Envisioning the Future of Cancer Science and Medicine
In the section, you will learn:
- Artificial intelligence has the potential to transform the future of cancer research and patient care by accelerating discoveries, streamlining drug development, and advancing precision oncology.
- Precision gene editing technologies may help shape the future of cancer research by enabling more advanced cancer models, discovering novel therapeutic targets, and developing more personalized treatments.
- Liquid biopsies can advance the future of cancer detection and treatment as powerful, minimally invasive tools for detecting cancer earlier and monitoring treatment response throughout the course of therapy.
- Next-generation treatments, including cancer vaccines and radiopharmaceutical therapies, could redefine the future of cancer care and offer new hope for difficult-to-treat cancers.
The pace of progress against cancer has accelerated tremendously in recent years, as underscored throughout this 15th edition and past versions of this annual report. Breakthrough discoveries and technological advances across the fields of medicine have substantially increased our understanding of cancer initiation and progression. This foundational knowledge is driving better strategies to reduce the risk of developing cancer, detect cancer at the earliest possible stage, and treat cancer effectively and more precisely—with fewer long-term side effects. As a result, cancer deaths are declining, and cancer survivors are living longer and fuller lives.
Technological and therapeutic advances against cancer and their impact on saving and improving lives of patients with cancer are a source of great optimism for cancer researchers, including AACR President, 2025–2026, Lillian L. Siu, MD, FAACR, FRCPC.
Below, we highlight some of the most exciting areas in cancer science and medicine that are poised to transform the future of cancer research and patient care.
A New Wave of Technologies
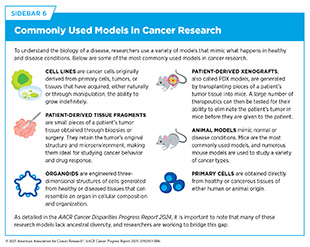
Emerging technologies are driving progress in cancer research by enabling scientists to investigate tumors with greater precision (see Sidebar 10). For example, spatial profiling is allowing researchers to map interactions of tumor and immune cells within intact tissues, offering new insight into the tumor microenvironment (TME) and its role in treatment resistance (1027)Gong D, et al. (2024) Cancer Cell, 42: 1653.. Technologies such as organoid systems, miniature organ-like structures grown from a patient’s own tumor cells, and three-dimensional (3D) tumor culture models (see Sidebar 6) are advancing our understanding of the disease by more closely mimicking cancer biology and accelerating the development of more effective, personalized therapies (1028)Verstegen MMA, et al. (2025) Nat Med, 31: 409.
(1029)Peng Z, et al. (2025) Mol Cancer, 24: 93.. The sections below highlight some of the most promising emerging technologies in cancer research.
New Advances in Artificial Intelligence
Artificial intelligence (AI) has the potential to transform cancer research and patient care by enabling faster, more accurate, and personalized approaches across the cancer continuum. AI technologies, including machine learning (ML), deep learning (DL), generative AI (GenAI), large language models (LLMs), and agentic AI, are now being applied to tasks such as analyzing histopathology images, identifying genomic alterations, and discovering new drug candidates (see Sidebar 54). This section will highlight some of the ways AI is being harnessed across the cancer care continuum, including its use in accelerating basic research discoveries, improving early detection, enhancing diagnostics, streamlining drug development, and supporting clinical decision-making. It will also address the importance of ensuring AI advances are effective and accessible to all patient populations.
Accelerating Basic Research Discoveries
AI is rapidly transforming basic research by helping scientists interpret biological data and uncover molecular patterns that drive cancer development. DL models and LLMs are now being applied across foundational areas of cancer research. One emerging application for AI in basic research is its use in enhancing the clinical relevance of knowledge gained from laboratory models of cancer. For example, researchers recently developed a DL-based method that integrates transcriptomic data from cell lines, patient-derived xenografts, and patient tumors simultaneously (see Sidebar 6) (1032)Dimitrieva S, et al. (2025) Sci Adv, 11: eadn5596.. This approach enables researchers to identify which laboratory models most closely resemble a patient’s tumors and ensure that findings from laboratory experiments are more likely to translate into meaningful clinical advances.
DL models are also advancing our ability to model gene regulation, enabling the prediction of gene expression directly from DNA sequences and aiding the interpretation of non-coding variants—genetic changes that occur outside protein-coding regions but can still affect how genes are regulated and expressed (1033)Barbadilla-Martinez L, et al. (2025) Nat Rev Genet.. Researchers have developed a DL model that analyzes transcriptomic data from more than 50,000 tumors across 18 cancer types to uncover patterns in gene activity that reflect important differences between cancers. These patterns help link specific molecular features to immune responses, cancer subtypes, and patient outcomes (1034)Qiu W, et al. (2025) Nat Biomed Eng, 9: 333.. As these technologies advance, their integration into cancer research will provide new insights into cancer biology and accelerate future discoveries.
Improving Detection and Diagnosis
AI-driven tools have the potential to offer faster, more accurate, and less invasive methods to detect and diagnose cancer. As one example, using over 163,000 low-dose computed tomography (CT) scans of the chest and 49 types of clinical records—such as pathology test results and demographic information—researchers recently developed an AI model to enhance lung cancer diagnosis. The model automates the analysis of 3D CT scans and integrates this information with clinical data. It assists radiologists—doctors trained to create and interpret medical images such as X-rays and CT scans—in identifying lung nodules and assessing cancer risk (1035)Niu C, et al. (2025) Nat Commun, 16: 1523..
