Executive Summary
We are making remarkable strides against the collection of diseases known as cancer. Breakthroughs across basic, translational, clinical, and population sciences—combined with rapid technological innovation—are transforming how we prevent, detect, diagnose, and treat cancer.
As the first and largest professional organization in the world dedicated to preventing and curing all cancers, the American Association for Cancer Research (AACR) remains committed to enhancing public understanding of cancer and advocating for robust, sustained, and predictable investment in medical research—investment that is essential to saving lives. This includes steadfast support for the US federal agencies that fuel progress against cancer, particularly the National Institutes of Health (NIH), National Cancer Institute (NCI), Food and Drug Administration (FDA), and Centers for Disease Control and Prevention (CDC).
However, the significant gains made in cancer science and medicine are under threat. Recent disruptions to the federal workforce, along with growing uncertainty about the future of medical research funding, pose a grave risk to the scientific enterprise. Any erosion of support for NIH would have a catastrophic impact on future discoveries and delay lifesaving advances for patients and families. It is therefore encouraging to see a bipartisan effort in the Senate not only to maintain current funding levels, but also to increase support for medical research and continue the federal investment in saving lives.
Now in its 15th edition, the AACR Cancer Progress Report series to Congress and the American public is a major cornerstone of our educational and advocacy efforts. Over the past decade and a half, these reports have showcased the extraordinary momentum in cancer research and how sustained federal investment has led to improvements in treatment options, survival, and quality of life for cancer patients. This year’s report continues that tradition—highlighting how medical research can transform the lives of cancer patients, including those of the nine courageous individuals featured in this report. It also makes clear that continued progress hinges on unwavering support for NIH, NCI, FDA, and CDC and on a stable research infrastructure that can deliver advances for the benefit of all patients affected by this dreadful disease.
Cancer in 2025
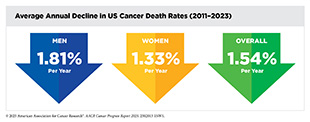
Significant progress against cancer has led to a steady decline in cancer death rates and a consistent increase in the number of individuals living longer, fuller lives after a diagnosis. In the United States, the overall cancer death rate decreased by 34 percent between 1991 and 2023, a reduction that translates into averting more than 4.5 million deaths from cancer. This reduction in cancer mortality is largely attributable to national public health efforts, including prevention initiatives, screening programs, and advancements in treatment for certain cancers.
The steady progress in reducing cancer mortality is largely driven by reduced smoking rates and subsequent decline in lung cancer deaths, a trend that has accelerated in recent years due to advances in treatment and early detection. Steady declines in death rates for colorectal cancer and female breast cancer since the 1990s have also played a key role in reducing the overall cancer mortality. More recently, advances in precision medicine have led to reductions in death rates for leukemia, melanoma, and kidney cancer, further contributing to overall progress. Thanks to the research-driven advances across the cancer care continuum, approximately 18.6 million individuals with a history of cancer were living in the United States as of January 1, 2025, and this number is projected to exceed 22 million by 2035.
While remarkable progress has been made, cancer remains a major public health challenge in the United States and globally. In the United States, more than two million new cases are projected for 2025 alone, underscoring the urgent need for continued investment in research, prevention, early detection, and equitable access to care.
One of the key challenges we face is the uneven progress across cancer types. For example, the overall 5-year relative survival rate remains just 13 percent for pancreatic cancer and 6 percent for glioblastoma multiforme, an aggressive form of brain cancer, compared to 92 percent for breast cancer and 98 percent for prostate cancer. Additionally, the burden of cancer continues to disproportionately affect racial and ethnic minority groups and other medically underserved populations in the United States. These disparities are driven by a long history of structural inequities and systemic injustices and are driven by complex and interrelated structural and social factors.
The burden of cancer and its economic toll, both on individuals and on US health care systems, are expected to rise in the coming decades, underscoring the urgent need for more research in medicine and public health to accelerate the pace of progress against cancer. The progress highlighted in this report was made as a direct result of the cumulative efforts of individuals working across the spectrum of medical research supported by federal funding. Importantly, public sector funding from NIH and NCI directly benefits patients through the development of lifesaving anticancer therapeutics and preventive interventions.
