—such as clonal hematopoiesis (CH), monoclonal gammopathy of undetermined significance (MGUS), and monoclonal B-cell lymphocytosis (MBL)—before they progress to become cancerous. Advances in genetic surveillance and liquid biopsy (see Liquid Biopsy in Cancer Care), along with long-term follow-up studies, are enabling earlier detection of these precancerous states, setting the stage for proactive interventions tailored to the biology of each blood cancer subtype.
Precancerous Conditions That May Become Myeloid Malignancies
As people age, hematopoietic stem cells accumulate genetic alterations. In some cases, these alterations allow one group of cells to grow faster than the rest, resulting in a condition called clonal hematopoiesis (CH) (828)Weeks LD, et al. (2023) Blood, 142: 2235.. There are two main types. Clonal hematopoiesis of indeterminate potential (CHIP) involves genetic changes but no symptoms, and clonal cytopenia of undetermined significance (CCUS) is characterized by low blood counts (cytopenias) that cannot be otherwise explained (828)Weeks LD, et al. (2023) Blood, 142: 2235.. CH is especially common among older adults; about 10 percent to 20 percent of people over age 70 have it (829)Jaiswal S, et al. (2019) Science, 366..
About 87 percent of CH cases involve alterations in a few key genes, for example, DNMT3A or TET2, that produce proteins involved in modifying the epigenome (see Epigenetic Changes) (828)Weeks LD, et al. (2023) Blood, 142: 2235.(830)Kunimoto H, et al. (2017) Int J Hematol, 106: 34.. The remaining cases carry mutations in genes, for example, TP53 or JAK2, involved in key cell processes, such as cell proliferation and growth (831)van Zeventer IA, et al. (2023) Cancer Cell, 41: 1017.. These are often found by accident when patients undergo genetic testing for unrelated medical reasons. Inherited mutations in genes, for example, CEBPA, RUNX1, and GATA2, can also predispose a person to CH (832)Tsai FD, et al. (2020) Blood, 136: 1615..
Though many people with CH never develop serious problems, having these mutations does increase the risk of developing blood cancers, especially leukemia and myelodysplastic syndromes (828)Weeks LD, et al. (2023) Blood, 142: 2235.. CH is also linked to other health problems, including heart disease, stroke, and chronic inflammation (833)Kislikova M, et al. (2023) Life (Basel), 13.(834)Ahmad H, et al. (2023) Annu Rev Med, 74: 249.(835)Uddin MDM, et al. (2022) Nat Commun, 13: 5350.. For example, people with CH caused by alterations in TET2 or JAK2 genes have two to four times the risk of cardiovascular disease (828)Weeks LD, et al. (2023) Blood, 142: 2235..
Researchers have created risk scores, such as the clonal hematopoiesis risk score (CHRS), to help differentiate a small number of CHIP/CCUS cases that pose a higher risk of developing into myeloid cancers from a large number of cases that have minimal risk of progressing into cancer (836)Weeks LD, et al. (2023) NEJM Evid, 2.. Because CH is a common finding with advanced age, integrating such risk scores into clinical decision-making can help prioritize patients whose CHIP has a higher likelihood of progressing into cancer. Although there are currently no routine treatments for CHIP/CCUS, recent clinical trials are investigating potential interventions, such as anti-inflammatory drugs for high-risk CCUS, or vitamin C, which functions in concert with TET2 protein, for CCUS in which the TET2 gene is altered (837)Dunn WG, et al. (2024) J Clin Invest, 134..
Precancerous Conditions That May Become Lymphoid Malignancies
Monoclonal B-cell lymphocytosis (MBL) is a condition in which a higher-than-normal number of identical B cells originating from the same parent cell are found in the blood. People with MBL may develop other B-cell diseases, such as CLL. MBL is classified into low-count and high-count forms, depending on whether the number of identical B cells is below or above a certain threshold. High-count MBL is more likely to progress to CLL, at a rate of about 1 percent to 2 percent per year, while low-count MBL rarely progresses and is usually found incidentally in healthy older adults (838)Strati P, et al. (2015) Blood, 126: 454.(839)Galigalidou C, et al. (2021) Front Oncol, 11: 769612.. Studies have shown that MBL is more common with age and in first-degree relatives of people with CLL, suggesting a genetic component. Certain infections, such as hepatitis C, may also increase the risk of developing MBL (838)Strati P, et al. (2015) Blood, 126: 454.(840)Liu Y, et al. (2025) Am Soc Clin Oncol Educ Book, 45: e473650..