Researchers have also developed a novel DL model to detect pancreatic tumors from CT images with over 94 percent accuracy, highlighting the potential for AI in identifying hard-to-detect cancers (1036)Mekala S, et al. (2025) Sci Rep, 15: 19787.. In another study, researchers using a DL model achieved more than 99 percent accuracy in diagnosing endometrial cancer from histopathologic images, showing how this approach can reduce diagnostic delays, catch cancer earlier, and improve patient outcomes (1037)Sheakh MA, et al. (2025) Computer Methods and Programs in Biomedicine Update, 7: 100181..
Recent advances in ML technologies are harnessing noninvasive biomarkers—measurable molecular markers found in body fluids that can signal the presence of disease or response to treatment—to improve early cancer detection. In one study, researchers used microbiome-based biomarkers (see Microbiome) with ML models to improve early detection of pancreatic cancer. Using information about the microbiome from blood samples, researchers developed ML models capable of distinguishing patients with pancreatic cancer from healthy individuals with over 95 percent accuracy (1038)Lee D, et al. (2025) Sci Rep, 15: 10995.. This represents a major advance for a cancer that is extremely difficult to detect early and has one of the highest mortality rates due to late-stage diagnosis. In another study, researchers developed an ML model to analyze samples from noninvasive blood-based liquid biopsies (see Liquid Biopsy in Cancer Care) that detected gliomas across all grades with high sensitivity (1039)Mathios D, et al. (2025) Cancer Discov..
Together, these examples demonstrate how AI-powered diagnostic tools are advancing earlier, less invasive detection of some of the deadliest cancers, particularly those that often go unnoticed until symptoms appear at more advanced stages. In addition to helping with early detection of difficult-to-detect cancers, AI is also accelerating improved detection of cancers for which effective screening methods already exist, such as helping read mammograms to detect breast cancer faster, more accurately, and efficiently (see Technological Innovations Advance Cancer Screening for Early Detection).
Recent studies show that AI can improve how patients are classified based on their tumor’s characteristics. For instance, researchers developed a DL model that accurately predicts EGFR and KRAS mutations—genetic alterations commonly associated with aggressive forms of the disease—in lung cancer from routine histology images, potentially eliminating the need for often costly conventional genetic sequencing (1040)Zhao Y, et al. (2025) Lancet Oncol, 26: 136..
In medulloblastoma, the most common malignant brain tumor in children, accurate molecular classification of tumors plays a critical role in guiding treatment decisions but typically requires invasive procedures and time-consuming molecular tests. In a recent study, researchers showed that ML algorithms trained on gene expression data classified medulloblastoma with high accuracy, including those with poor prognosis and high metastasis rates (1041)Hourfar H, et al. (2025) Clin Oncol (R Coll Radiol), 40: 103789..
In another study, researchers developed an AI model that used spatial transcriptomics to automate histologic and molecular diagnosis of central nervous system tumors. The model accurately predicted tissue histology and tumor subtypes with more than 89 percent accuracy, demonstrating its potential to precisely identify and diagnose subtypes, even when the tissue sample is sparse or unsuitable for conventional molecular testing (1042)Ritter M, et al. (2025) Nat Cancer, 6: 292.. Beyond a single cancer type, a pan-cancer classification model has also been developed, which differentiated more than 170 tumor types across all organ sites. Achieving over 97 percent precision across diverse sample types, this approach shows how AI can expand the use of DNA methylation–based tumor classification beyond brain tumors and support diagnosis across a broad range of cancers (1043)Yuan D, et al. (2025) Nat Cancer..
AI applications are also showing promise in predicting when and where in the body cancer will spread and whether it will come back. For example, a recent study showed that ML models predicted aggressive properties of brain tumors from MRI scans of patients, a task that even experienced radiologists have difficulty performing consistently, offering a noninvasive way to identify patients with brain metastases earlier and inform treatment decisions based on likely tumor behavior (1044)Najafian K, et al. (2024) Neurooncol Adv, 6: vdae200..
As another example, researchers have recently developed a fully automated AI system that detects and classifies bone metastases on CT scans with high sensitivity and accuracy. The model significantly reduced the time radiologists need to accurately diagnose bone metastases, while improving detection rates, demonstrating its potential to support routine cancer staging and early identification of metastases (1045)Zhang Y, et al. (2025) Nat Commun, 16: 4444.. Similarly, ML models were able to predict when and where breast cancer was likely to spread, including both local recurrences and distant metastases, thus informing prognosis and helping to guide treatment decisions (1046)Kovacs KA, et al. (2025) Sci Rep, 15: 4868.. These models offer a new approach to risk assessment and enable the selection of patients who may benefit from more intensive systemic treatments.
Advancing New Treatments
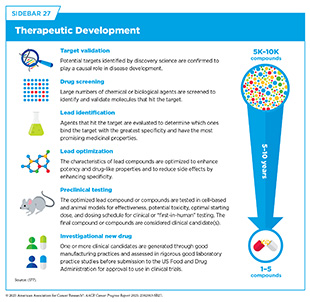
AI is reshaping cancer drug development by streamlining aspects of cancer science and medicine that have traditionally been time-consuming, expensive, and high-risk. By analyzing vast datasets, modeling complex biological systems, and uncovering meaningful insights, AI is helping researchers identify key drivers of disease and design new therapies more efficiently. These innovations have the potential to accelerate drug discovery while delivering safer, more targeted treatments to patients. Early drug development involves target identification—determining the genes, proteins, or pathways most critical to cancer progression—and drug discovery—finding or designing drugs that can act on those targets (see Sidebar 27). Innovations such as AlphaFold, which earned the 2024 Nobel Prize in Chemistry for leveraging AI to predict the 3D structure of proteins, are enabling researchers to model the structure of millions of proteins with high accuracy and opening new paths to therapeutic development (1047)Callaway E (2024) Nature, 634: 525.. At the same time, AI is helping scientists predict and address drug resistance by integrating multi-omic and clinical data to develop more effective, personalized treatment strategies (1048)Mao Y, et al. (2025) Mol Cancer, 24: 123..