Recent funding cuts and proposed budget reductions will stall progress, disrupt the research enterprise, and jeopardize future discoveries. Without strong federal support for medical research, the United States risks falling behind in scientific innovation and losing momentum in efforts to prevent, treat, and ultimately cure cancer. Continued federal investments in NIH, NCI, FDA, and CDC are essential to preserve US leadership in medical research, support the next generation of cancer researchers, and ensure that future breakthroughs translate into longer, healthier lives for everyone.
Understanding the Path to Cancer Development
Decades of basic research have established cancer as a collection of diseases characterized by the uncontrolled growth of cells. Cancer development is driven by disruptions of the molecular and cellular functions that regulate how cells grow, divide, and survive. Many of these changes are often just the first step in a complex, multistep process that is influenced by changes both inside and outside the cell.
Research has shown that different cancer types share certain characteristics or hallmarks, including the ability of cancer cells to acquire changes that make their genome unstable, divide limitlessly, grow uncontrollably, escape cell death, spread to other tissues in the body, evade destruction by the immune system, and increase nutrients and oxygen supply to tumors.
One of the hallmarks of cancer cells is alterations in the DNA sequence, also called genetic mutations. There are two types of genetic mutations associated with cancer: germline and somatic. Germline mutations are inherited and passed down from parents to children and contribute to about 10 percent of all cancer cases. The remaining 90 percent of all cancer cases stem from somatic mutations, which are acquired throughout a person’s lifetime and can arise in multiple ways, such as due to errors made during cell division, or in response to environmental exposures, lifestyle factors, or because of chronic health conditions. In addition to DNA mutations, other changes inside the cell—including RNA variations, protein modifications, and epigenetic alterations—often work together to drive cancer initiation and progression.
Influences outside the cell also play a critical role in cancer development. These external factors include the circulatory and immune systems, the nervous system, and the microbiome. As the disease progresses, cancer cells acquire additional characteristics that allow them to shape and respond to their surroundings, creating the tumor microenvironment (TME). Research has shown that the TME affects the growth of cancer cells, and cancer cells influence the TME to promote their survival.
Technological advances have made it possible to study cancer at the levels of single cells and molecules, revealing that each patient’s cancer is unique. This foundational insight is the basis for precision medicine, or personalized medicine, which is broadly defined as treating patients based on molecular characteristics that distinguish them from other individuals with the same disease. Rapid developments in precision medicine are yielding new and effective ways to make a diagnosis, plan treatment, and predict and monitor treatment response.
Reducing the Risk of Cancer Development
Advances in basic, translational, and clinical research and population sciences have broadened our understanding of factors that contribute to an individual’s risk of developing cancer. Approximately 40 percent of all cancers in the United States are attributable to modifiable causes. These risk factors include tobacco use, excess body weight, physical inactivity, excess exposure to ultraviolet (UV) radiation, alcohol consumption, environmental factors, and cancer-causing pathogenic infections.
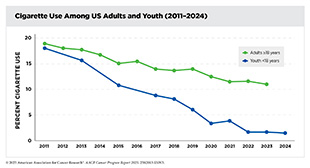
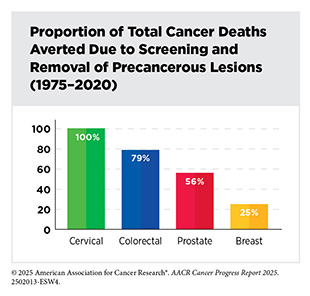
Between 1975 and 2020, approximately 5.94 million cancer deaths were averted due to public health interventions focused on tobacco control, national screening programs, and vaccination initiatives. While smoking rates have declined significantly, the rising prevalence of other risk factors, particularly obesity among children and adults as well as alcohol consumption, remains a significant public health concern. If left unaddressed, these trends can jeopardize the progress made against cancer over the last 50 years.