Researchers are working to understand how and why MBL sometimes turns into CLL. High-count MBL shares many of the same genetic features as CLL, including specific genetic alterations and abnormal B-cell receptors—proteins present on the surface of B cells. Low-count MBL has fewer of these changes and may result from normal immune responses (838)Strati P, et al. (2015) Blood, 126: 454.(839)Galigalidou C, et al. (2021) Front Oncol, 11: 769612.. Advances in single-cell analysis have shown that even first waves of identical cells that originate from a single parent cell can negatively impact the immune system and blood production, especially in high-risk MBL cases (841)Serafin A, et al. (2025) Hematol Oncol, 43 Suppl 2: e70084.. However, not all MBL cases follow the same path. Some remain stable for years, while others progress into CLL with significant health risks. This has raised questions about whether low- and high-count MBL are truly part of the same disease process. More long-term studies are needed to monitor how MBL behaves over time and to identify which individuals are most at risk for progression to leukemia (840)Liu Y, et al. (2025) Am Soc Clin Oncol Educ Book, 45: e473650..
Precancerous Conditions That May Become Multiple Myeloma
Multiple myeloma often begins silently as a condition called monoclonal gammopathy of undetermined significance (MGUS) and sometimes progresses through an intermediate stage known as smoldering multiple myeloma (SMM) (842)Hevroni G, et al. (2024) Blood Rev, 68: 101242.(843)Cordas Dos Santos DM, et al. (2024) Nat Rev Cancer, 24: 867.(844)Kyle RA, et al. (2018) N Engl J Med, 378: 241.(845)Murray D, et al. (2019) Blood Cancer J, 9: 102.. MGUS is a common condition, affecting about 3 percent of people over age 50 and around 5 percent of those over the age of 70. It typically progresses very slowly, with a risk of becoming multiple myeloma of about 1 percent per year.
Researchers have created several models that help determine the risk an individual with MGUS or SMM has of developing multiple myeloma (843)Cordas Dos Santos DM, et al. (2024) Nat Rev Cancer, 24: 867.. These risk assessment models range from simpler 2/20/20 models to newer PANGEA models. The 2/20/20 models, developed by the International Myeloma Working Group, consider levels and ratios of certain proteins made by plasma cells, as well as the number of plasma cells in the bone marrow (846)Mateos MV, et al. (2020) Blood Cancer J, 10: 102.. These models are widely used in the clinic but do not offer risk assessment over time. In contrast, emerging tools like the PANGEA models also incorporate genetic alterations and other biomarkers, allowing personalized risk assessment over time (847)Cowan A, et al. (2023) Lancet Haematol, 10: e203.. However, current clinical use of these models is limited because of the lack of experts needed for implementation.
Advances in genomics have revealed that early precursors of multiple myeloma exhibit fewer mutations, but progression is marked by increasing genomic alterations and the heterogeneous nature of the intermediate states (848)Alberge JB, et al. (2025) Nat Genet, 57: 1493.. For example, SMM contains a mix of precancerous MGUS as well as cancerous multiple myeloma–like cell populations (843)Cordas Dos Santos DM, et al. (2024) Nat Rev Cancer, 24: 867.. Furthermore, mutations in genes involved in cell proliferation and DNA repair are often linked with high-risk SMM and quicker progression of the precancerous state into cancerous multiple myeloma (843)Cordas Dos Santos DM, et al. (2024) Nat Rev Cancer, 24: 867.(848)Alberge JB, et al. (2025) Nat Genet, 57: 1493.. Studies that combine whole-genome sequencing and single-cell technologies and that follow patients with MGUS, SMM, and multiple myeloma over long periods of time are further refining our understanding of the interplay of influences inside the cell (such as genetic alterations) with those outside the cell (such as the immune system).