AI-driven target identification harnesses large amounts of biological data to determine key vulnerabilities in cancer. A recent study applied AI-powered software to transcriptomic data from adenoid cystic carcinoma (ACC)—a rare type of salivary gland cancer with few treatment options—and identified the protein PRMT5, an enzyme that modifies other proteins to control gene expression, as a promising therapeutic target. They showed that inhibition of PRMT5 suppressed tumor growth in multiple preclinical models and reduced the expression of key drivers of ACC progression, providing a strong rationale for future clinical development of PRMT5 inhibitors as a targeted therapy for ACC patients (1049)Mishra V, et al. (2025) J Exp Clin Cancer Res, 44: 11..
In another study, researchers developed a DL model that integrates multi-omic data and information on known pathways from public databases to predict cancer-driving genes. By modeling interactions between genes, proteins, and RNAs, the tool revealed 57 cancer-driving gene candidates across multiple tumor types, including three genes that had not been identified by other models (1050)Su X, et al. (2025) Nat Biomed Eng, 9: 371.. These findings highlight the potential of AI to uncover novel, clinically relevant targets, paving the way for the development of more effective and personalized therapies.
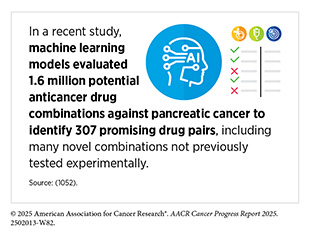
AI can also assist with more efficient drug design, predicting drug behavior, and accelerating the identification of effective drug combinations and repurposed therapies. In one study, researchers developed an ML-driven approach to guide combination drug screening by prioritizing which of the 1.4 million possible pairwise combinations of 206 drugs to test in pediatric cancer cell lines. The model accurately predicted previously unknown drug pairings and identified effective combinations confirmed by follow-up experiments after testing only 4 percent of the 1.4 million possible combinations—a task that would have taken a team of trained researchers years to complete (1051)Tosh C, et al. (2025) Nat Commun, 16: 156.. This adaptive screening strategy could help streamline the discovery of promising drug combinations and substantially shorten the timeline for future experimental validation.
AI can also expand treatment options for cancer through drug repurposing—the use of existing drugs approved for non-cancer conditions to treat cancer. Researchers recently developed an ML model that uses patient-specific genomic data, including mutations in non-coding regions of the genome, to identify drugs originally intended for other diseases. By using tumor data from over 5,000 patients, this model identified actionable drugs approved to treat conditions other than cancer for more than 30 percent of cases and successfully validated several predictions in laboratory tests using tumor cells from patients with pancreatic and esophageal cancers (1053)Dong X, et al. (2025) NPJ Precis Oncol, 9: 36..
These findings highlight how AI models can help overcome long-standing barriers in drug discovery by rapidly narrowing down large experimental workflows and guiding the development of more personalized cancer treatments.
Enhancing Clinical Decision-Making
AI-driven technologies are improving clinicians’ ability to predict patient outcomes and personalize treatment plans. Digital twins—virtual, continuously updated models of individual patients—are one such technology. Digital twins integrate real-world data from electronic health records (EHRs) and clinical evaluations, alongside multi-omics and lifestyle information, to simulate disease progression, predict treatment responses, and guide personalized treatment based on a patient’s own unique biology. They also show promise in clinical trial settings, particularly for pediatric and rare cancers, where they can function as synthetic control arms to augment or replace conventional controls when patient enrollment is limited (1054)Mollica L, et al. (2024) ESMO Real World Data and Digital Oncology, 5: 100056..
Another emerging application for AI is in cancer surgery. In a recent study, an AI-powered 3D reconstruction system significantly improved lung surgery planning by more accurately identifying critical anatomic features, such as blood vessels and bronchi, thus reducing errors by over 40 percent and preoperative planning time by 25 percent (1055)Chen X, et al. (2025) Nat Commun, 16: 4086..
At the same time, LLMs are proving valuable for diagnostic and treatment decisions. In a randomized controlled trial, physicians using GPT-4, a large multimodal DL model, outperformed those using conventional resources alone in clinical management tasks, such as considering diagnostic decisions, treatment options, and factors like patient comfort and preferences (1056)Goh E, et al. (2025) Nat Med, 31: 1233.. Researchers have also recently developed a fully automated clinical AI agent that integrates GPT-4 with precision oncology tools, including models for genomic analysis, histopathology interpretation, image analysis, and web-based search tools. By combining these capabilities, the agent guided clinical decision-making with high accuracy. When tested on 20 realistic, simulated patient cases, this system recommended appropriate treatments and care plans in over 90 percent of cases, nearly tripling the accuracy of GPT-4 alone (1057)Ferber D, et al. (2025) Nat Cancer.. Together, these advances are paving the way for smarter clinical support for patients that can deliver more personalized care and shape the future of cancer treatment.
Muscle-invasive bladder cancer (MIBC) is an aggressive form of bladder cancer in which the tumor grows into the muscle layer of the bladder wall. Because of its higher risk of spreading beyond the bladder, patients are often given chemotherapy before surgery to shrink the tumor and eliminate cancer cells that may have spread. However, not all patients respond to this treatment, which can cause serious side effects. Researchers have recently developed a DL model that integrates tumor images and gene expression data to predict how patients will respond to chemotherapy. The model accurately identified patients likely to benefit from treatment and revealed key molecular and visual features of tumors associated with treatment response (1058)Bai Z, et al. (2025) NPJ Digit Med, 8: 174.. This approach may help tailor treatment plans for patients with MIBC and reduce unnecessary exposure to toxic therapies.