In the United States, the prevalence of obesity among adults was 40.3 percent between 2021 and 2023. There is emerging evidence that weight loss interventions, such as bariatric surgery and glucagon-like peptide-1 (GLP-1) medications (e.g., Wegovy, Ozempic), reduce the risk of developing obesity-related cancers. When combined with a healthy diet and regular physical activity, these interventions can significantly promote weight loss in individuals with obesity, subsequently lowering the risk of cancers such as liver, colon, pancreatic, esophageal, and gallbladder.
While addressing modifiable risk factors is crucial in reducing the risk of developing certain cancers, psychosocial and systemic factors cannot be overlooked. Psychosocial factors, such as stress, have been linked to increased cancer risk. Stress may directly contribute to cancer development by promoting inflammation, disrupting hormone balance, and impairing immune function. It may also indirectly raise cancer risk by encouraging unhealthy behaviors such as smoking, excessive alcohol consumption, and poor dietary habits.
Environmental risk factors, which are often unavoidable, are present in the air, water, and food, and can contribute to cancer development. The rising incidence of lung cancer among individuals who have never smoked is increasingly linked to exposure to carcinogens found in air pollution. Another class of environmental carcinogens that is of increasing concern is per- and poly-fluoroalkyl substances (PFAS), which are a group of endocrine-disrupting chemicals used in household products, food packaging, textiles, and flame retardants. PFAS persist in the environment, increasing long-term exposure to these harmful substances. Exposure to PFAS has been linked to an increased risk for cancers of the kidney, liver, and thyroid.
In recent years, wildfires in the Western United States and Canada have grown more frequent and severe due to climate change. Individuals at greatest risk of exposures to the carcinogenic byproducts from wildfire suppression are those living near affected areas as well as firefighters or first responders involved in containment efforts. Exposure to these carcinogens increases the risk for multiple cancers including lung cancer and gliomas.
As our understanding of cancer risk factors evolve, it is essential to invest in collaborative and translational research that builds upon existing knowledge and accelerates the pace of progress in cancer prevention and control. Additionally, lawmakers should prioritize developing policies that protect all segments of the US population, especially those unable to avoid risk factors due to their geographic location or occupation.
Cancer Screening for Early Detection
Cancer screening means checking for signs of cancer or for cells that might become cancerous in people who have no symptoms of the disease. The goal is to find cancer early, when it is most treatable and the chances of better outcomes are the highest. A growing body of evidence shows that screening not only saves lives, but it also helps reduce the overall burden of cancer across the population.
In the United States, experts on the US Preventive Services Task Force (USPSTF) develop guidelines for individuals at an average risk of developing cancers of the breast, cervix, colon and rectum, and prostate, as well as for individuals at a higher risk of developing lung cancer. These recommendations are based on several factors, including a person’s age, sex, family history, lifestyle, and environmental exposures. The USPSTF regularly reviews new scientific evidence and, if needed, revises these screening guidelines.
Screening is a process that also involves follow-up care if something abnormal is detected. Unfortunately, many people, especially those in medically underserved communities, face barriers that prevent them from getting screened or receiving timely follow-up care. These barriers include lack of insurance, limited access to quality care, mistrust of the medical system, experiences of discrimination, and poor communication with providers.
Researchers have identified proven strategies that are helping more people get screened, such as incorporating patient navigation, using electronic health records to send reminders, making it easier to access testing, and building trust through approaches that are tailored to the needs of different communities.
Early detection of cancer is a very active area of research, with growing excitement around new technologies. During the 12 months covered by this report, FDA has approved several devices and software that use artificial intelligence (AI) to detect cancer earlier and more accurately. Another area of rapid progress is the development of minimally invasive screening approaches, with FDA approvals of a blood-based and a stool-based test for colorectal cancer screening, and of a self-collection device that enables at-home sample collection for cervical cancer screening. Innovations in imaging, as well as a better understanding of genetic alterations that increase a person’s cancer risk, are also improving early detection of cancer. These advances hold promise, but experts emphasize the need for long-term studies to ensure these tools improve outcomes without causing harm or widening existing cancer disparities.