Multiple clinical trials are evaluating a range of therapies to intercept MGUS and SMM before they progress into multiple myeloma (see Table 14). For example, trials using lenalidomide and newer T-cell–redirecting therapies, such as daratumumab, have shown promising responses in patients with high-risk SMM, including significantly lowered risk of progress to active myeloma or death, improved progression-free survival, and higher overall survival (849)Lonial S, et al. (2020) J Clin Oncol, 38: 1126.(850)Mateos MV, et al. (2013) N Engl J Med, 369: 438.(851)Landgren CO, et al. (2020) Leukemia, 34: 1840..
As intercepting precancerous hematologic conditions becomes more routine in the clinic, risk of overtreatment and concerns of toxicities associated with treatments remain. Because these precancerous conditions can take a long time to develop into cancer, long-term clinical trials are also lacking. Experts have called upon prioritizing clinical trial design that incorporates precise identification of at-risk populations, robust safety and dose optimization, and meaningful metrics that can capture early treatment benefits without compromising long-term outcomes (852)Ghobrial IM, et al. (2024) Blood Cancer Discov, 5: 146.. As the field evolves, the integration of emerging clinical, molecular, and immune-based risk assessment tools will be essential to redefine standards for surveillance and early treatment.
Modernizing Clinical Trial Design for Blood Cancers
Clinical trials for hematologic malignancies have long been limited by traditional study designs that often fail to match the complexity and heterogeneity of these diseases. Standard designs—rigid phases, fixed eligibility criteria, and narrow endpoints—have slowed the pace of therapeutic innovation, particularly in rare subtypes and biologically diverse conditions, such as DLBCL, AML, and multiple myeloma. An analysis of clinical trials for hematologic cancers conducted between 2015 and 2020 found that only 21 percent of the trials were randomized, and the vast majority of randomized trials used a surrogate endpoint, such as how many patients would respond to a treatment, rather than a clinical endpoint most relevant to patients, such as overall survival (853)Wesson W, et al. (2022) Eur J Cancer, 167: 152.. Furthermore, trials often struggle with underenrollment and face challenges in translating early-phase activity into long-term benefit.
To overcome these challenges, researchers are developing innovative models that simulate heterogeneity of the cancer among patients, predict trial outcomes, and inform which treatment to use before the clinical trial even starts. As one recent example, researchers integrated both heterogeneity within the cancer of a patient and heterogeneity among different patients into a model to predict how patients might respond to the treatment (854)Pomeroy AE, et al. (2025) Blood Cancer Discov, 6: 254.. To validate the model, researchers used real-world data from nine clinical trials evaluating the efficacy of drug combinations to treat DLBCL. The model not only accurately predicted findings of two successful trials that improved survival, but also forecasted the failure of seven trials that did not improve outcomes for patients (854)Pomeroy AE, et al. (2025) Blood Cancer Discov, 6: 254.. Such predictive models could make future trials more efficient, accurately predict which new drug combinations are most likely to work, and increase the chances of finding treatments that cure DLBCL, as well as other blood cancers (855)Goldstein JS, et al. (2025) Blood Cancer Discov, 6: 153..
In parallel, the clinical trial community is increasingly embracing adaptive clinical trials (see Sidebar 28). For example, large trial networks like Beat AML and MyeloMATCH (see Sidebar 47) in the United States are organized around treating patients based on the molecular features of their blood cancer, and allowing for quick clinical decisions to match patients with therapies based on rapid genomic profiling (857)Tyner JW, et al. (2018) Nature, 562: 526.. Recent findings from the Beat AML trial show that 48 percent of AML patients above the age of 60—the age group that has a high risk of an AML diagnosis but limited treatment options available—carry a mutation that is potentially targetable for treatment (858)Hoff FW, et al. (2025) Blood Cancer J, 15: 55.. These collaborative trials accelerate progress against cancer by reducing duplicated efforts and increasing the likelihood of meaningful outcomes for patients with rare subtypes of cancer.