AI is also being used to predict which patients will respond favorably to immunotherapy. In a recent multicenter study of patients with advanced lung cancer, researchers developed a DL model that analyzes pathology slides to predict who is most likely to benefit from immune checkpoint inhibitors (ICIs). The model’s predictions were comparable to current clinical biomarkers, such as PD-L1, and improved when the two approaches were combined, highlighting that this model could help guide treatment decisions and improve patient outcomes (637)Rakaee M, et al. (2025) JAMA Oncol, 11: 109..
Researchers have also applied techniques from natural language processing (NLP)—a field of AI that includes tools, such as LLMs, that allow computers to understand and use human language—to extract information from EHRs and clinical notes to combine with genomic, demographic, and treatment data. Using data from nearly 25,000 cancer patients, this approach improved predictions of cancer outcomes, such as survival and risk of metastasis, compared to traditional staging or genomic data alone (1059)Jee J, et al. (2024) Nature, 636: 728..
In another study, researchers trained a DL model on data from over 15,000 patients across 38 types of solid tumors to identify indicators of how cancer will progress, and which treatment may be most effective. They uncovered more than 1,300 novel connections between diverse data types—such as body composition, lab tests, and tumor genetics—to uncover mechanisms that help determine how a patient’s disease is likely to progress (1060)Keyl J, et al. (2025) Nat Cancer, 6: 307.. These findings show the promise of AI in aiding clinicians in prioritizing and optimizing critical patient-specific information and treatment strategies.
Leveraging a novel ML platform that combines drug response data with molecular features from the patient’s own cancer cells, researchers have uncovered biomarkers of chemotherapy resistance and identified potential alternative treatments for colorectal cancer patients. The platform accurately predicted clinical outcomes across four diverse cohorts and proposed alternative therapies for treatment-resistant tumors (1061)Chia S, et al. (2025) Cell Rep Med, 6: 102053.. Another AI tool integrated multi-omic data from lung cancer patients to predict personalized treatment responses, suggest new drug uses, and estimate how well treatments might work and their potential side effects (1062)Butt RN, et al. (2025) Sci Rep, 15: 15080..
AI-guided platforms can also aid in determining how to adjust treatment doses over time. In a recent study, researchers developed an AI tool to guide individualized chemotherapy dosing recommendations based on a patient’s treatment response data (1063)Blasiak A, et al. (2025) NPJ Precis Oncol, 9: 49.. The platform enabled real-time dose adjustments and was adaptable to patient-specific changes such as toxicity levels and tumor response, offering a promising path toward safer, more personalized cancer chemotherapeutic regimens.
Fairness and Oversight
As AI begins to reshape cancer research and patient care, it introduces new layers of complexity that can both help and hinder clinical decision-making, raising serious concerns about fairness, transparency, and accountability. Researchers have also highlighted the lack of standardized benchmarks in using AI in medicine. Without shared datasets, evaluation methods, or consistent performance metrics, tools may get optimized for narrow clinical contexts—for example, working well only in one hospital’s patient population or imaging protocols—without generalizing to broader use (1064)Mahmood F (2025) Nat Med, 31: 1060.. However, efforts to ensure broad inclusion and oversight extend beyond technical validation. There is a need for shared responsibility between AI developers, regulators, and health care institutions to address bias and avoid discriminatory outcomes from AI-powered decision tools (1065)Ratwani RM, et al. (2024) JAMA, 332: 1051..
While AI holds great promise for improving diagnosis, personalized treatment, and increasing access to care for cancer patients, it may inadvertently worsen disparities. For example, underrepresentation of diverse populations in AI training datasets and unequal access to AI tools, particularly in low-income and rural areas, may exacerbate existing health disparities (1066)Dankwa-Mullan I, et al. (2025) Curr Oncol Rep, 27: 95.. Building public confidence in AI will require clear communication, robust data governance, and active engagement with all stakeholders. The future of AI in cancer care will depend on both technological innovation and collective action to ensure these tools are transparent, equitable, and trusted by those that will use them.
CRISPR in Cancer Research and Drug Development
CRISPR-based systems are powerful and versatile gene editing tools that allow researchers to precisely modify DNA. Researchers have harnessed these systems not only to deepen our understanding of mechanisms that drive human diseases but also to explore new ways to treat them. For example, CRISPR can be used to re-create genetic mutations to see how they affect the body, fix or replace faulty genes, add tags to track how genes behave, turn genes on or off without changing the DNA itself, or engineer immune cells to fight disease.
Traditional CRISPR tools are made up of two parts, a programmable RNA guide that can find a specific site in the DNA and a Cas protein that acts like molecular scissors to cut both strands of DNA at that site. After DNA has been cut, the cell’s natural DNA repair processes can be harnessed to make changes with high precision and accuracy.
Building on these foundational CRISPR-Cas systems, next-generation CRISPR tools, like base and prime editors, have been developed that allow even more refined edits to be made, down to single letters of the genetic code. Base editors work like pencils that can erase and rewrite a single letter of DNA. These tools work best for changing one nucleotide base to another, for example, switching an adenine (A) to guanine (G) or cytosine (C) to thymine (T) (see Influences Inside the Cell). Prime editors are more like word processors and can make a wider range of changes, including adding or deleting small pieces of DNA, or swapping multiple letters at once.
These innovative tools are supporting advances in research and clinical development through disease modeling, drug screening, and the development of new therapies. In the context of cancer, CRISPR is being used to uncover how mutations drive tumor development, explore vulnerabilities in cancer cells, and create personalized treatment approaches. The following section describes how gene editing is reshaping our understanding of cancer and accelerating the discovery of innovative, more effective therapies.