Unifying Cancer Science and Medicine: A Continuum of Innovation for Impact
The dedicated efforts of researchers across the spectrum of cancer science and medicine are driving breakthroughs in clinical care that are improving survival and enhancing quality of life for patients in the United States and worldwide. Clinical trials are a cornerstone of medical research, providing the evidence needed to determine whether new cancer treatments are safe and effective. Advances in our understanding of the genetic drivers of cancer have led to innovative approaches to clinical trial design and execution. For patients with cancer, participating in clinical studies can be life-changing, as reflected in the personal stories of Michelle Anderson-Benjamin and Bob Fortin.
It is essential that clinical trial participants reflect the diversity of the US population that may use and ultimately benefit from these treatments. Yet, participation in cancer clinical trials remains low, with significant sociodemographic disparities among those enrolled. Addressing these challenges requires collaboration among researchers, clinicians, and policymakers to remove barriers and promote equitable access to research opportunities.
Surgery, radiotherapy, and chemotherapy are three of the five foundational pillars of cancer treatment. While effective, these therapies can have long-term side effects. Ongoing clinical studies are exploring whether less intensive use of these modalities can provide comparable outcomes for some patients while improving their quality of life. In parallel, more refined uses, such as molecularly targeted radiotherapeutics and optimized chemotherapeutic formulations, are being integrated into standard care.
Fueled by research, innovative personalized cancer treatments are rapidly moving from the laboratory to the clinic, offering patients safer and more effective options than ever before. Between July 1, 2024 and June 30, 2025, FDA approved 20 new therapeutics for treating various cancer types, one new device for treating lung cancer, and expanded indications for eight previously approved therapies to include new cancer types.
These advances include several groundbreaking immunotherapies. For example, afamitresgene autoleucel, a first-in-class adoptive cell therapy, known as T-cell receptor (TCR) T-cell therapy, is offering new hope to patients with synovial sarcoma, such as Quinn Johnsen.
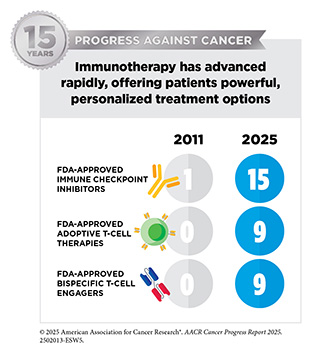
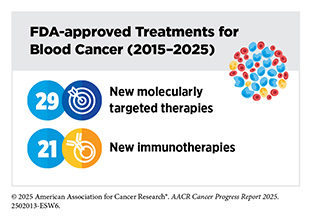
A new therapeutic antibody, zolbetuximab-clzb (Vyloy), targeting a novel protein on stomach cancer cells is now benefiting individuals with gastroesophageal cancer, such as Greg Myers. These approvals reflect the growing potential of immunotherapy to tackle rare and intractable cancers. The dramatic rise in the use of immune checkpoint inhibitors—immunotherapies that help restore the immune system’s ability to recognize and attack cancer cells—further underscores this potential. Since the first AACR Cancer Progress Report was released in 2011, the number of FDA-approved ICIs has grown from just one for melanoma to 15 therapies now used to treat more than 20 cancer types.
Equally impressive are the innovative newly approved molecularly targeted therapies that are reshaping and improving cancer care. A new combination regimen comprising avutometinib and defactinib is now providing effective treatment for certain types of ovarian cancer, as seen in the experience of Mary Catherine Riley. Patients with low-grade glioma, such as Alex Hepner, are benefiting from vorasidenib (Voranigo), the first targeted therapy that inhibits mutated forms of the proteins IDH1 and IDH2 in brain tumors. For patients such as Dawn Varrati, diagnosed with the aggressive bile duct cancer cholangiocarcinoma, the approval of zanidatamab-hrii (Ziihera), a new bispecific antibody, has opened new treatment possibilities. These cutting-edge therapies exemplify how deeper biological insights are translating into more precise and effective treatments for patients with difficult-to-treat cancers.
While these developments mark significant progress against cancer, much work remains to ensure that all patient populations have access to the latest innovations in cancer treatment.