Similar efforts, such as the HARMONY Alliance in Europe, are establishing integrated data resources for blood cancers, enabling continuous learning across trials through real-world data sharing, profiling of a patient’s tumor characteristics, and grouping of patients based on their tumor’s characteristics to test specific treatments (859)Lang KM, et al. (2020) Trials, 21: 437.. Researchers are also introducing improvements to adaptive clinical trials, such as stopping the trial early if a drug is not working or accelerating patient recruitment if a drug is showing promise (860)Giovagnoli A (2021) Int J Environ Res Public Health, 18.(861)Berry DA (2011) Nat Rev Clin Oncol, 9: 199.. Such trials are being piloted in acute leukemia and lymphoid malignancies, in which cancer spreads rapidly, so the time to decide on the right treatment is short. Quick treatment decisions are also pivotal because of the heterogeneous nature of these malignancies, which can render treatments ineffective in a short time because of the development of resistance (862)van der Maas NG, et al. (2024) Blood Cancer J, 14: 56.(863)Thall PF (2020) Contemp Clin Trials, 90: 105860..
Another encouraging development is the growing use of molecular features of the tumor to identify subtypes within a cancer and artificial intelligence–driven models to differentiate tumors within and among patients and guide how patients are grouped in clinical trials, especially for combination therapies. As one example, strong evidence indicates that near lack of minimal residual disease (MRD, also called measurable residual disease)—when a very small number of cancer cells remain in the body during or after treatment—can be used as a regulatory endpoint in multiple myeloma clinical trials (865)Landgren O, et al. (2025) Blood Cancer Discov, 6: 13..
For example, overall response rates are now routinely exceeding 90 percent in multiple myeloma clinical trials, and the median progression-free survival (see Sidebar 32) is approaching 5 years in newly diagnosed patients. While these improvements in patient outcomes reflect rapid advances against the disease, they have rendered traditional endpoints like overall response rate (see Sidebar 32) less effective for timely drug evaluation (865)Landgren O, et al. (2025) Blood Cancer Discov, 6: 13., necessitating the use of other endpoints, such as the MRD status. An analysis of data from over 13,000 patients across more than 20 trials shows that patients who were MRD-negative had improved overall survival (866)Shi Q, et al. (2025) J Clin Oncol, 43: 1289.(867)Munshi NC, et al. (2020) Blood Adv, 4: 5988.. Following this evidence, in April 2024, FDA unanimously endorsed MRD negativity as an early endpoint that is reasonably likely to predict clinical benefit, paving the way for accelerated drug approvals in multiple myeloma (865)Landgren O, et al. (2025) Blood Cancer Discov, 6: 13.. This milestone also reflects a successful collaboration between academia, industry, and regulators, and offers a blueprint for validating intermediate endpoints in other hematologic cancers.
Two recent studies showed that using MRD status can help tailor therapy in multiple myeloma and potentially avoid unnecessary stem cell transplants. Patients who responded well to a four-drug combination—including isatuximab, carfilzomib, lenalidomide, and dexamethasone—did just as well continuing on medication as those who went on to transplant (869)Perrot A, et al. (2025) N Engl J Med, 393: 425.. The same regimen also led to high rates of deep remission with manageable side effects in a broader group of patients (801)Perrot A, et al. (2025) Blood, 146: 52.. These results support a more individualized, evidence-based approach to using drug combinations in early treatment.
Clinical trials for blood cancers are shifting from rigid, inefficient designs to flexible, data-driven systems. Tools like simulation modeling, adaptive trial structures, and biomarker-guided enrollment are reshaping how treatments are tested. This is especially critical for curable diseases like pediatric leukemias, in which smarter trial design can accelerate access to lifesaving therapies. While hurdles remain, the field is clearly moving toward faster, more precise, and patient-centered discovery.
Next Section: Supporting Cancer Patients and Survivors Previous Section: Unifying Cancer Science and Medicine—A Continuum of Innovation for Impact