Basic Research Discoveries
CRISPR gene editing has rapidly evolved into one of the most transformative tools in basic research. The ability of CRISPR systems to precisely modify genes in living cells has made it possible for researchers to model human diseases and uncover the genetic underpinnings of complex biological processes with unprecedented speed and accuracy.
In a recent study, researchers used base and prime editors to scan tens of thousands of mutations in the EGFR gene, a gene frequently mutated in lung cancer, revealing previously unknown variants that drive tumor growth or treatment resistance (1067)Belli O, et al. (2024) Nat Biotechnol.. This approach not only generated new insights into the genetic drivers of cancer but also demonstrated how precision editing can inform treatment decisions and accelerate the development of personalized therapies.
In another study, researchers used CRISPR to introduce cancer-driving mutations in experimental human brain organoids (see Sidebar 6), which retain the structural complexity of the developing brain. These organoids provide a powerful platform for studying brain tumor development and can be used to investigate the specific roles of individual gene variants (1068)Pagliaro A, et al. (2025) Nat Protoc.. Advances in basic research using CRISPR technologies will continue to enable the development of more biologically relevant experimental models and deepen insights into cancer biology.
Drug Development
By enabling researchers to systematically investigate the molecular pathways that drive cancer growth, resistance, and immune evasion, CRISPR is contributing to advances in drug development strategies. With its unparalleled precision and adaptability to large experimental designs, CRISPR allows researchers to perform genome-wide functional screens that can accelerate the development of precision cancer therapies in ways not previously possible.
Recently, CRISPR screening has been used to identify novel immune-related targets that boost the effectiveness of chimeric antigen receptor (CAR) T-cell therapy and reveal mechanisms of immune evasion that tumors use to resist treatment, advancing the search for more effective immunotherapies (1069)Du Y, et al. (2025) Oncogene, 44: 409..
Researchers have also developed a CRISPR screening platform to identify synthetic lethal interactions, which occur when two otherwise tolerated gene changes occur simultaneously and lead to cell death, across multiple cancer types. By pairing these results with large-scale drug sensitivity data from tumor cell line screens, they identified gene alterations that sensitize cancer cells to specific drugs, offering a systematic approach to match cancer patients with targeted therapies and advancing synthetic lethality-based treatment strategies (1070)Fong SH, et al. (2025) Nat Genet, 57: 154..
These findings demonstrate how CRISPR technologies can be used to bridge basic discovery and actionable clinical insight to accelerate cancer drug development.
Next-Generation Treatments
Recent innovations in CRISPR-based technologies are rapidly advancing toward the clinic, offering tools to correct genetic defects, reprogram immune cells, and remodel the TME (see Tumor Microenvironment) directly in patients. Across solid tumors and blood cancers, these technologies are being explored not only to enhance existing therapeutic strategies, such as immunotherapies and adoptive cell therapies, but also as a treatment itself.
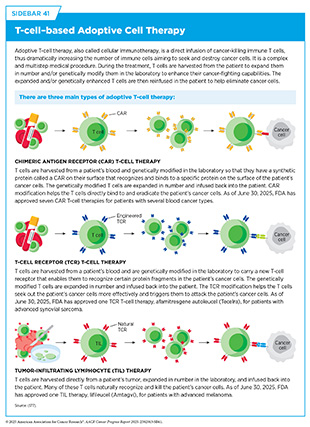
In several recent studies, CRISPR-based systems have been used to improve the effectiveness of established treatments by activating the immune system. For example, researchers have enhanced the efficacy of adoptive T-cell therapies (see Sidebar 41) by increasing how long T cells can be active and how quickly they remove cancer cells in both blood cancer and solid tumors (1071)Patel RP, et al. (2024) Sci Immunol, 9: eadn6509.. In addition, researchers have genetically engineered natural killer (NK) cells to resist immunosuppressive signals in the TME and improve clinical performance of immunotherapy (1072)Rea A, et al. (2025) Nat Immunol, 26: 582.. Tumor-infiltrating lymphocytes (TILs) modified with CRISPR to block proteins that weaken the immune system are also showing early promise in clinical trials (1073)Lou E, et al. (2025) Lancet Oncol, 26: 559.. These advances contribute to a growing trend of using CRISPR to reprogram immune cells for greater antitumor potency (1074)Feng X, et al. (2024) Exp Hematol Oncol, 13: 102.(1075)Tarannum M, et al. (2025) Nat Biotechnol, 43: 516.(1076)Khoshandam M, et al. (2024) Genes Dis, 11: 101121..
Researchers are also exploring the potential of CRISPR itself as an anticancer treatment. Base and prime editors provide a way to precisely rewrite faulty DNA without cutting it, offering the potential to fix cancer-causing mutations with remarkable accuracy (1077)Xu W, et al. (2024) J Transl Med, 22: 1133.. RNA-targeting CRISPR strategies are also being used to turn off genes that help cancer evade the immune system without changing DNA, opening the door to more tailored immune-based treatments.
At the same time, researchers are also developing advanced delivery systems that send CRISPR directly to tumors. For example, researchers have recently developed nanoparticles that cross the blood–brain barrier, a protective layer of tightly packed cells that protects the brain from harmful substances, and activate gene editing only within glioblastoma tumor cells (1078)Li Q, et al. (2025) ACS Appl Mater Interfaces, 17: 4480.. In another study, an EGFR-targeted nanoparticle was developed that delivers CRISPR to head and neck cancers, resulting in complete disappearance of tumors in some animal models (1079)Masarwy R, et al. (2025) Adv Sci (Weinh), 12: e2411032..