A Decade of Progress: Transformative Advances in Blood Cancer
Blood cancers, also known as hematologic malignancies, are a group of cancers that begin in blood-forming tissues of the body, such as the bone marrow. These cancers are broadly categorized as leukemia, lymphoma, and multiple myeloma, and pose a significant public health challenge in the United States and globally. In 2025, an estimated 192,070 new cases will be diagnosed, and 56,110 deaths will occur in the United States from the three most common blood cancer types (leukemia, lymphoma, and multiple myeloma); 1,393,600 new cases and 745,000 deaths are estimated globally.
Thanks to research-driven scientific breakthroughs and technological innovations, death rates from blood cancers have declined significantly, and many people diagnosed today are living longer and fuller lives. As a result, once considered among the most difficult cancers to treat, blood cancers are increasingly becoming manageable or even curable for many patients. As an example, mortality rates for non-Hodgkin lymphoma (NHL)—the most common type of blood cancer in the United States—have declined by 47 percent between 1997 and 2023. As of January 1, 2025, over 1.67 million survivors of blood cancers were living in the United States.
Deeper understanding of molecular underpinnings of blood cancers, propelled by significant federal investments in discovery science, led to the development and approval of one of the first molecularly targeted therapeutics, imatinib (Gleevec), for the treatment of chronic myelogenous leukemia (CML). Imatinib not only transformed clinical care of patients with CML, but it also catalyzed a revolution in precision medicine that has significantly improved health outcomes for millions of patients with cancer. A remarkable example of progress in blood cancer precision medicine is the recent approval of revumenib, the first menin-targeted therapy, offering a new treatment option for patients with acute myeloid leukemia (AML), such as John C. (Jack) Moorman.
Progress against blood cancers has also paved the way for breakthroughs in other areas of medicine. Many treatments originally developed for leukemia or lymphoma are now being evaluated, and in some cases successfully used, for solid tumors. Some discoveries in blood cancers have even had applications in treating autoimmune diseases and rare genetic disorders.
Despite rapid strides against blood cancers over the past decade, advances in precision medicine are not benefiting everyone equally. In the United States, certain populations, such as residents of rural areas and medically underserved populations, still face significant barriers to accessing the latest treatments. Similarly, in many low- and middle-income countries, people with blood cancers often go undiagnosed, untreated, or they receive care that is outdated due to lack of resources and infrastructure.
Although challenges such as access to cutting-edge treatments remain, the pace of progress against blood cancers has never been more rapid. Researchers are innovating clinical trial design to inform clinical decisions, such as whether to monitor or intercept blood cancer and whether a patient would benefit from stopping the treatment. These innovations are leading the way to develop similar approaches for solid tumors. Continued federal investment in research and greater collaboration across all sectors are vital to ensure access to quality care and secure a brighter future for all patients with blood cancers.
Supporting Cancer Patients and Survivors
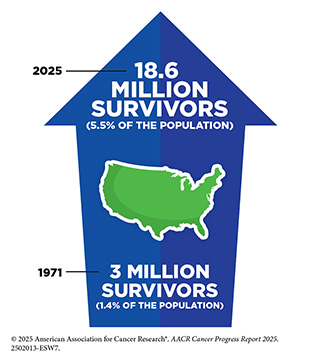
According to NCI, a person is considered a cancer survivor from the time of cancer diagnosis through the balance of their life. Unprecedented advances in cancer treatments over the past decade have led to more patients living longer and fuller lives after a cancer diagnosis. As of January 1, 2025, more than 18.6 million adults and children with a history of cancer were living in the United States, which equates to about 5.5 percent of the population. This is a substantial improvement from 50 years ago, when cancer survivors constituted only 1.4 percent of the US population. The number of cancer survivors is projected to grow to over 22 million by 2035.
Depending on the type and stage of cancer, as well as the age at which an individual is diagnosed, cancer survivorship can encompass a wide range of unique experiences. For example, some people may live cancer free for the remainder of life following successful treatment, while others may manage cancer as a chronic illness requiring ongoing treatment and monitoring, experience persistent side effects, or face a recurrence or a new cancer diagnosis. Understanding and addressing the physical, psychosocial, and financial challenges faced by cancer survivors, supporting their quality of life, and ensuring that care is accessible and equitable are important priorities in cancer survivorship research.