Although these innovations are highly promising, it is important to note that most CRISPR-based therapies are still in early stages of development. These technologies must undergo rigorous testing in clinical trials to determine their safety, efficacy, and long-term effects in the human body. Continued research is essential to ensure that these powerful tools can be safely and effectively integrated into cancer treatment.
Liquid Biopsy in Cancer Care
A biopsy involves the removal of cells or tissues from a patient to help physicians diagnose a condition, such as cancer, or assess how it is responding to treatment. Traditionally, biopsies are invasive and limited to a single timepoint. In contrast, a liquid biopsy—the analysis of blood or other body fluids—offers a less invasive alternative by capturing tumor-derived material that is routinely shed into the bloodstream during cancer development and treatment. This includes circulating tumor cells (CTCs), cell-free DNA (cfDNA) such as circulating tumor DNA (ctDNA), and exosomes—tiny, membrane-bound vesicles that transport proteins, RNA, and other molecules between cells. These materials can be analyzed to uncover molecular alterations associated with cancer. Although they are currently used primarily alongside traditional tissue biopsies, liquid biopsies may also be useful for detecting tumors located in areas that are difficult to biopsy. Unlike traditional biopsies, liquid biopsies can be easily repeated during the course of clinical care, which may enable dynamic, real-time insights into tumor evolution and therapeutic responses. As such, liquid biopsy approaches can enhance early detection (see Advances in Minimally Invasive Screening Approaches), diagnosis, treatment selection, and monitoring of disease progression and recurrence (see Sidebar 55). The following section highlights recent research exploring how liquid biopsies are being leveraged across the cancer continuum.
Diagnosing Cancer and Predicting Metastases
Emerging research in liquid biopsy is revealing powerful new strategies for detecting cancer earlier and diagnosing the disease more accurately than ever before. Liquid biopsies at diagnosis have been studied for both liquid and solid tumors. As an example, researchers have developed a sequencing method that detects ctDNA with high precision and accuracy from a small blood sample in children newly diagnosed with leukemia, offering a noninvasive alternative to bone marrow biopsy while capturing a broad spectrum of tumor-specific alterations (1080)Lei S, et al. (2025) Leukemia, 39: 420..
Liquid biopsies can also identify patients at the highest risk for cancer spread. In individuals with pancreatic cancer, detection of mutant KRAS ctDNA was strongly associated with metastatic tumors that were difficult to detect by conventional methods and poor survival outcomes, offering new insights into disease biology and aiding decisions about surgery and treatment intensity (1081)Leiting JL, et al. (2025) Ann Surg Oncol, 32: 4453.. Together, these studies underscore the growing impact of liquid biopsy and ctDNA analysis in expanding the reach of cancer diagnosis and improving risk stratification for metastatic progression.
Monitoring Treatment Response and Predicting Resistance
Liquid biopsy can also be used to monitor how tumors respond to treatment and to detect the emergence of resistance. Mutations in the KRAS gene are common in colorectal and pancreatic cancers, with G12C being a clinically important subtype now targetable by approved therapies. In a recent study, ctDNA profiling was used to assess how tumors with KRAS G12C mutations respond to treatment. Researchers found that nearly half of the patients had additional mutations that may interfere with the effectiveness of KRAS G12C inhibitors (1082)Jazieh K, et al. (2025) Clin Cancer Res, 31: 899.. These findings underscore the importance of pre-treatment molecular profiling to identify changes in the tumor that might reduce treatment effectiveness.
Monitoring dynamic ctDNA changes during treatment can signal emerging resistance or treatment success. In non–small cell lung cancer, early disappearance of detectable KRAS G12C ctDNA following treatment with sotorasib—a targeted therapy that blocks the KRAS G12C protein—was linked to better outcomes, while persistent ctDNA predicted poor response (88)Skoulidis F, et al. (2025) Nat Med.. In another study, a greater than two-fold drop in ctDNA after one chemotherapy cycle was indicative of sensitivity to platinum-based treatment in small cell lung cancer and was associated with longer survival (1083)Murciano-Goroff YR, et al. (2024) JCO Precis Oncol, 8: e2400216..
Early changes in ctDNA levels after starting treatment have also been used to predict treatment outcome and monitor immune response. In a phase II clinical trial, early changes in tumor-agnostic ctDNA features within the first week of starting treatment with the ICI pembrolizumab predicted both progression-free survival and overall survival across solid tumors (1084)Stutheit-Zhao EY, et al. (2024) Cancer Discov, 14: 1048..
Together, these findings position ctDNA as a critical tool for predicting resistance and assessing treatment effectiveness, with ongoing research also exploring its use in pediatric cancers (1085)Janssen FW, et al. (2024) NPJ Precis Oncol, 8: 172..
Detecting Minimal Residual Disease and Recurrence
An emerging application of liquid biopsy that holds significant promise for improving cancer care is its ability to detect minimal residual disease (MRD) and recurrence after treatment. Recent research has demonstrated that the presence or absence of ctDNA following surgery or systemic therapy can serve as a powerful indicator of patient outcomes and guide posttreatment decisions.
In colorectal cancer, a recent study showed that patients with detectable ctDNA after surgical removal of the tumor had worse survival outcomes, while sustained ctDNA clearance during additional chemotherapy predicted long-term benefit (1086)Nakamura Y, et al. (2024) Nat Med, 30: 3272.. Clinical trials have also shown that ctDNA measurement as a marker of MRD can guide treatment decisions in early-stage disease. In colorectal cancer, researchers found that using ctDNA to inform adjuvant chemotherapy decisions significantly reduced unnecessary treatment without compromising long-term survival (1087)Tie J, et al. (2025) Nat Med, 31: 1509..
Similarly, in metastatic kidney cancer, researchers showed that tracking ctDNA over time can offer important insights for patients receiving standard immunotherapy or targeted drugs. They found that patients whose ctDNA remained undetectable had much longer periods without disease progression, while those with detectable ctDNA were more likely to experience relapse, revealing its potential to monitor remission and relapse in real time (1088)Basu A, et al. (2024) JCO Precis Oncol, 8: e2400667..
In another study in melanoma, ctDNA detected after surgery identified patients at highest risk of recurrence, and its continued presence during follow-up treatment signaled early relapse and poor survival despite standard therapy (1089)Syeda MM, et al. (2025) Lancet Oncol, 26: 641.. In cervical cancer, results from a phase III clinical trial showed that detection of ctDNA after treatment predicted relapse and survival. Personalized ctDNA tests could detect signs of cancer recurrence months before it appeared on scans, while patients with detectable ctDNA after treatment were significantly more likely to experience relapse (1090)Mayadev J, et al. (2025) Ann Oncol.. These studies demonstrate how liquid biopsy approaches are helping clinicians personalize posttreatment care, offering a less invasive, highly sensitive tool to detect recurrence and plan clinical care.
Although liquid biopsy has shown promise in enhancing current methods of cancer detection and monitoring, challenges remain that limit its routine clinical use (see Sidebar 55). Researchers have highlighted key barriers to ctDNA adoption, some of which include a variable amount of detectable material and lack of standardized protocols (1091)Bartolomucci A, et al. (2025) NPJ Precis Oncol, 9: 84.. Overcoming these hurdles through continued research will be essential to fully unlock the potential of liquid biopsies to personalize cancer care and guided treatment.
New Frontiers in Cancer Treatment
In recent years, researchers have made remarkable progress in transforming how cancer is treated. Among the most promising advances are next-generation cellular immunotherapies and molecularly targeted therapies, which are expanding therapeutic possibilities for patients with aggressive or treatment-resistant cancers (see Sidebar 37).
For example, in a recent study, researchers engineered allogeneic CAR T cells—immune cells derived from healthy donors rather than the patient—to express an HIV (human immunodeficiency virus) protein called Nef, which has the natural ability to protect the engineered cells from the patient’s immune system, thus improving their survival (1092)Perica K, et al. (2025) Nature, 640: 793.. This strategy could pave the way for more accessible, durable, and potent T-cell therapies for a broader population of patients. Researchers are also exploring strategies for CAR T-cell therapies targeting the most intractable solid tumors, such as glioblastoma. Recent trials have shown encouraging results using CAR T cells in brain tumors targeting multiple tumor-specific proteins, novel surface proteins, and combinatorial approaches (649)Bagley SJ, et al. (2025) Nat Med.(1093)Park S, et al. (2024) NPJ Precis Oncol, 8: 279..
Another area of rapid gains has been in KRAS-targeted therapies, whereby decades of basic research have paved the way for the next generation of treatment advances (see Basic Research Decoding Cancer’s Complexities). Building on the success of FDA-approved inhibitors of KRAS protein with the G12C mutation, researchers are now developing molecules that target other KRAS mutations and degrade KRAS proteins entirely, and immune-based strategies—such as vaccines, engineered T cells, and combination treatments—to help the immune system better recognize and attack KRAS-driven cancers (see Sidebar 56). The sections below highlight some of the most exciting emerging areas in cancer treatment.
Cancer Vaccines
Researchers are developing new types of cancer vaccines that prevent cancer in high-risk individuals and treat existing disease by training the immune system to recognize and attack tumor-specific targets. Vaccines play a critical role in disease prevention, with vaccines against the hepatitis B virus (HBV) and human papillomavirus (HPV) already reducing the global burden of liver and cervical cancers by targeting oncogenic viruses before cancer develops. Building on these successes, researchers are now exploring the development of preventive vaccines against cancers that target genetic mutations or molecular characteristics of cancer itself (see Genetic Insights Advancing Cancer Risk Reduction and Interception).
For example, an ongoing trial is using a KRAS-targeted peptide vaccine in individuals with KRAS mutations who are at higher risk of developing pancreatic cancer (1094). Peptides—short chains of amino acids that join together to form proteins—are often used in vaccines to stimulate specific immune responses. Preventive cancer vaccines offer an advantage in the likelihood of a strong, lasting response over therapeutic vaccines (1095)Graciotti M, et al. (2025) Nat Rev Drug Discov, 24: 134.. As research in this field continues to advance, these vaccines hold great potential to reduce cancer risk, ease the burden of continuous monitoring, and reshape how we approach cancer prevention.
Therapeutic cancer vaccines activate a patient’s immune system to recognize and destroy cancer after it has developed, often by targeting unique markers found on cancer cells. These vaccines can complement existing therapies and may be especially effective when used in combination with ICIs, chemotherapy, or molecularly targeted therapies (1096)Zaidi N, et al. (2025) Nat Rev Cancer.(1097)Martini DJ, et al. (2025) Cancer Discov.. The only FDA-approved therapeutic cancer vaccine currently is sipuleucel-T (Provenge), which was approved in 2010 for treating advanced prostate cancer.
One therapeutic cancer vaccine strategy uses an RNA-based approach that can be tailored to encode multiple tumor-specific neoantigens—mutated proteins that are unique to cancer cells. In a recent clinical trial in patients with pancreatic cancer, an individualized RNA vaccine, given alongside the ICI atezolizumab and chemotherapy, triggered vaccine-specific T-cell responses in half of the patients, resulting in delayed cancer recurrence and favorable survival outcomes (1098)Sethna Z, et al. (2025) Nature, 639: 1042..