Research has shown that maintaining a healthy diet, staying physically active, limiting alcohol intake, and quitting smoking can help mitigate the physical challenges associated with a cancer diagnosis. Researchers are also using other evidence-based strategies, including palliative care, behavioral health interventions, patient-reported outcomes, and patient navigation, to help reduce the adverse impact of a cancer diagnosis on the physical and emotional health of cancer survivors. Understanding the challenges experienced by cancer survivors, as well as how to reduce or eliminate them, is an active and continually evolving area of research.
Challenges experienced by patients and survivors of cancer also extend to friends and family members who are often informal caregivers. It is estimated that over four million caregivers are caring for an adult cancer patient in the United States. These caregivers support cancer survivors in various ways, such as arranging transportation for medical appointments, helping with day-to-day activities, coordinating care, and providing emotional support. While fulfilling the caregiving role, this can be physically, emotionally, and financially demanding. Caregivers are at an increased risk for psychological distress, and many face disruptions to their employment, education, or personal well-being. As the number of cancer survivors continues to grow, advancing survivorship and caregiver support through research, policy, and practice is essential to improving outcomes for all those affected by cancer.
Envisioning the Future of Cancer Science and Medicine
Breakthrough discoveries and technological advances across the fields of medicine have substantially increased our understanding of cancer initiation and progression. This foundational knowledge is driving better strategies to reduce the risk of developing cancer, detect cancer at the earliest possible stage, and treat cancer effectively and more precisely—with fewer long-term side effects. As a result, cancer deaths are declining, and cancer survivors are living longer and fuller lives.
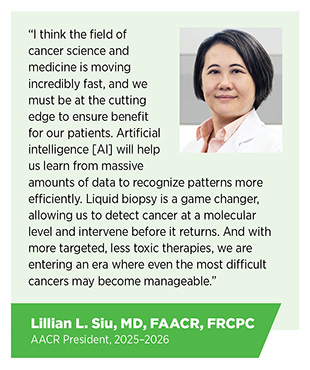
Technological and therapeutic advances against cancer and their impact on saving and improving lives of patients are a source of great optimism for cancer researchers, including AACR President, 2025–2026, Lillian L. Siu, MD, FAACR, FRCPC.
Emerging technologies are driving progress in cancer research by enabling researchers to investigate tumors with greater precision and gain deeper biological insights. AI is transforming cancer research and patient care by enabling faster, more accurate, and personalized approaches across the cancer continuum. AI is now being applied to analyze histopathology images, identify genomic alterations, discover new drug candidates, improve early detection, streamline drug development, and support clinical decision-making.
CRISPR gene editing tools are advancing cancer research and drug development by enabling disease modeling, high-throughput drug screening, and the development of new therapeutic strategies. CRISPR-based systems are being used to uncover which mutations drive tumor development, identify vulnerabilities in cancer cells, and design personalized treatment approaches.
Another rapidly advancing technology is liquid biopsy, the analysis of blood or other body fluids for tumor-derived material routinely shed during cancer development and treatment. Liquid biopsy offers a minimally invasive alternative to the traditional tissue biopsy and is being integrated into efforts to enhance early detection, improve diagnosis, inform treatment selection, and monitor disease progression or recurrence.
Emerging therapies, many of which are still the focus of ongoing research, are advancing the frontiers of precision oncology and are poised to transform the future of cancer care. One exciting area is the development of cancer vaccines that either prevent cancer in high-risk individuals or treat existing disease by training the immune system to recognize and attack tumor-specific targets. Preventive cancer vaccines that target the molecular characteristics of cancer hold great promise to reduce cancer risk, lessen the burden of ongoing surveillance, and reshape how we approach cancer prevention. Therapeutic cancer vaccines are designed to activate a patient’s immune system after cancer has developed, often by targeting unique proteins found on cancer cells.
Another promising class of therapies is radiopharmaceuticals, including radioligand therapies (RLTs), which deliver cytotoxic radiation directly to cancer cells. RLTs combine a radioactive compound with a targeting agent that binds to specific proteins expressed on the surface of cancer cells, enabling the delivery of high-energy radiation to tumors while sparing surrounding healthy tissues and offering a highly localized, tumor-specific radiotherapy approach.
Advancing Cancer Science and Medicine Through Evidence-based Policies
Federal government policies play a critical role in advancing cancer research, developing safe and effective new treatments, improving patient outcomes, and supporting the next generation of researchers and physician-scientists. Sustained investments over decades in vitally important agencies such as NIH, NCI, FDA, and CDC have led to significant progress across the cancer care continuum—from improvements in prevention and early detection to breakthroughs in treatment and survivorship. These agencies support the discovery of lifesaving therapies, strengthen the clinical trial infrastructure, train the scientific workforce, and expand access to cancer screening and care. Federal funding of medical research also spurs our nation’s economy. Every $1 in NIH funding returns $2.56 in economic activity.
The proposed $17.97 billion reduction to NIH funding in the administration’s FY 2026 budget would severely undermine this progress and jeopardize the nation’s leadership in medical research. Such drastic reductions would stall scientific discovery, limit access to promising new therapies for patients such as Richard Schlueter, and discourage the next generation of researchers at a critical time.
Therefore, AACR was thrilled that the Senate Appropriations Committee voted in a bipartisan manner on July 31, 2025, to advance the FY 2026 Labor, Health and Human Services, and Education (Labor-HHS-Ed) Appropriations Subcommittee bill and provide $47.2 billion for NIH and $7.374 billion for NCI, reflecting funding increases in these agencies of $400 million and $150 million, respectively.
According to a recent poll, 77 percent of Americans oppose reducing federal funding for medical research. By rejecting the administration’s proposed cuts to NIH, the US Senate aligned with the public’s priorities, recognizing the vital role of NIH in accelerating progress against cancer and countless other diseases affecting millions of Americans. These bipartisan actions are essential to protecting our nation’s scientific enterprise, preserving public trust in science, and sustaining the lifesaving research on which patients and families depend.
AACR Call to Action
For more than 50 years, bipartisan investment in medical research has driven historic progress against cancer, delivering earlier detection, better treatments, and more time for millions of patients and families. That progress is now in jeopardy. Prolonged funding uncertainty and administrative and political interference are weakening cancer research, undermining scientific integrity, and eroding the infrastructure that turns discovery into patient care.
Promising signs of bipartisan resolve in Congress show that this damage can be stopped and progress restored. The fight against cancer is at a decisive moment, and lawmakers have the power to ensure that promising science moves forward, discoveries reach patients, and hope for a future without cancer is not lost.
AACR urges Congress to take immediate action to:
- Restart clinical trials and restore canceled research grants to ensure that patients are not turned away from lifesaving studies and that promising science is not lost at a critical stage.
- Support the federal research infrastructure to repair the damage caused by mass reductions in workforce, frozen contracts, and suspended peer review. Discovery has stalled, and scientific capacity is breaking down.
- Protect public health programs that prevent cancer to avoid losing ground on screening, HPV vaccination, tobacco cessation, and early interventions. These efforts save lives.
- Ensure that new treatments reach patients without delay to prevent promising therapies from being trapped in bureaucratic limbo while families wait for help that may come too late.
- Foster early-career and early-stage scientists and stabilize research careers to stop the exodus of postdoctoral researchers and junior investigators who are abandoning science or being recruited overseas. When they leave, they take future cures with them.
- Defend the independence and integrity of science by reversing the August executive order that politicizes federal grantmaking, thus restoring safeguards that keep research free from political interference and ensuring that grantmaking, peer review, and public policies are guided by scientific evidence rather than ideology.
- Reassert America’s global leadership in medical innovation to preserve decades of progress and prevent other nations from overtaking the United States in the race for the next generation of cures.
- Provide no less than $51.303 billion for NIH and $7.934 billion for NCI in fiscal year 2026 to sustain the scientific workforce, power new breakthroughs against cancer and other human diseases, and uphold a national commitment to the patients and families who are relying on lifesaving progress.
Cancer touches every family, every community, and every generation. At this defining moment, Congress owes it to every patient, every survivor, and every family to protect the progress we have made and deliver on the promise of a future without cancer.
Next Section: Snapshot of a Year of Progress Previous Section: Message from AACR