Dendritic cell (DC)-based vaccines represent another strategy for cancer treatment. In a pilot study of patients with early-stage HER2-positive breast cancer, injection of a DC vaccine targeting HER2 was found to be safe and immunologically active, driving increased T-cell infiltration in tumors and potentially enhancing complete response rates when given before chemotherapy (1099)Soliman H, et al. (2025) NPJ Breast Cancer, 11: 29.. In another trial, a DC vaccine with personalized mutant KRAS peptides is being tested in patients with pancreatic cancer, demonstrating feasibility and laying the foundation for further study of DC vaccines in one of the deadliest cancer types (1100)Bear AS, et al. (2024) J Clin Invest, 134..
Neoantigen-targeting peptide vaccines are another promising therapeutic cancer vaccine strategy. A personalized neoantigen vaccine trial in patients with kidney cancer who were considered at high risk of recurrence after having their tumors completely removed through surgery demonstrated strong immune system activation, targeting key driver mutations and inducing durable T-cell responses, with all nine vaccinated patients remaining disease-free after a follow-up of more than 3 years (1101)Braun DA, et al. (2025) Nature, 639: 474.. In another trial, a shared antigen vaccine, which targets mutations or proteins commonly found across patients’ tumors, is being tested in patients with resected pancreatic and colorectal cancer. This vaccine targets mutant KRAS peptides and is being evaluated in combination with nivolumab and ipilimumab, investigating potential combined effects between vaccine-induced immune response and ICIs (1102)Liang KL, et al. (2025) Cancers (Basel), 17..
Researchers have also recently shown promising results from a trial that used personalized vaccines targeting neoantigen peptides predicted in individuals with high-risk solid tumors and hematologic malignancies. All trial participants showed positive neoantigen-specific T-cell and B-cell immune responses. The study also revealed that even tumors with relatively few genetic mutations could be effectively targeted using tailored neoantigen selection, and that vaccine-induced immune responses correlated with long-term survival in some patients (1103)Saxena M, et al. (2025) Cancer Discov, 15: 930..
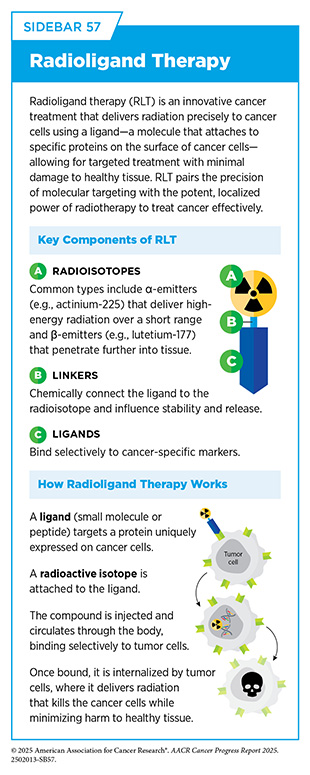
Radiopharmaceutical Therapy
Radiopharmaceutical therapies, including radioligand therapies (RLTs), are a rapidly evolving class of cancer therapeutics that use radioactive compounds to deliver cytotoxic radiation directly to cancer cells. RLTs combine a radioactive compound with a targeting agent that binds to specific proteins found on cancer cells. Once bound, high-energy radiation is delivered directly to the cancer cells while avoiding surrounding healthy cells, offering a highly localized, tumor-specific approach to treatment (see Sidebar 57). As of June 30, 2025, FDA has approved two RLTs— lutetium-177 dotatate (Lutathera) for neuroendocrine tumors in 2018 and lutetium-177 vipivotide tetraxetan (Pluvicto) for metastatic prostate cancer in 2022 (see Applying Precision to Radiation Therapy).
The effectiveness of these therapies has brought about a wave of innovation and clinical exploration. The success of Pluvicto in targeting prostate-specific membrane antigen (PSMA)–expressing prostate tumors has led to a four-fold increase in radiopharmaceutical use in metastatic prostate cancer since 2021, and radiopharmaceuticals now represent the second most common treatment for these patients after chemotherapy (497)Global Oncology Trends 2025. Accessed: June 11, 2025.. The field is also advancing rapidly in breast cancer, for which clinical trials are exploring investigational RLTs in combination with bispecific antibodies and immunotherapies to overcome tumor heterogeneity and enhance immune activation (562)Giugliano F, et al. (2025) Cancer Treat Rev, 136: 102940..
Emerging technologies are further expanding the potential of radiopharmaceuticals by refining delivery methods and exploring novel isotopes. In a first-in-human trial for glioblastoma, instead of using a radioactive isotope bound to a ligand, researchers used a pressure-driven technique to deliver a novel beta-emitting isotope, rhenium-186, encapsulated into nanoliposomes—tiny, hollow spheres made from natural substances like those found in cell membranes—directly into brain tumors. This method bypasses the blood–brain barrier and enables high local radiation doses, exceeding those achievable with external beam radiation therapy, with minimal systemic toxicity. Patients who received more than 100 Gy (units of ionizing radiation dose) experienced a median survival of 17 months—compared to 8 months with standard of care treatment—highlighting the importance of precise, localized delivery in treating difficult-to-access tumors (1104)Brenner AJ, et al. (2025) Nat Commun, 16: 2079..
In the past decade, more than 160 clinical trials have been launched globally to evaluate RLTs across a range of tumor types (497)Global Oncology Trends 2025. Accessed: June 11, 2025.. With ongoing advances in research, radiopharmaceuticals hold promise as the next generation of precision therapies for patients with cancer.
Next Section: Advancing Cancer Science and Medical Research Through Evidence-Based Policies Previous Section: Supporting Cancer Patients and Survivors